Response Surface Methodology Modelling of an Aqueous Two-Phase System for Purification of Protease from Penicillium candidum (PCA 1/TT031) under Solid State Fermentation and Its Biochemical Characterization
Abstract
:1. Introduction
2. Results
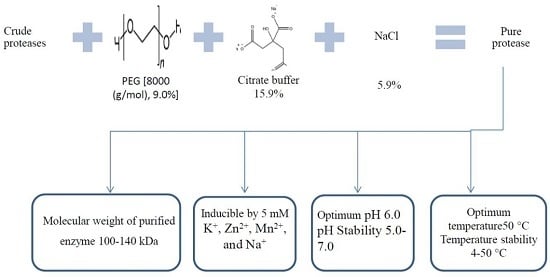
2.1. Optimizing the Protease Purification Using an Aqueous Two-Phase System
2.1.1. Fitting of the RSM Models
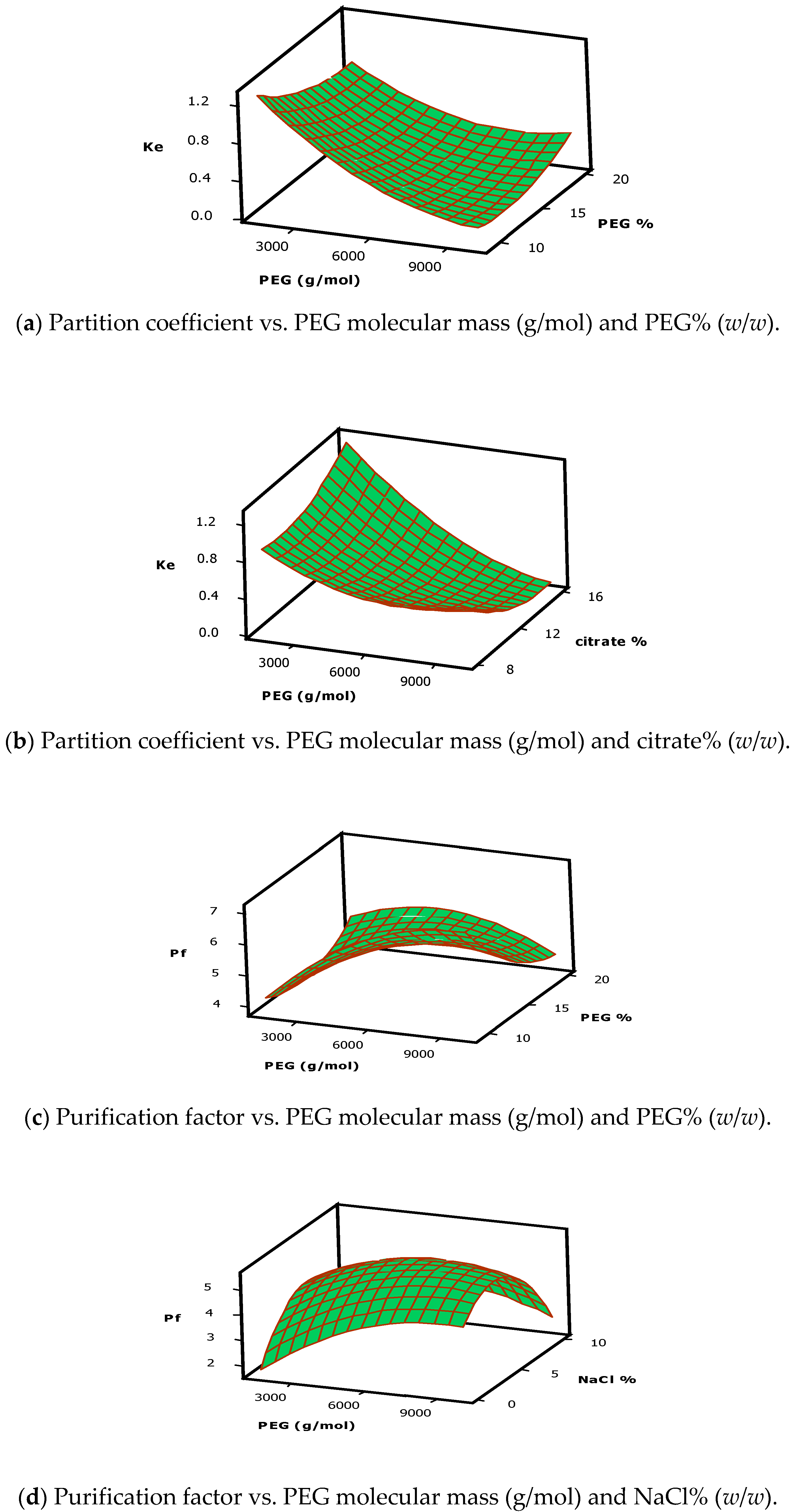
2.1.2. Partition Coefficient (Y1)
2.1.3. Purification Factor (Y2)
2.1.4. Yields (Y3)
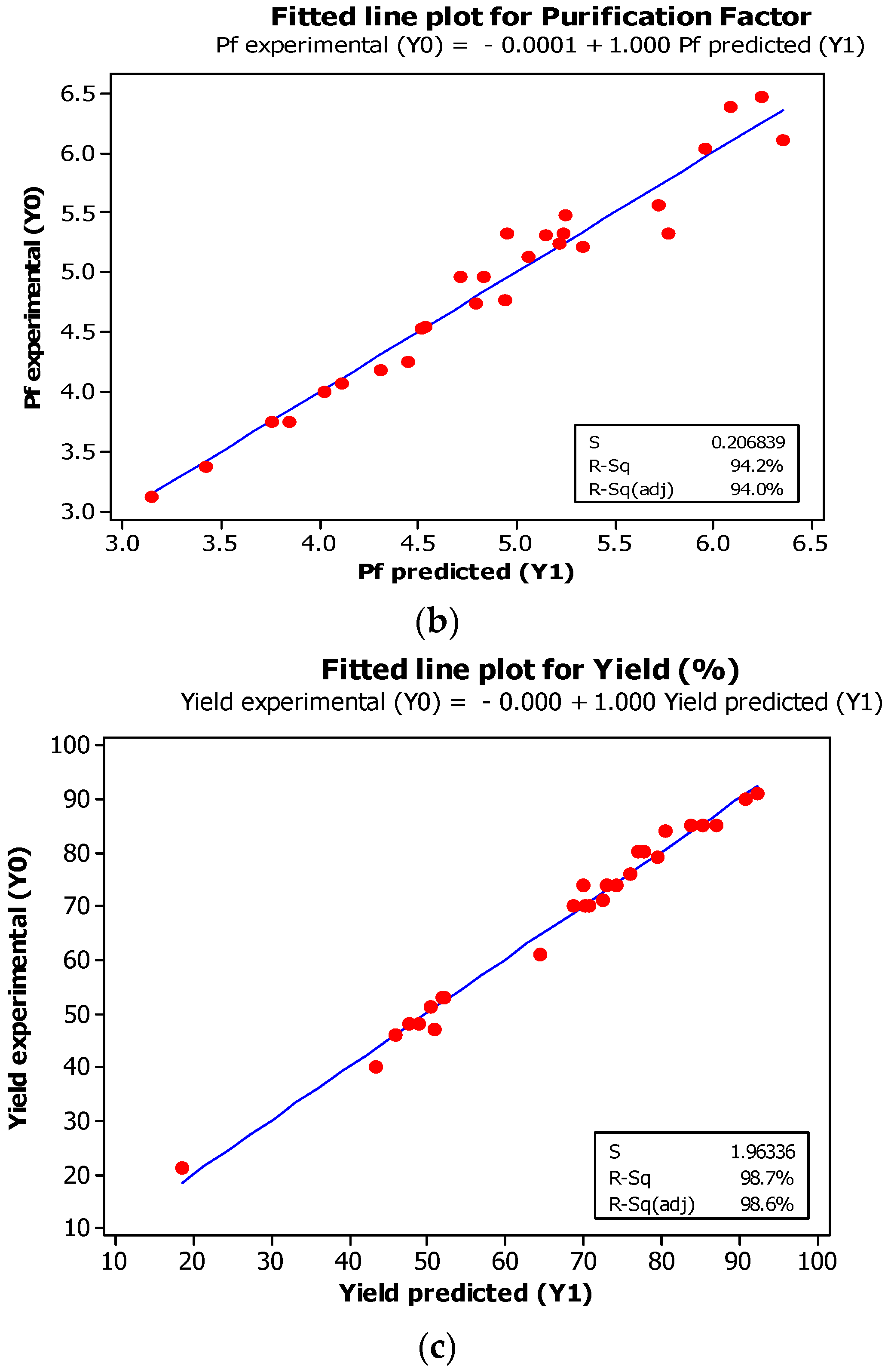
2.1.5. Experimental Validation of the Models
2.2. Characterization of Protease from P. candidum (PCA 1/TT031) in Aqueous Solutions
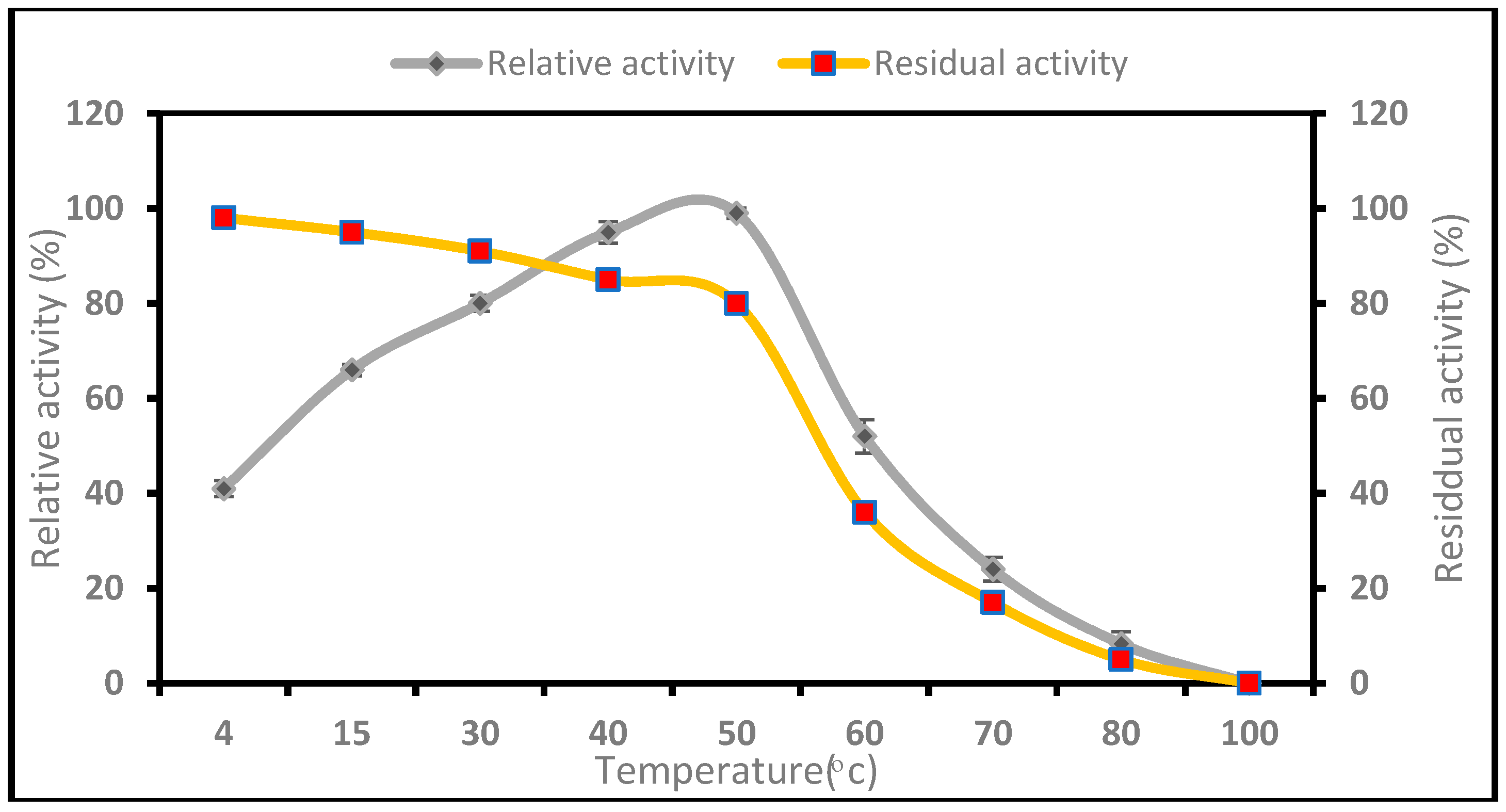
2.2.1. Influence of Temperature on the Activity and Stability of P. candidum (PCA 1/TT031) Protease
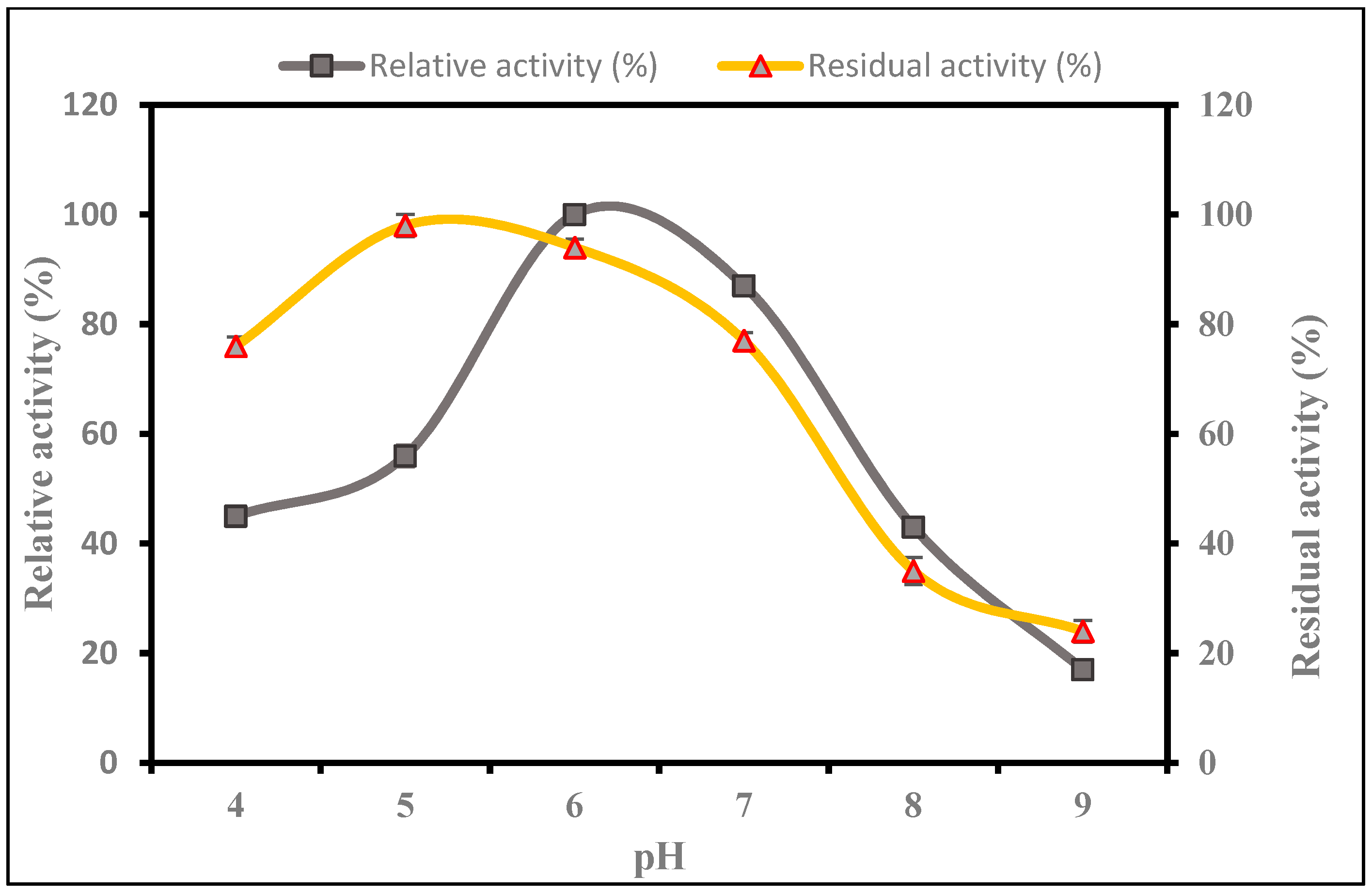
2.2.2. Influence of pH on the Activity and Stability of P. candidum (PCA 1/TT031) Protease
2.2.3. Influence of Inhibitors and Metal Ions on the Activity of P. candidum (PCA 1/TT031) Protease
2.2.4. SDS-PAGE Assessment of Purified Protease and ATPS
3. Discussion
3.1. Protease Purification Using the Aqueous Two-Phase System
3.1.1. Partition Coefficient (Y1)
3.1.2. Purification Factor (Y2)
3.1.3. Yields (Y3)
3.1.4. Experimental Validation of the Models
3.2. Characterization of Protease from P. candidum (PCA 1/TT031) in Aqueous Solutions
3.2.1. Influence of Temperature on the Activity and Stability of P. candidum (PCA 1/TT031) Protease
3.2.2. Influence of pH on the Activity and Stability of P. candidum (PCA 1/TT031) Protease
3.2.3. Influence of Metal Ions and Inhibitors on the Activity of P. candidum (PCA 1/TT031) Protease
3.2.4. SDS-PAGE Assessment of Purified Protease and ATPS
4. Methods and Materials
4.1. Materials
4.2. Production of Protease by Solid-State Fermentation
4.3. Enzyme Extraction
4.4. Proteolytic Assay
4.5. Protein Determination
4.6. Optimizing the Protease Purification Using an Aqueous Two-Phase System
4.6.1. ATPS Preparation
4.6.2. Calculations
4.6.3. Experimental Design
4.6.4. Variance Analysis
4.6.5. Optimization and Validation of the Experimental Process
4.7. Protease Characterization
4.7.1. Influence of Temperature on Activity and Stability of P. candidum (PCA 1/TT031) Protease
4.7.2. Influence of pH on Activity and Stability of P. candidum (PCA 1/TT031) Protease
4.7.3. Influence of Metal Ions and Inhibitors on Activity of P. candidum (PCA 1/TT031) Protease
