Enhanced Production of Anthraquinones and Phenolic Compounds and Biological Activities in the Cell Suspension Cultures of Polygonum multiflorum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Establishment of Callus Culture
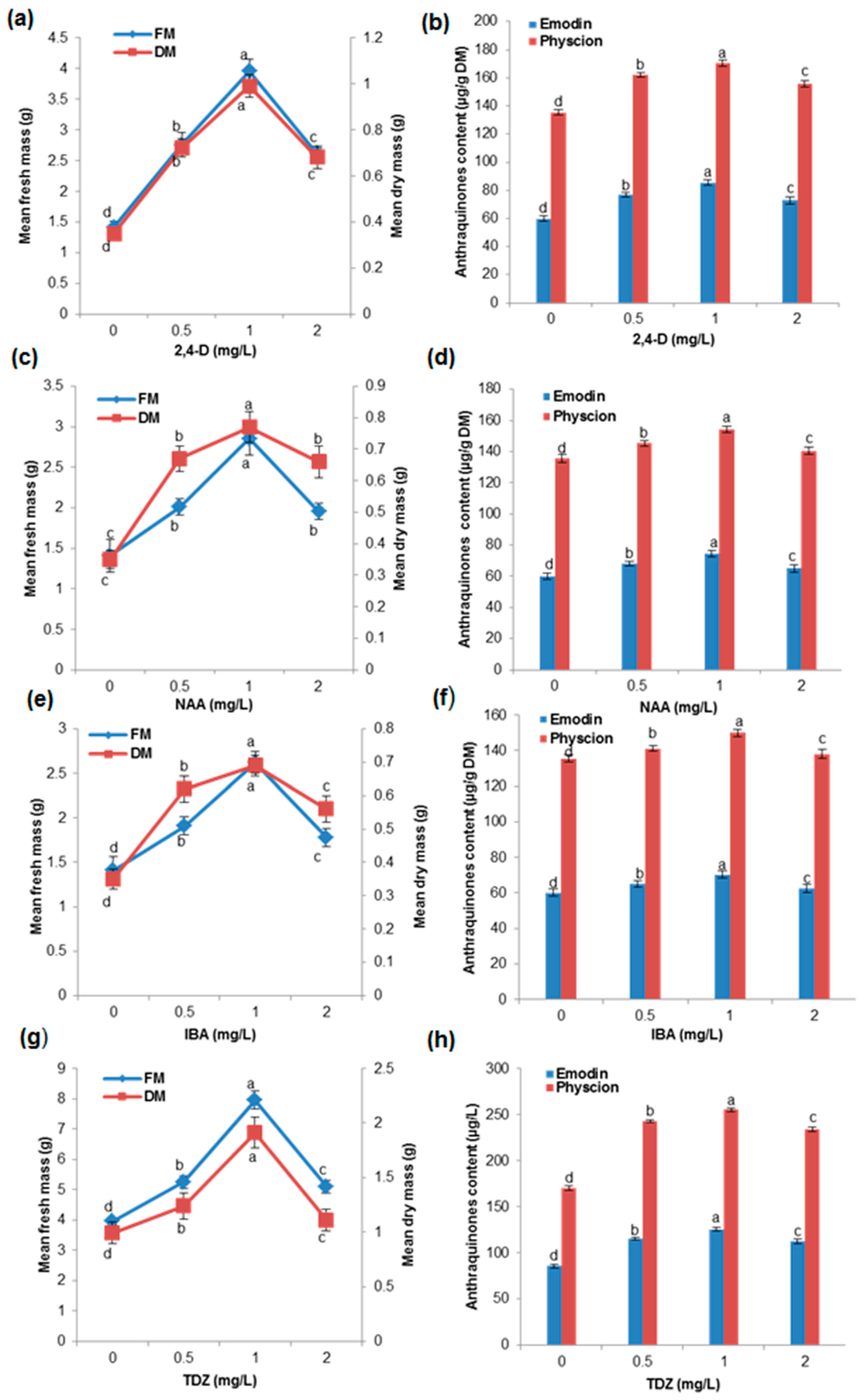
2.2. Effects of Different Concentration of Auxins and Cytokinins on Biomass Accumulation and AQ Production in the Cell Suspension Culture
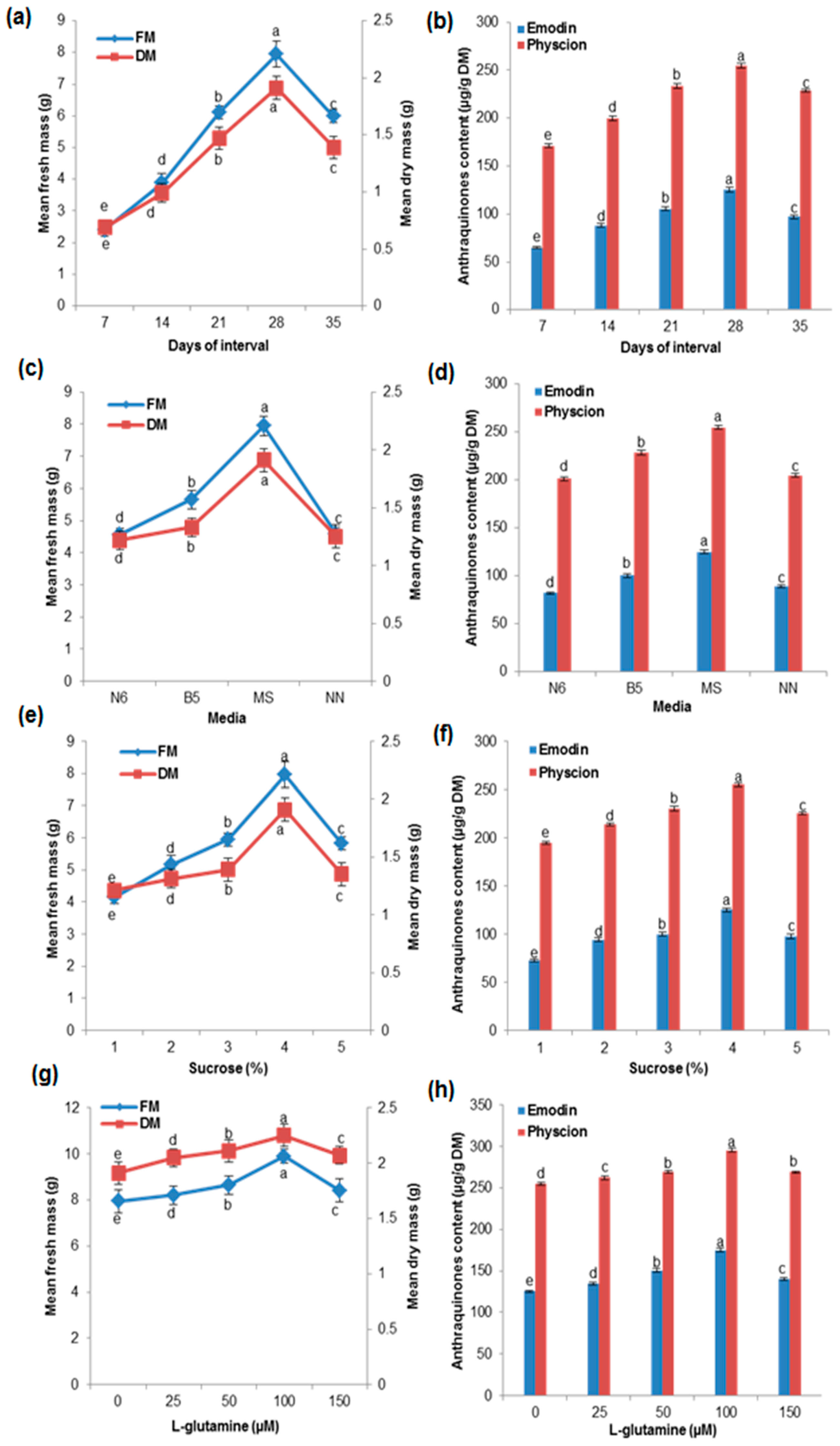
2.3. Effects of Growth Kinetics on Biomass Accumulation and AQ Production in the Cell Suspension Culture
2.4. Effects of Various Media and Different Concentrations of Sucrose and Glutamine on Biomass Accumulation and AQ Production in the Cell Suspension Culture
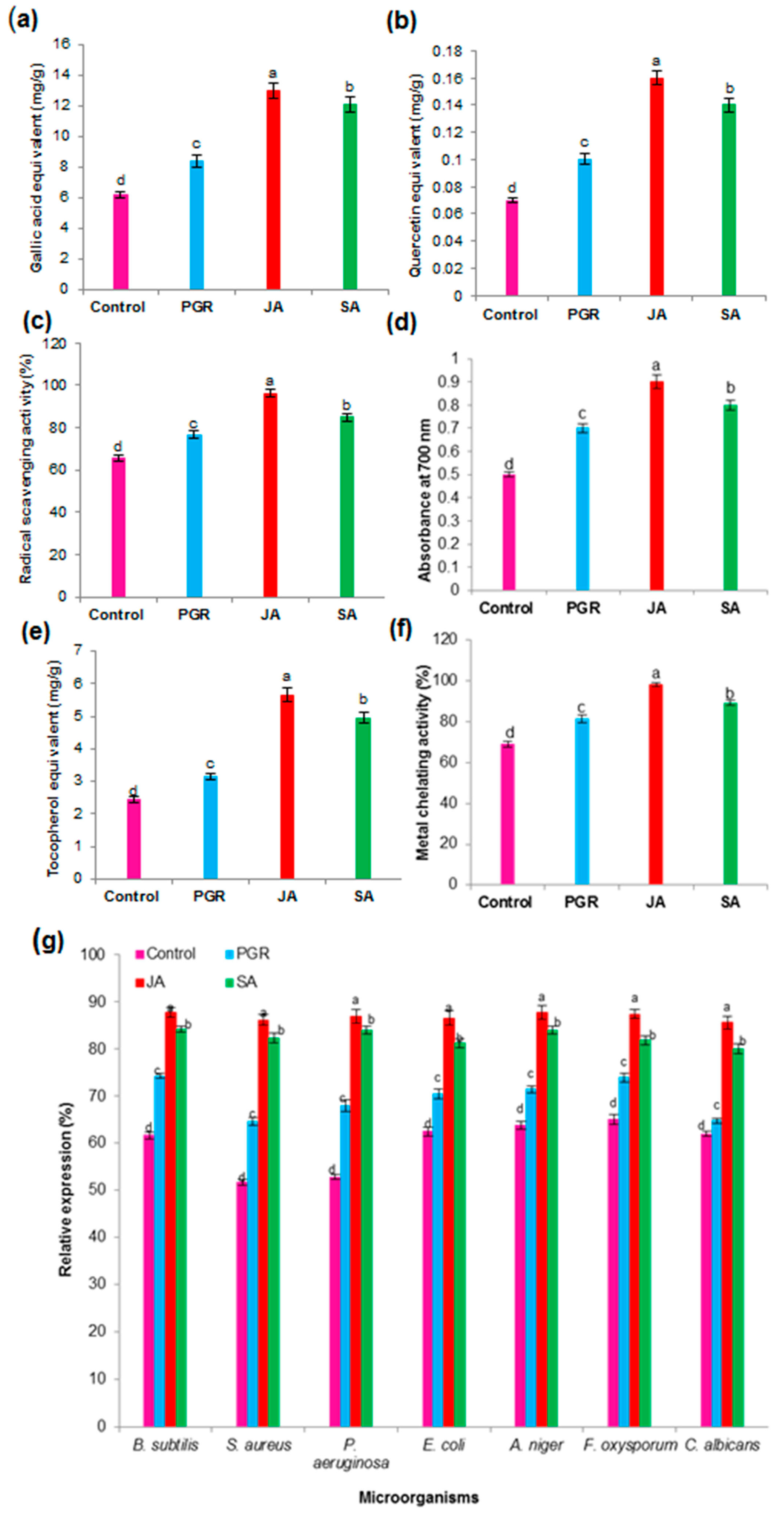
2.5. Effects of Elicitors (JA and SA) on Biomass Accumulation and AQ Production in the Cell Suspension Culture
2.6. Phenolic Compound Profiles in the Cell Suspension Culture
2.6.1. Individual Phenolic Compounds
2.6.2. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in the Cell Suspension Culture
2.6.3. Antioxidant Activity in the Cell Suspension Culture
2.6.4. Antimicrobial Activity in the Cell Suspension Culture
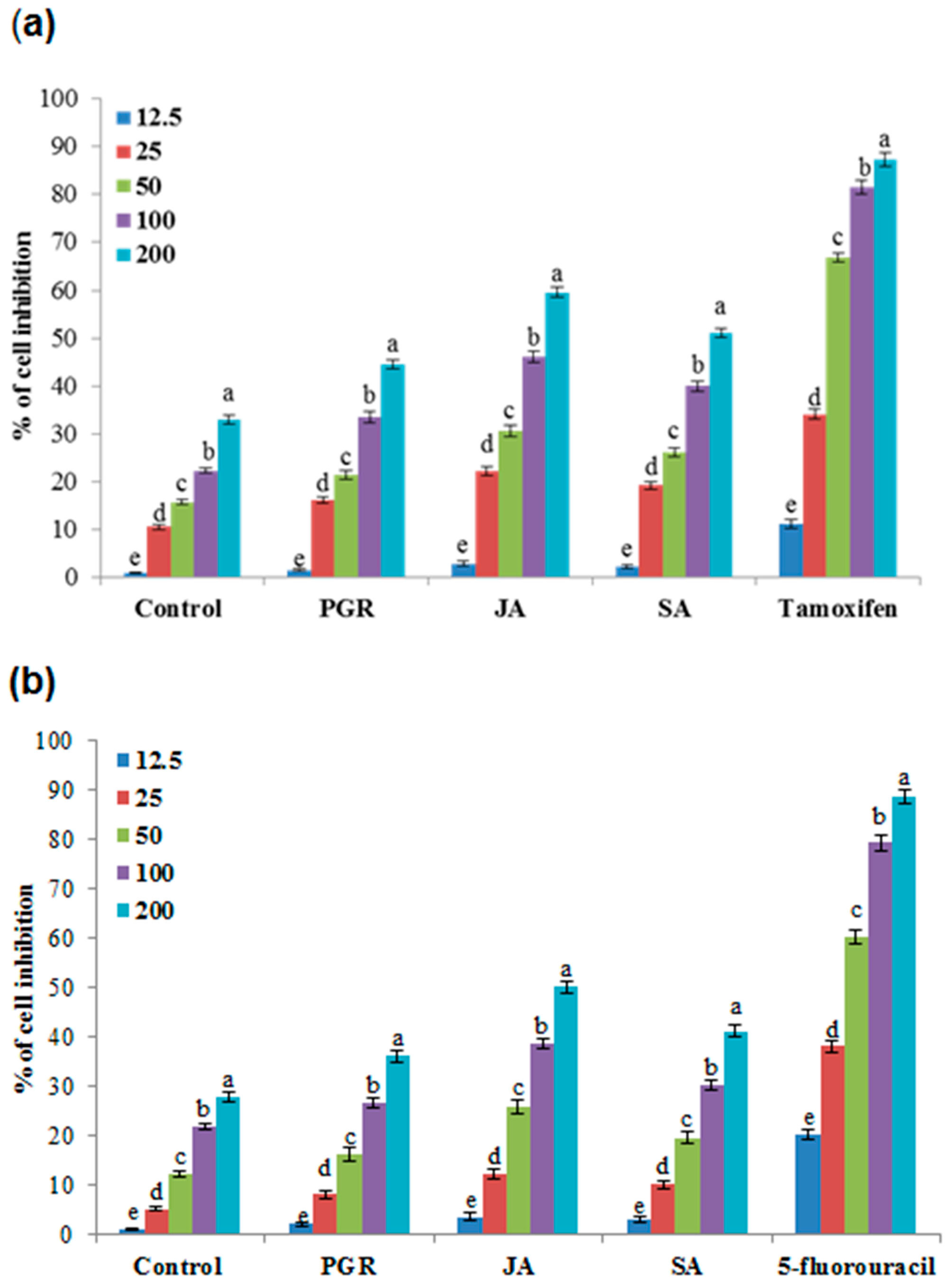
2.6.5. Anticancer Activity in the Cell Suspension Culture
3. Materials and Methods
3.1. Plant Materials
3.2. Establishment of Callus and Cell Suspension Cultures
3.3. Effects of Auxins, Cytokinins, Amino Acid (Glutamine), Media, and Carbon (Sucrose) Sources for Biomass Accumulation and AQ Production
3.4. Effects of JA and SA Elicitation for Biomass Accumulation and AQ Production
3.5. Extraction and Estimation of Anthraquinones (AQs) by Using High-Performance Liquid Chromatography (HPLC)
3.6. Extraction and Estimation Individual Phenolic Compounds by Using Ultra-HPLC
3.7. Estimation of Total Phenolic Content and Total Flavonoid Content
