Melatonin Suppresses the Growth of Ovarian Cancer Cell Lines (OVCAR-429 and PA-1) and Potentiates the Effect of G1 Arrest by Targeting CDKs
Abstract
:1. Introduction
2. Results and Discussion
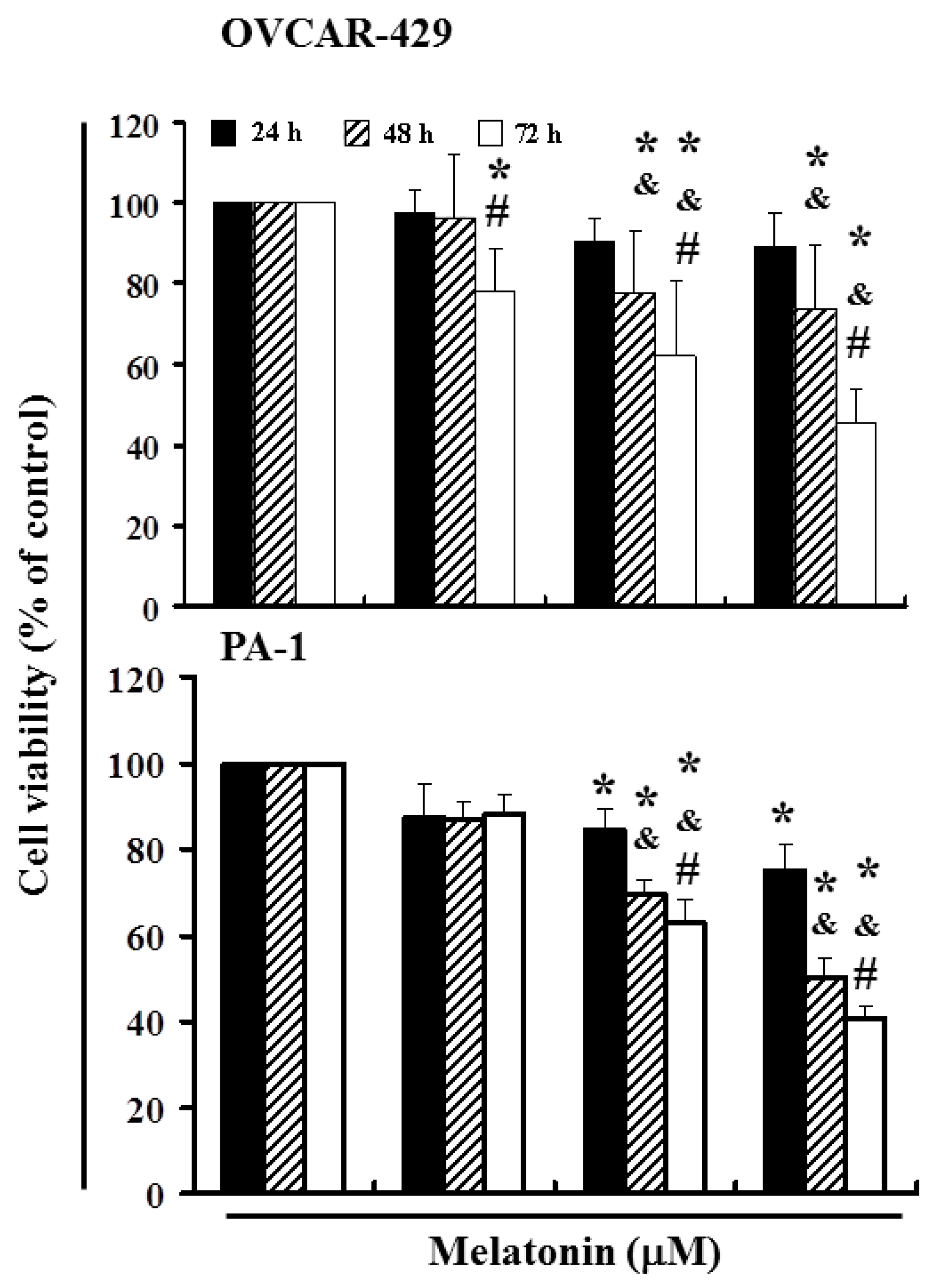
2.1. Melatonin Can Mediate the Survival of Ovarian Cancer Cells and Thereby Inhibit Proliferation
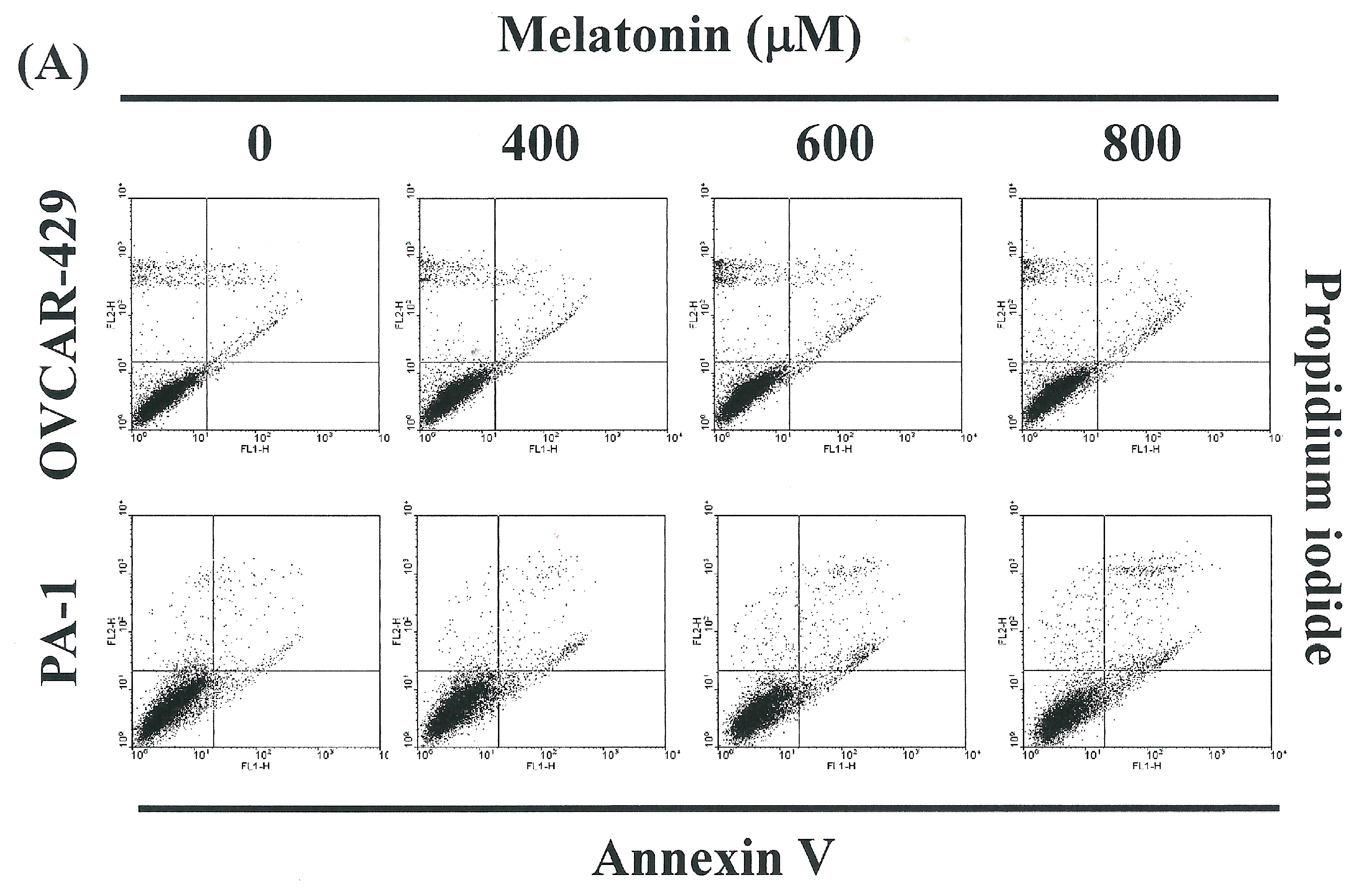
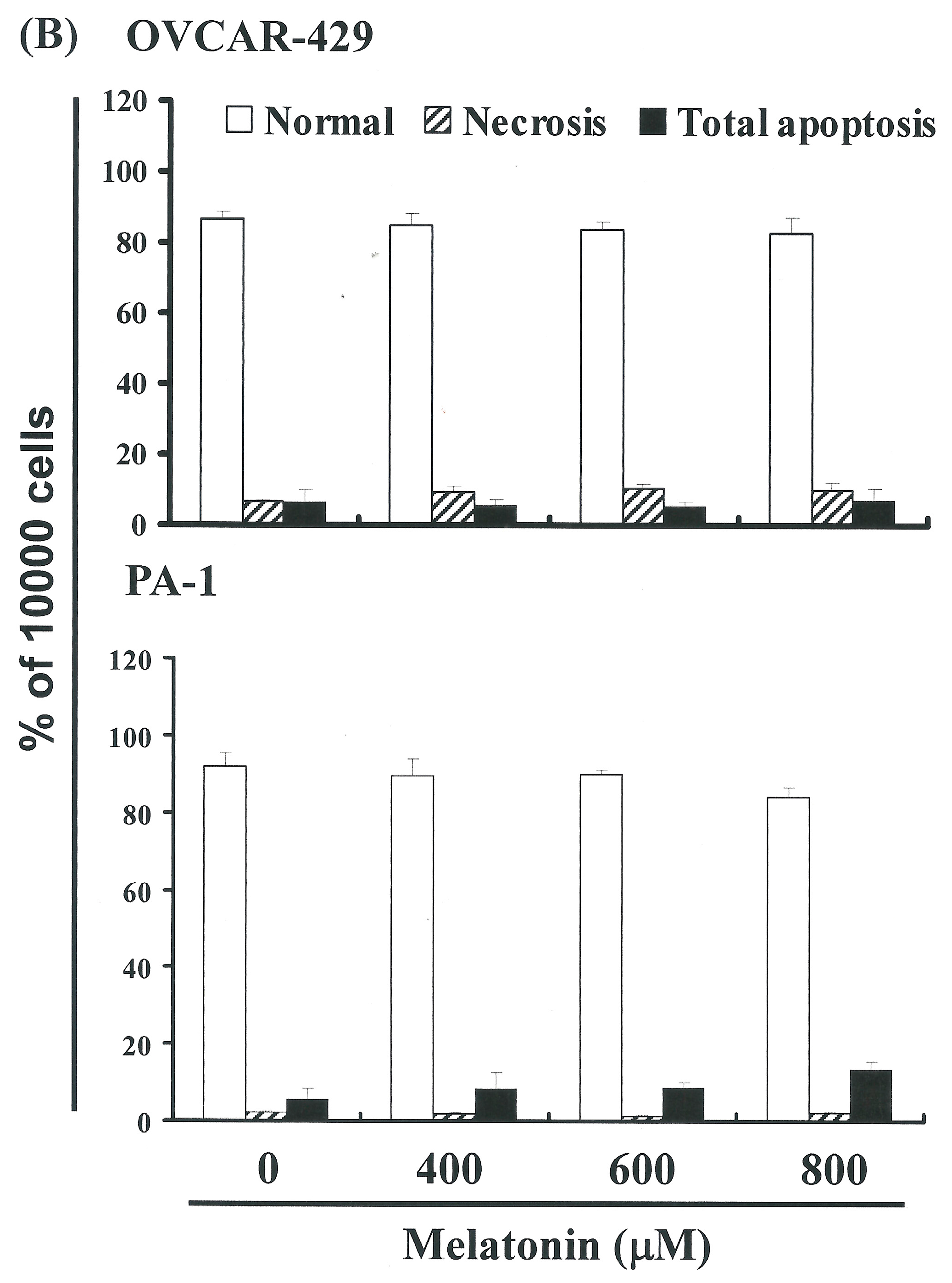
2.2. Non-Melatonin-Induced Apoptosis/Necrosis of OVCAR-429 and PA-1 Cell Lines
2.3. Melatonin-Induced Accumulation of Melatonin-Treated Cells in the G1 Phase
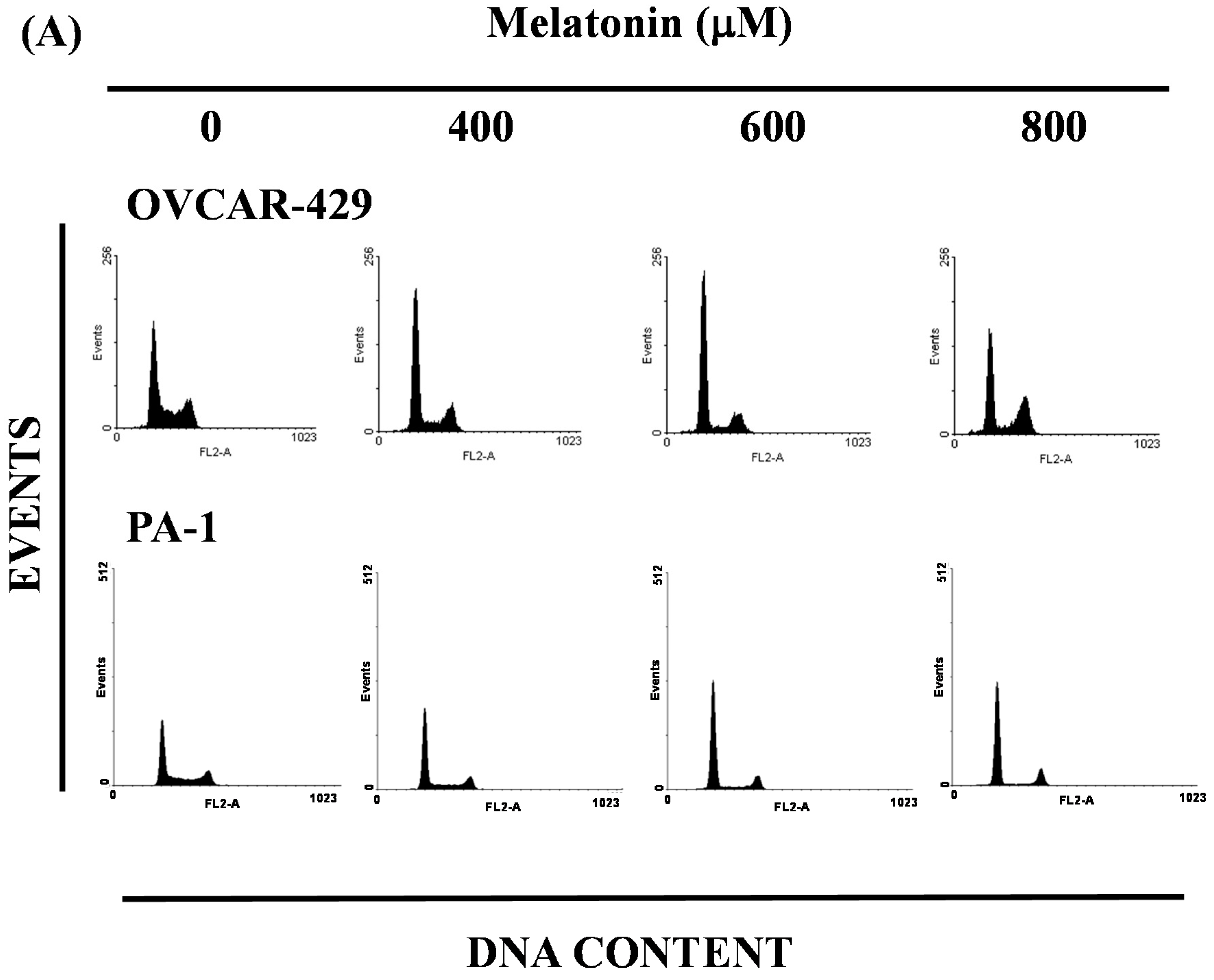

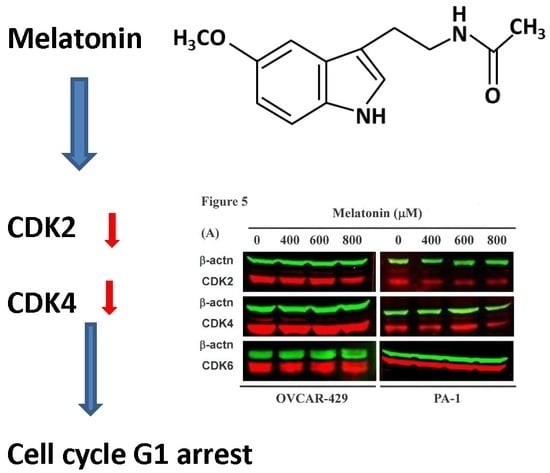
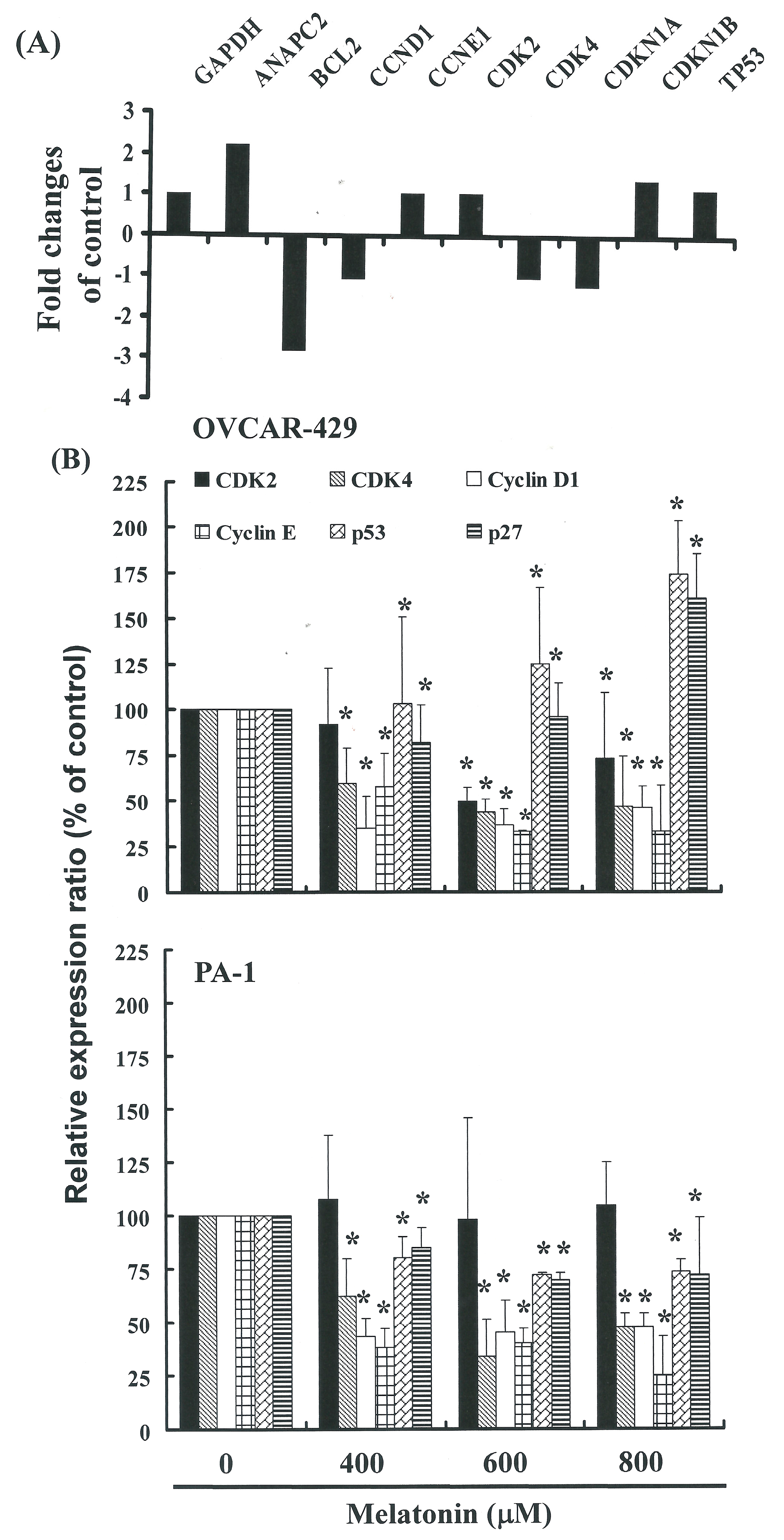
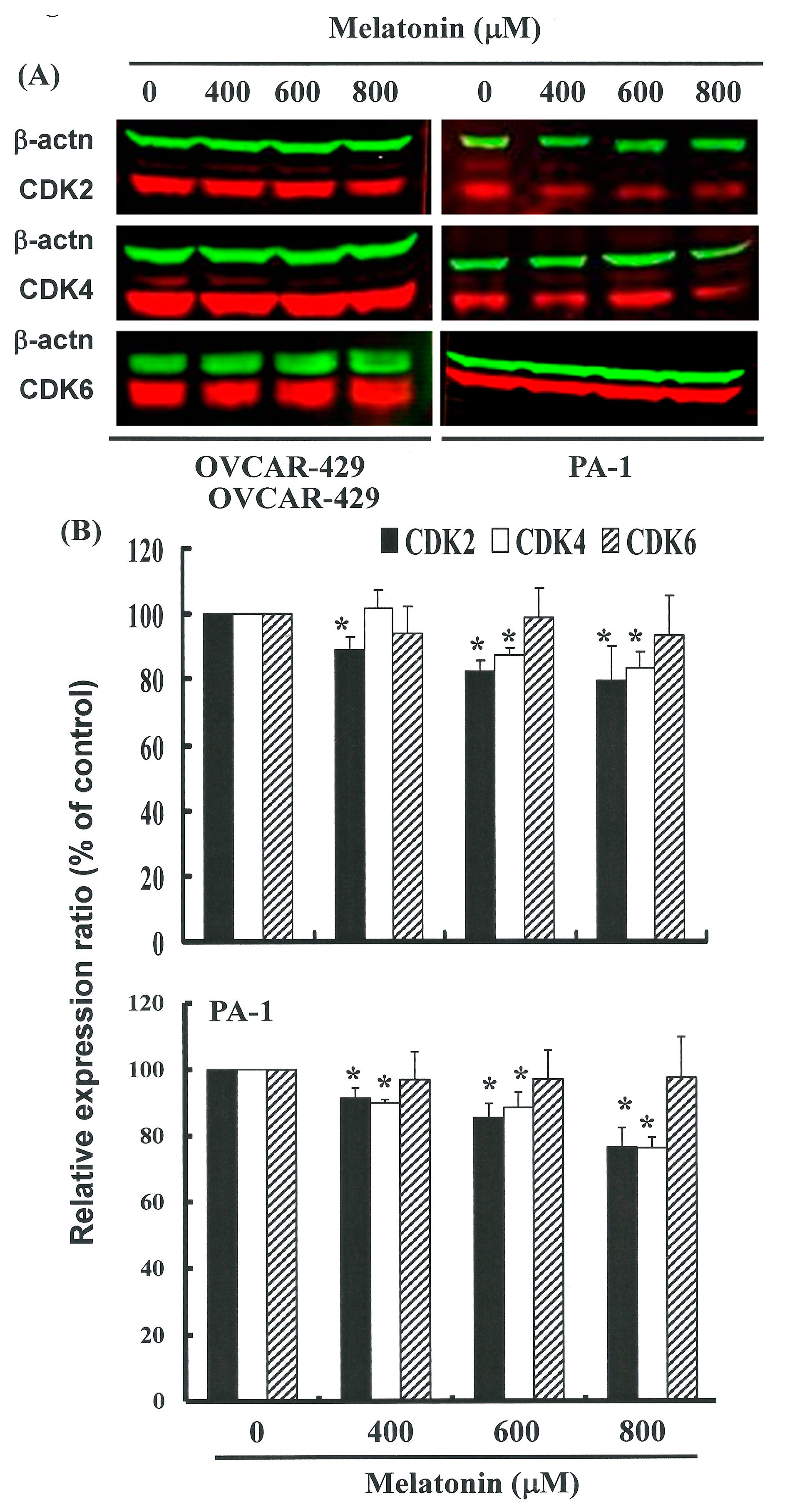
2.4. G1 Phase Arrest in Melatonin-Treated Cells via down Regulation of CDK2 and 4
3. Experimental Section
3.1. Materials
3.2. Cells
3.3. Cell Proliferation Assay
3.4. Measurement of Apoptosis
3.5. Cell Cycle Analysis