4.7.4. SDS–PAGE Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suganthi, C.; Mageswari, A.; Karthikeyan, S.; Anbalagan, M.; Sivakumar, A.; Gothandam, K. Screening and optimization of protease production from a halotolerant Bacillus licheniformis isolated from saltern sediments. J. Genet. Eng. Biotechnol. 2013, 11, 47–52. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.; Cihan, A.C.; Chaulagain, B.P. Microbial enzymes and their applications in industries and medicine. BioMed Res. Int. 2013. [Google Scholar] [CrossRef]
- Robinson, R.K. Dairy Microbiology Handbook: The Microbiology of Milk and Milk Products; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Císarová, M.; Tancinová, D.; Barboráková, Z.; Masková, Z.; Felsöciová, S.; Kucerková, V. Potential production of cyclopiazonic acid by penicillium camemberti strains isolated from camembert type cheese. J. Microb. Biotechnol. Food Sci. 2012, 2, 434–435. [Google Scholar]
- Gripon, J.; Auberger, B.; Lenoir, J. Metalloproteases from Penicillium caseicolum and P. roqueforti: Comparison of specificity and cemical characterization. Int. J. Biochem. 1980, 12, 451–455. [Google Scholar] [CrossRef]
- Chrzanowska, J.; Kolaczkowska, M.; Dryjański, M.; Stachowiak, D.; Polanowski, A. Aspartic proteinase from Penicillium camemberti: purification, properties, and substrate specificity. Enzym. Microb. Technol. 1995, 17, 719–724. [Google Scholar] [CrossRef]
- Lenoir, J.; Auberger, B.; Gripon, J. Characteristics of the proteolytic system of penicillium-caseicolum. 3. Characterization of an acid protease. Lait 1979, 59, 244–268. [Google Scholar] [CrossRef]
- Boutrou, R.; Aziza, M.; Amrane, A. Enhanced proteolytic activities of Geotrichum candidum and Penicillium camembertii in mixed culture. Enzym. Microb. Technol. 2006, 39, 325–331. [Google Scholar] [CrossRef]
- Desmazeaud, M.; Le Bars, D. Role of proteolytic enzymes of Streptococcus lactis, Penicillium roqueforti, and Penicillium caseicolum during cheese ripening. J. Dairy Sci. 1977, 60, 1532–1538. [Google Scholar]
- Cerning, J.; Gripon, J.; Lamberet, G.; Lenoir, J. Biochemical activities of Penicillium used in cheese making (Penicillium roqueforti, Penicillium camemberti). Lait 1987. [Google Scholar] [CrossRef]
- Ahiko, K.; Iwasawa, S.; Ulda, M.; Miyata, N. Studies on acid carboxypeptidase from Penicillium caseicolum. I. Purification and properties of acid carboxypeptidase. Rep. Res. Lab. Snow Brand Milk Prod. Co. 1981, 77, 127–134. [Google Scholar]