3.8. Biological Activities
3.8.1. Antioxidant Activities
3.8.2. Antimicrobial Activities
3.8.3. MTT Assay
3.9. Experimental Design and Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.; Wu, W.; Hou, K.; Chen, J.; Zhao, Z. Deep sequencing reveals transcriptome re-programming of Polygonum multiflorum thunb. roots to the elicitation with methyl jasmonate. Mol. Genet. Genom. 2016, 291, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Zhang, J.; Qiu, X.H.; Bai, J.Q.; Gao, Y.H.; Xu, W. Application of ultra-high-performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry for the qualitative and quantitative analysis of Polygonum multiflorum Thumb. and its processed products. Molecules 2016, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Sharma, N.; Sharma, U.K.; Singh, N.P.; Bhushan, S.; Sharma, M.; Sinha, A.K.; Ahuja, P.S. Qualitative and quantitative analysis of anthraquinone derivatives in rhizomes of tissue culture-raised Rheum emodi Wall Plants. J. Plant Physiol. 2010, 167, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D.; Panneerselvam, R.; Gopi, R.; Hong-bo, S. Elicitation of pharmacologically active phenolic compounds from Rauvolfia serpentina Benth. Ex. Kurtz. Ind. Crops Prod. 2013, 45, 406–415. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Metabolic engineering approaches for production of biochemicals in food and medicinal plants. Curr. Opin. Biotechnol. 2014, 26, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Giri, L.; Dhyani, P.; Rawat, S.; Bhatt, I.D.; Nandi, S.K.; Rawal, R.S.; Pande, V. In vitro production of phenolic compounds and antioxidant activity in callus suspension cultures of Habenaria edgeworthii: A rare Himalayan medicinal orchid. Ind. Crops Prod. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Perassolo, M.; Smith, M.E.; Giulietti, A.M.; Talou, J.R. Synergistic effect of methyl jasmonate and cyclodextrins on anthraquinone accumulation in cell suspension cultures of Morinda citrifolia and Rubia tinctorum. Plant Cell Tissue Organ Cult. 2016, 124, 319–330. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, J.J. Manipulation of ginsenoside heterogeneity in cell cultures of Panax notoginseng by addition of jasmonates. J. Biosci. Bioeng. 2002, 93, 48–53. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Hagège, D.; Courtois, D.; Joseph, C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2013, 113, 25–39. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Komaraiah, P.; Kavi Kishor, P.B.; Carlsson, M.; Magnusson, K.E.; Mandenius, C.F. Enhancement of anthraquinone accumulation in Morinda citrifolia suspension cultures. Plant Sci. 2005, 168, 1337–1344. [Google Scholar] [CrossRef]
- Shukor, M.F.A.; Ismail, I.; Zainal, Z.; Noor, N.M. Development of a Polygonum minus cell suspension culture system and analysis of secondary metabolites enhanced by elicitation. Acta Physiol. Plant. 2013, 35, 1675–1689. [Google Scholar] [CrossRef]
- Shao, L.; Zhao, S.J.; Cui, T.B.; Liu, Z.Y.; Zhao, W. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glycoside biosynthesis by suspension cells cultures of Polygonum multiflorum Thunb and production enhancement by methyl jasmonate and salicylic acid. Molecules 2012, 17, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Stalman, M.; Koskamp, A.M.; Luderer, R.; Vernooy, J.H.J.; Wind, J.C.; Wullems, G.J.; Croes, A.F. Regulation of anthraquinone biosynthesis in cell cultures of Morinda citrifolia. J. Plant Physiol. 2003, 160, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, H. Production of biologically active phenolic acids in Aronia melanocarpa (Michx.) Elliott in vitro cultures cultivated on different variants of the Murashige and Skoog medium. Plant Growth Regul. 