3.6. Western Blot Assay
3.7. Gene Expression Profiling (GEP)
3.8. RT-PCR
3.9. Real-Time PCR
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suh, D.H.; Lee, K.H.; Kim, K.; Kang, S.; Kim, J.W. Major clinical research advances in gynecologic cancer in 2014. J. Gynecol. Oncol. 2015, 26, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Okeke, T.C.; Anyaehie, U.B.; Ezenyeaku, C.C. Premature menopause. Ann. Med. Health Sci. Res. 2013, 3, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N. Fertility preservation in female cancer patients: An overview. J. Hum. Reprod. Sci. 2015, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kamieniak, M.M.; Muñoz-Repeto, I.; Rico, D.; Osorio, A.; Urioste, M.; García-Donas, J.; Hernando, S.; Robles-Díaz, L.; Ramón Y Cajal, T.; Cazorla, A.; et al. DNA copy number profiling reveals extensive genomic loss in hereditary BRCA1 and BRCA2 ovarian carcinomas. Br. J. Cancer 2013, 108, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Olaparib: A review of its use as maintenance therapy in patients with ovarian cancer. BioDrugs 2015, 29, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Guerlotte, J.; Cogne, M.; Greve, P.; Collin, J.P.; Voisin, P. Transcriptional regulation of hydroxyindole O-methyltransferase in the chicken pineal gland: Day/night changes and long-term effects of light and darkness. Biochem. J. 1993, 290, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.E.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.M. Role of melatonin in embryo fetal development. J. Med. Life 2014, 7, 488–492. [Google Scholar] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Kaur, G. Potential role of melatonin in prevention and treatment of oral carcinoma. Indian J. Dent. 2014, 5, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G.; Brainard, G.C.; Blask, D.E.; Lockley, S.W.; Motta, M.E. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 2014, 64, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, J.; Leja-Szpak, A. Melatonin influences pancreatic cancerogenesis. Histol. Histopathol. 2014, 29, 423–431. [Google Scholar] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Antoniello, N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: Review and remarks. Aging Clin. Exp. Res. 2013, 25, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Proietti, S.; Cucina, A.; Reiter, R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: A review. Expert Opin. Ther. Targets 2013, 17, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Pariente, J.A.; Rodríguez, A.B. Oxidative stress and immunosenescence: Therapeutic effects of melatonin. Oxid. Med. Cell. Longev. 2012, 2012, 670294. [Google Scholar] [CrossRef] [PubMed]
- Martín-Renedo, J.; Mauriz, J.L.; Jorquera, F.; Ruiz-Andrés, O.; González, P.; González-Gallego, J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J. Pineal Res. 2008, 45, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Dorigo, O.; Berek, J.S.; Kohrt, H. Immunotherapeutic approaches to ovarian cancer treatment. J. Immunother. Cancer 2015, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Tuefferd, M.; Couturier, J.; Penault-Llorca, F.; Vincent-Salomon, A.; Broët, P.; Guastalla, J.P.; Allouache, D.; Combe, M.; Weber, B.; Pujade-Lauraine, E.; et al. HER2 status in ovarian carcinomas: A multicenter GINECO study of 320 patients. PLoS ONE. 2007, 2, e1138. [Google Scholar] [CrossRef] [PubMed]
- De Jonage-Canonico, M.B.; Lenoir, V.; Martin, A.; Scholler, R.; Kerdelhué, B. Long term inhibition by Estradiol or Progesterone of Melatonin secretion after administration of a mammary carcinogen, the dimethyl benz(a)anthracene, in Sprague-Dawley female rat; inhibitory effect of Melatonin on mammary carcinogenesis. Breast Cancer Res. Treat. 