- Bockelmann, W. Secondary cheese starter cultures. Technol. Cheesemaking 2010, 193. [Google Scholar] [CrossRef]
- Matsuoka, H.; Fuke, Y.; Kaminogawa, S.; Yamauchi, K. Purification and debittering effect of aminopeptidase II from Penicillium caseicolum. J. Agric. Food Chem. 1991, 39, 1392–1395. [Google Scholar] [CrossRef]
- Fuke, Y.; Kaminogawa, S.; Matsuoka, H.; Yamauchi, K. Purification and properties of aminopeptidase I from Penicillium caseicolum. J. Dairy Sci. 1988, 71, 1423–1431. [Google Scholar] [CrossRef]
- Michelson, P. Cheese: Exploring Taste and Tradition; Gibbs Smith: Salt Lake City, UT, USA, 2010. [Google Scholar]
- Ahmed, I.; Zia, M.A.; Iftikhar, T.; Iqbal, H.M. Characterization and detergent compatibility of purified protease produced from Aspergillus niger by utilizing agro wastes. BioResources 2011, 6, 4505–4522. [Google Scholar]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Karataş, H.; Uyar, F.; Tolan, V.; Baysal, Z. Optimization and enhanced production of α-amylase and protease by a newly isolated Bacillus licheniformis ZB-05 under solid-state fermentation. Ann. Microb. 2013, 63, 45–52. [Google Scholar] [CrossRef]
- Sukumprasertsri, M.; Unrean, P.; Pimsamarn, J.; Kitsubun, P.; Tongta, A. Fuzzy logic control of rotating drum bioreactor for improved production of amylase and protease enzymes by Aspergillus oryzae in solid-state fermentation. J. Microbial. Biotechnol. 2013, 23, 335–342. [Google Scholar] [CrossRef]
- Rahimpour, F.; Hatti-Kaul, R.; Mamo, G. Response surface methodology and artificial neural network modelling of an aqueous two-phase system for purification of a recombinant alkaline active xylanase. Proc. Biochem. 2016, 51, 452–462. [Google Scholar] [CrossRef]
- Tinoco-Valencia, R.; Serrano-Carreón, L.; Martínez-Morales, F.; Trejo-Hernándezb, M.R.; Rito-Palomaresa, M. Pleurotus ostreatus laccase recovery from residual compost using aqueous two-phase systems. J. Chem. Technol. Biotechnol. 2016. [Google Scholar] [CrossRef]
- Souza, R.L.; Ventura, S.P.; Soares, C.M.; Coutinho, J.A.; Lima, Á.S. Lipase purification using ionic liquids as adjuvants in aqueous two-phase systems. Green Chem. 2015, 17, 3026–3034. [Google Scholar] [CrossRef]
- Xavier, L.; Freire, M.S.; Vidal-Tato, I.; González-Álvarez, J. Application of aqueous two phase systems based on polyethylene glycol and sodium citrate for the recovery of phenolic compounds from Eucalyptus wood. Maderas. Ciencia Y Tecnología 2015, 17, 345–354. [Google Scholar] [CrossRef]