2014, 72, 51–58. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Kapil Dev, G.; Jeyaraj, M.; Rajesh, M.; Muthuselvam, M.; Selvaraj, N.; Manickavasagam, M.; Ganapathi, A. A promising approach on biomass accumulation and withanolides production in cell suspension culture of Withania somnifera (L.) Dunal. Protoplasma 2013, 250, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Maria, L.; Adam, K.; Daniel, G. Plant growth regulators affect biosynthesis and accumulation profile of isoflavone phytoestrogens in high-productive in vitro cultures of Genista tinctoria. Plant Cell Tissue Organ Cult. 2014, 118, 419–429. [Google Scholar]
- Raj, D.; Kokotkiewicz, A.; Luczkiewicz, M. Effect of plant growth regulators on the accumulation of indolizidine alkaloids in Securinega suffruticosa callus cultures. Plant Cell Tissue Organ Cult. 2015, 123, 39–45. [Google Scholar] [CrossRef]
- Mok, M.C.; Gabelman, W.H.; Skoog, F. Carotenoid synthesis in tissue cultures of Daucus carota. J. Am. Soc. Hortic. Sci. 1976, 101, 442–449. [Google Scholar]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Simoes-Gurgel, C.; Cordeiro, L.D.S.; de Castro, T.C.; Callado, C.H.; Albarello, N.; Mansur, E. Establishment of anthocyanin production cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tissue Organ Cult. 2011, 106, 537–545. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H.; Ihsan-ul-haq, A. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crops Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Nagella, P.; Murthy, H.N. Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour. Technol. 2010, 101, 6735–6739. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Guo, Z.G.; Zeng, Z.L. Improved accumulation of phenylethanoid glycosides by precursor feeding to suspension culture of Cistanche salsa. Biochem. Eng. J. 2007, 33, 88–93. [Google Scholar] [CrossRef]