2003, 79, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, W.S.; Travlos, G.S.; Risinger, J.I.; Barrett, J.C. Melatonin does not inhibit estradiol-stimulated proliferation in MCF-7 and BG-1 cells. Carcinogenesis 1998, 19, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, K.; Pula, B.; Zemla, A.; Kobierzycki, C.; Kedzia, W.; Nowak-Markwitz, E.; Spaczynski, M.; Zabel, M.; Podhorska-Okolow, M.; Dziegiel, P. Expression of the MT1 melatonin receptor in ovarian cancer cells. Int. J. Mol. Sci. 2014, 15, 23074–23089. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, S.J.; Kim, B.; Yun, S.M.; Choi, do. Y.; Kim, S.H. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J. Pineal Res. 2012, 52, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Yanaihara, N.; Anglesio, M.S.; Ochiai, K.; Hirata, Y.; Saito, M.; Nagata, C.; Iida, Y.; Takakura, S.; Yamada, K.; Tanaka, T.; et al. Cytokine gene expression signature in ovarian clear cell carcinoma. Int. J. Oncol. 2012, 41, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, Y.; Fujita, Y.; Kuwabara, H.; Nagasaki, M.; Komai, T.; Oda, T. Activation of T cell death-associated gene 8 regulates the cytokine production of T cells and macrophages in vitro. Eur. J. Pharmacol. 2012, 683, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Markowska, M.; Mrozkowiak, A.; Pawlak, J.; Skwarło-Sońta, K. Intracellular second messengers involved in melatonin signal transduction in chicken splenocytes in vitro. J. Pineal Res. 2004, 37, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and central effects of melatonin on blood pressure regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-J.; Chang, C.-C.; Chen, Y.-T.; Lai, C.-S.; Hsu, Y.-C. Melatonin Suppresses the Growth of Ovarian Cancer Cell Lines (OVCAR-429 and PA-1) and Potentiates the Effect of G1 Arrest by Targeting CDKs. Int. J. Mol. Sci. 2016, 17, 176. https://doi.org/10.3390/ijms17020176