- Tubio, G.; Picó, G.A.; Nerli, B.B. Extraction of trypsin from bovine pancreas by applying polyethyleneglycol/sodium citrate aqueous two-phase systems. J. Chromatogr. B 2009, 877, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Garza-Madrid, M.; Rito-Palomares, M.; Serna-Saldívar, S.O.; Benavides, J. Potential of aqueous two-phase systems constructed on flexible devices: Human serum albumin as proof of concept. Process Biochem. 2010, 45, 1082–1087. [Google Scholar] [CrossRef]
- Ooi, C.W.; Tey, B.T.; Hii, S.L.; Ariff, A.; Wu, H.S.; Lan, J.C.W.; Juang, R.S.; Kamal, S.M.M.; Ling, T.C. Direct purification of Burkholderia pseudomallei lipase from fermentation broth using aqueous two-phase systems. Biotechnol. Bioproc. Eng. 2009, 14, 811–818. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Simpson, B.K.; Kishimura, H. Partitioning and recovery of proteinase from tuna spleen by aqueous two-phase systems. Proc. Biochem. 2005, 40, 3061–3067. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Sadeghi, R.; Hamidi, A.A. Liquid–liquid equilibria of an aqueous two-phase system containing polyethylene glycol and sodium citrate: Experiment and correlation. Fluid Phase Equilibria 2004, 219, 149–155. [Google Scholar] [CrossRef]
- Raja, S.; Murty, V.R.; Thivaharan, V.; Rajasekar, V.; Ramesh, V. Aqueous two phase systems for the recovery of biomolecules—A review. Sci. Technol. 2011, 1, 7–16. [Google Scholar] [CrossRef]
- Pérez, R.L.; Loureiro, D.B.; Nerli, B.B.; Tubio, G. Optimization of pancreatic trypsin extraction in PEG/citrate aqueous two-phase systems. Protein Expr. Purif. 2015, 106, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ratanapongleka, K. Recovery of biological products in aqueous two phase systems. Int. J. Chem. Eng. Appl. 2010, 1, 191. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Kapoor, M. Extracellular endo-mannanase from Bacillus sp. CFR1601: Economical production using response surface methodology and downstream processing using aqueous two phase system. Food Bioprod. Process 2013, 91, 672–681. [Google Scholar] [CrossRef]
- Singh, P.; Shera, S.S.; Banik, J.; Banik, R.M. Optimization of cultural conditions using response surface methodology versus artificial neural network and modeling of l-glutaminase production by Bacillus cereus MTCC 1305. Bioresour. Technol. 2013, 137, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, A. Application of response surface methodology to the modeling of cellulase purification by solvent extraction. Adv. Biosci. Biotechnol. 2012, 3, 408–416. [Google Scholar] [CrossRef]