- Wang, Y.; Weathers, P.J. Sugars proportionately affect artemisinin production. Plant Cell Rep. 2007, 26, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Mewis, I.; Smetanska, I.M.; Müller, C.T.; Ulrichs, C. Specific poly-phenolic compounds in cell culture of Vitis vinifera L. cv. Gamay Fréaux. Appl. Biochem. Biotechnol. 2011, 164, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Min, J.Y.; Kim, Y.D.; Kang, Y.M.; Park, D.J.; Jung, H.N.; Kim, S.W.; Choi, M.S. Effects of methyl jasmonate and salicylic acid on the production of bilobalide and ginkgolides in cell cultures of Ginkgo biloba. In Vitro Cell Dev. Biol. Plant 2006, 42, 44–49. [Google Scholar] [CrossRef]
- Riedel, H.; Akumo, D.N.; Thaw Saw, N.M.M.; Kütük, O.; Neubauer, P.; Smetanska, I. Elicitation and precursor feeding influence phenolic acids composition in Vitis vinifera suspension culture. Afr. J. Biotechnol. 2012, 11, 3000–3008. [Google Scholar]
- Dong, J.; Zhan, Y. Effects of several physiochemical factors on cell growth and gallic acid accumulation of Acer ginnala Maxim cell suspension culture. Afr. J. Biotechnol. 2011, 10, 7831–7839. [Google Scholar]
- Szopa, A.; Ekiert, H. In vitro cultures of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine)—A potential biotechnological rich source of therapeutically important phenolic acids. Appl. Biochem. Biotechnol. 2012, 166, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of vine vigor on grape (Vitis vinifera L. Cv. Pinot Noir) anthocyanins. 1. Anthocyanin concentration and composition in fruit. J. Agric. Food Chem. 2007, 55, 6575–6584. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, J.; Yao, L.; Lu, Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour. Bioprocess. 2015, 2, 5. [Google Scholar] [CrossRef]