Shen C-J, Chang C-C, Chen Y-T, Lai C-S, Hsu Y-C. Melatonin Suppresses the Growth of Ovarian Cancer Cell Lines (OVCAR-429 and PA-1) and Potentiates the Effect of G1 Arrest by Targeting CDKs. International Journal of Molecular Sciences. 2016; 17(2):176. https://doi.org/10.3390/ijms17020176
Chicago/Turabian StyleShen, Ching-Ju, Chi-Chang Chang, Yi-Tz Chen, Chung-Sheng Lai, and Yi-Chiang Hsu. 2016. "Melatonin Suppresses the Growth of Ovarian Cancer Cell Lines (OVCAR-429 and PA-1) and Potentiates the Effect of G1 Arrest by Targeting CDKs" International Journal of Molecular Sciences 17, no. 2: 176. https://doi.org/10.3390/ijms17020176
APA StyleShen, C.-J., Chang, C.-C., Chen, Y.-T., Lai, C.-S., & Hsu, Y.-C. (2016). Melatonin Suppresses the Growth of Ovarian Cancer Cell Lines (OVCAR-429 and PA-1) and Potentiates the Effect of G1 Arrest by Targeting CDKs. International Journal of Molecular Sciences, 17(2), 176. https://doi.org/10.3390/ijms17020176