- Deepak, V.; Kalishwaralal, K.; Ramkumarpandian, S.; Babu, S.V.; Senthilkumar, S.; Sangiliyandi, G. Optimization of media composition for Nattokinase production by Bacillus subtilis using response surface methodology. Bioresour. Technol. 2008, 99, 8170–8174. [Google Scholar] [CrossRef] [PubMed]
- Albertson, P. Partition of Cell Particles and Macromolecules, 3rd ed.; Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Karkaş, T.; Önal, S. Characteristics of invertase partitioned in poly (ethylene glycol)/magnesium sulfate aqueous two-phase system. Biochem. Eng. J. 2012, 60, 142–150. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Porto, C.S.; Porto, T.S.; Nascimento, K.S.; Teixeira, E.H.; Cavada, B.S.; Lima-Filho, J.L.; Porto, A.L. Partition of lectin from Canavalia grandiflora Benth in aqueous two-phase systems using factorial design. Biochem. Eng. J. 2011, 53, 165–171. [Google Scholar] [CrossRef]
- Navapara, R.D.; Avhad, D.N.; Rathod, V.K. Application of response surface methodology for optimization of bromelain extraction in aqueous two-phase system. Sep. Sci. Technol. 2011, 46, 1838–1847. [Google Scholar] [CrossRef]
- Benavides, J.; Rito-Palomares, M. Practical experiences from the development of aqueous two-phase processes for the recovery of high value biological products. J. Chem. Technol. Biotechnol. 2008, 83, 133–142. [Google Scholar] [CrossRef]
- Chow, Y.H.; Yap, Y.J.; Tan, C.P.; Anuar, M.S.; Tejo, B.A.; Show, P.L.; Ariff, A.B.; Ng, E.-P.; Ling, T.C. Characterization of bovine serum albumin partitioning behaviors in polymer-salt aqueous two-phase systems. J. Biosci. Bioeng. 2015, 120, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Shkel, I.A.; Knowles, D.; Record, M.T. Separating chemical and excluded volume interactions of polyethylene glycols with native proteins: Comparison with PEG effects on DNA helix formation. Biopolymers 2015, 103, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: phase separation and applications. J. Chromatogr. A 2012, 1238, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Ling, T.C.; Walker, S.; Lyddiatt, A. Partitioning of haemoglobin and bovine serum albumin from whole bovine blood using aqueous two-phase systems. Sep. Purif. Technol. 2012, 90, 182–188. [Google Scholar] [CrossRef]