- Vuong, T.V.; Franco, C.; Zhang, W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014, 1, 15–21. [Google Scholar] [CrossRef]
- Taurino, M.; Ingrosso, I.; D’amico, L.; de Domenico, S.; Nicoletti, I.; Corradini, D.; Santino, A.; Giovinazzo, G. Jasmonates elicit different sets of stilbenes in Vitis vinifera cv. Negramaro cell cultures. SpringerPlus 2015, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 2007, 12, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Abbasi, B.H.; Ali, G.S. Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult. 2015, 120, 1099–1106. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Kim, E.H.; Kim, S.H.; Chung, I.M. Production of anthraquinones, phenolic compounds and biological activities from hairy root cultures of Polygonum multiflorum Thunb. Protoplasma 2014, 251, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.M.; Marraiki, N. Antimicrobial activity and phytochemical analyses of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt. Saudi J. Biol. Sci. 2010, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Ali, W.; Eom, S.H.; Hentschel, U.; Roitsch, T. Synthesis of distinctly different sets of antimicrobial activities by elicited plant cell suspension cultures. Plant Cell Tissue Organ Cult. 2010, 106, 105–113. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C. The N6 medium and its applications to anther culture of cereal crops. In Proceedings of the Symposium Plant Tissue Culture, Beijing, China, 25–30 May 1978; Science Press: Beijing, China, 1978; pp. 43–50. [Google Scholar]
- Su, X.Q.; Zhang, G.J.; Ma, Y.; Chen, M.; Chen, S.H.; Duan, S.M.; Wan, J.Q.; Hashimoto, F.; Lv, H.P.; Li, J.H.; et al. Isolation, identification, and biotransformation of teadenol a from solid state fermentation of pu-erh tea and in vitro antioxidant activity. Appl. Sci. 2016, 6, 161. [Google Scholar] [CrossRef]