- Nascimento, T.P.; Sales, A.E.; Porto, C.S.; Brandão, R.M.P.; de Campos-Takaki, G.M.; Teixeira, J.A.C.; Porto, T.S.; Porto, A.L.F.; Converti, A. Purification of a fibrinolytic protease from Mucor subtilissimus UCP 1262 by aqueous two-phase systems (PEG/sulfate). J. Chromatogr. B 2016, 1025, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Amid, M.; Manap, M.Y.A.; Shuhaimi, M. Purification of a novel protease enzyme from kesinai plant (Streblus asper) leaves using a surfactant–salt aqueous micellar two-phase system: A potential low cost source of enzyme and purification method. Eur. Food Res. Technol. 2013, 237, 601–608. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Ottomar, K.W.; Ngonyani, D.M.; Lyddiatt, A. Influence of system and molecular parameters upon fractionation of intracellular proteins from Saccharomyces by aqueous two-phase partition. Enzym. Microb. Technol. 1991, 13, 24–32. [Google Scholar] [CrossRef]
- Barbosa, J.M.P.; Souza, R.L.; Fricks, A.T.; Zanin, G.M.; Soares, C.M.F.; Lima, Á.S. Purification of lipase produced by a new source of Bacillus in submerged fermentation using an aqueous two-phase system. J. Chromatogr. B 2011, 879, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ni, L.; Han, J.; Wang, Y.; Li, Y.; Li, Y.; Tian, Y. Simultaneous aqueous two-phase flotation of sodium chlorophyllin and removal of sugars from saponified solution of bamboo leaves. Chem. Eng. Process 2016, 101, 41–49. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Yu, C.; Li, C.; Yan, Y.; Liu, Y.; Wang, L. Separation, concentration and determination of chloramphenicol in environment and food using an ionic liquid/salt aqueous two-phase flotation system coupled with high-performance liquid chromatography. Anal. Chim. Acta 2011, 685, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mehrnoush, A.; Mustafa, S.; Sarker, M.Z.I.; Yazid, A.M.M. Optimization of serine protease purification from mango (Mangifera indica cv. Chokanan) peel in polyethylene glycol/dextran aqueous two phase system. Int. J. Mol. Sci. 2012, 13, 3636–3649. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Raghavarao, K. Aqueous two phase extraction for purification of C-phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- Porto, T.; e Silva, G.M.; Porto, C.; Cavalcanti, M.; Neto, B.; Lima-Filho, J.; Converti, A.; Porto, A.; Pessoa, A. Liquid–liquid extraction of proteases from fermented broth by PEG/citrate aqueous two-phase system. Chem. Eng. Process 2008, 47, 716–721. [Google Scholar] [CrossRef]