| No. | Phenolic Compounds | Concentration (µg/g DM) | |||
|---|---|---|---|---|---|
| Control | PGR | JA | SA | ||
| Hydroxybenzoic acid | |||||
| 1 | Gallic acid | 681.45 ± 2.0 d | 765.20 ± 1.5 c | 819.12 ± 2.0 a | 802.00 ± 1.5 b |
| 2 | Protocatechuic acid | 302.15 ± 2.5 d | 351.00 ± 1.0 c | 425.41 ± 2.2 a | 414.15 ± 1.0 b |
| 3 | β-Resorcylic acid | 172.00 ± 1.6 d | 235.50 ± 1.2 c | 354.43 ± 1.5 a | 340.15 ± 1.0 b |
| 4 | Syringic acid | 101.75 ± 1.0 d | 145.25 ± 1.5 c | 280.22 ± 1.8 a | 255.00 ± 2.0 b |
| 5 | Gentisic acid | 185.15 ± 1.5 d | 221.85 ± 1.0 c | 325.00 ± 2.0 a | 301.50 ± 1.5 b |
| Total | 1442.50 d | 1718.80 c | 2204.18 a | 2112.80 b | |
| Hydroxycinnamic acid | |||||
| 6 | Caffeic acid | 165.25 ± 2.0 d | 211.10 ± 1.0 c | 265.55 ± 2.0 b | 285.40 ± 1.0 a |
| 7 | p-Coumaric acid | 95.00 ± 1.0 d | 120.00 ± 1.5 c | 205.10 ± 1.5 b | 217.32 ± 1.2 a |
| 8 | Ferulic acid | 312.45 ± 2.5 d | 345.15 ± 2.0 c | 415.50 ± 2.5 b | 441.20 ± 1.4 a |
| 9 | Chlorogenic acid | 72.05 ± 1.0 d | 105.50 ± 1.1 c | 211.12 ± 1.0 b | 215.35 ± 1.0 a |
| 10 | m-Coumaric acid | 40.00 ± 1.2 c | 83.16 ± 1.0 b | 106.00 ± 1.0 a | 105.10 ± 1.5 a |
| 11 | o-Coumaric acid | 102.20 ± 1.5 c | 145.00 ± 1.2 b | 202.10 ± 1.0 a | 202.00 ± 1.0 a |
| Total | 786.95 d | 1009.91 c | 1405.36 b | 1466.37 a | |
| Flavonols | |||||
| 12 | Myricetin | 245.25 ± 2.0 c | 271.62 ± 1.5 b | 385.50 ± 1.2 a | 384.10 ± 1.5 a |
| 13 | Quercetin | 312.10 ± 1.5 d | 395.30 ± 2.1 c | 455.55 ± 1.0 a | 431.15 ± 2.0 b |
| 14 | Kaempferol | 105.50 ± 1.0 d | 152.10 ± 1.0 c | 215.40 ± 2.0 a | 201.55 ± 1.0 b |
| 15 | Naringenin | 82.00 ± 1.0 d | 95.50 ± 1.5 c | 145.15 ± 1.0 a | 120.55 ± 1.5 b |
| 16 | Rutin | 525.50 ± 2.0 d | 592.15 ± 2.5 c | 675.00 ± 2.0 a | 621.10 ± 2.5 b |