- Hatti-Kaul, R. Aqueous two-phase systems. Mol. Biotechnol. 2001, 19, 269–277. [Google Scholar] [CrossRef]
- Andrews, B.; Schmidt, A.; Asenjo, J. Correlation for the partition behavior of proteins in aqueous two-phase systems: Effect of surface hydrophobicity and charge. Biotechnol. Bioeng. 2005, 90, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.R.; Ferreira, L.A.; Mikheeva, L.M.; Teixeira, J.A.; Zaslavsky, B.Y. Origin of salt additive effect on solute partitioning in aqueous polyethylene glycol-8000–sodium sulfate two-phase system. J. Chromatogr. A 2014, 1337, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fuke, Y.; Matsuoka, H. The purification and characterization of prolyl aminopeptidase from Penicillium camemberti. J. Dairy Sci. 1993, 76, 2478–2484. [Google Scholar] [CrossRef]
- Germano, S.; Pandey, A.; Osaku, C.A.; Rocha, S.N.; Soccol, C.R. Characterization and stability of proteases from Penicillium sp. produced by solid-state fermentation. Enzym. Microb. Technol. 2003, 32, 246–251. [Google Scholar] [CrossRef]
- Rodrigues, P.; Lima, C.A.; José Luiz Filho, L.; Teixeira, J.A.; Porto, A.L.; Cunha, M.G. Production and characterization of protease from Penicillium aurantiogriseum URM 4622. VALNATURA-A Europe-Latin América post-graduate research network in the VALorization of NATURAL resources. City 2008, 97–104. [Google Scholar]
- Agrawal, D.; Patidar, P.; Banerjee, T.; Patil, S. Production of alkaline protease by Penicillium sp. under SSF conditions and its application to soy protein hydrolysis. Process Biochem. 2004, 39, 977–981. [Google Scholar] [CrossRef]
- Seong, C.N.; Choi, S.K. InhA-like protease secreted by Bacillus sp. S17110 inhabited in turban shell. J. Microbiol. 2007, 402–408. [Google Scholar]
- Sant'Anna, G.; Freire, D.; Gomes, P.; Bon, E. Lipase production by a new promising strain of Penicillium restrictum. Revista de Microbiologia 1997, 28, 6–12. [Google Scholar]
- Sandhya, C.; Sumantha, A.; Szakacs, G.; Pandey, A. Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process Biochem. 2005, 40, 2689–2694. [Google Scholar] [CrossRef]
- Peres, C.M.; Alves, M.; Hernandez-Mendoza, A.; Moreira, L.; Silva, S.; Bronze, M.R.; Vilas-Boas, L.; Peres, C.; Malcata, F.X. Novel isolates of lactobacilli from fermented Portuguese olive as potential probiotics. LWT-Food Sci. Technol. 2014, 59, 234–246. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yavari, M.; Pazuki, G.; Vossoughi, M.; Mirkhani, S.; Seifkordi, A. Partitioning of alkaline protease from Bacillus licheniformis (ATCC 21424) using PEG–K2HPO4 aqueous two-phase system. Fluid Phase Equilibria 2013, 337, 1–5. [Google Scholar] [CrossRef]