| 17 | Biochanin A | 112.15 ± 1.5 c | 125.00 ± 1.0 b | 162.50 ± 2.0 a | 160.00 ± 1.0 a |
| 18 | Formononetin | 121.00 ± 1.8 c | 145.20 ± 1.2 b | 180.15 ± 1.0 a | 180.51 ± 1.5 a |
| 19 | Hesperidin | 132.55 ± 2.2 d | 165.00 ± 1.5 c | 195.50 ± 1.2 a | 187.00 ± 1.0 b |
| Total | 1636.05 d | 1941.87 c | 2414.75 a | 2285.96 b | |
| Other Phenolic compounds | |||||
| 20 | Pyrogallol | 925.00 ± 3.0 d | 1012.44 ± 2.5 c | 1121.15 ± 3.2 a | 1101.00 ± 2.0 b |
| 21 | Resveratrol | 212.00 ± 1.5 d | 305.22 ± 2.0 c | 414.50 ± 1.0 a | 400.25 ± 1.5 b |
| 22 | Veratric acid | 135.25 ± 1.0 d | 194.10 ± 1.0 c | 245.21 ± 1.5 a | 212.15 ± 1.0 b |
| 23 | Vanillin | 145.55 ± 1.0 b | 146.00 ± 1.0 b | 172.15 ± 1.2 a | 171.00 ± 2.0 a |
| Total | 1417.80 d | 1657.76 c | 1953.01 a | 1884.40 b | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiruvengadam, M.; Rekha, K.; Rajakumar, G.; Lee, T.-J.; Kim, S.-H.; Chung, I.-M. Enhanced Production of Anthraquinones and Phenolic Compounds and Biological Activities in the Cell Suspension Cultures of Polygonum multiflorum. Int. J. Mol. Sci. 2016, 17, 1912. https://doi.org/10.3390/ijms17111912
Thiruvengadam M, Rekha K, Rajakumar G, Lee T-J, Kim S-H, Chung I-M. Enhanced Production of Anthraquinones and Phenolic Compounds and Biological Activities in the Cell Suspension Cultures of Polygonum multiflorum. International Journal of Molecular Sciences. 2016; 17(11):1912. https://doi.org/10.3390/ijms17111912
Chicago/Turabian StyleThiruvengadam, Muthu, Kaliyaperumal Rekha, Govindasamy Rajakumar, Taek-Jun Lee, Seung-Hyun Kim, and Ill-Min Chung. 2016. "Enhanced Production of Anthraquinones and Phenolic Compounds and Biological Activities in the Cell Suspension Cultures of Polygonum multiflorum" International Journal of Molecular Sciences 17, no. 11: 1912. https://doi.org/10.3390/ijms17111912