- Antov, M.G.; Peričin, D.M.; Dašić, M.G. Aqueous two-phase partitioning of xylanase produced by solid-state cultivation of Polyporus squamosus. Process Biochem. 2006, 41, 232–235. [Google Scholar] [CrossRef]
- Joglekar, A.; May, A. Product excellence through design of experiments. Cereal Foods World 1987, 32, 857. [Google Scholar]
- Xu, Q.; Shen, Y.; Wang, H.; Zhang, N.; Xu, S.; Zhang, L. Application of response surface methodology to optimise extraction of flavonoids from Fructus sophorae. Food Chem. 2013, 138, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Tan, C.P. Response surface methodology and multivariate analysis of equilibrium headspace concentration of orange beverage emulsion as function of emulsion composition and structure. Food Chem. 2009, 115, 324–333. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.; Yusof, S. Effect of Arabic gum, xanthan gum and orange oil on flavor release from diluted orange beverage emulsion. Food Chem. 2008, 107, 1161–1172. [Google Scholar] [CrossRef] [Green Version]




| Regression Coefficient | Partition Coefficient (Y1) | Purification Factor (Y2) | Yield (Y3) |
|---|---|---|---|
| b0 | 4.25371 | 8.00997 | 122.231 |
| b1 | −0.00019 | 0.000124 | 0.007 |
| b2 | −0.18962 | −0.72459 | −4.359 |
| b3 | −0.20842 | −0.61406 | −10.700 |
| b4 | −0.14749 | 0.99890 | −2.721 |
| b12 | 0.00000 | −0.00000 | −0.000 |
| b22 | 0.00426 | 0.01864 | 0.099 |
| b32 | 0.01023 | 0.00242 | 0.281 |
| b42 | 0.01415 | −0.07165 | −0.140 |
| b12 | 0.00001 | −0.00004 | 0.000 |
| b13 | −0.00001 | 0.00001 | 0.000 |
| b14 | 0.00000 | −0.00003 | 0.000 |
| b23 | 0.00170 | 0.03580 | 0.023 |
| b24 | −0.00082 | −0.02791 | −0.127 |
| b34 | 0.00063 | 0.02063 | 0.350 |
| R2 | 0.96 | 0.94 | 0.98 |
| R2 (adj.) | 0.92 | 0.87 | 0.97 |
| Regression (p-value) | 0.000 a | 0.000 a | 0.000 a |
| Ariables | Main Effects | Quadratic Effects | Interaction Effects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variable | X1 | X2 | X3 | X4 | X12 | X22 | X32 | X42 | X1X2 | X1X3 | X1X4 | X2X3 | X2X4 | X3X4 | |
| Partition coefficient (Y1) | p-value | 0.010 a | 0.005 a | 0.024 a | 0.015 a | 0.000 a | 0.013 a | 0.003 a | 0.000 a | 0.016 a | 0.002 a | 0.432 | 0.533 | 0.707 | 0.834 |
| F-ratio | 9.05 | 11.24 | 6.55 | 7.82 | 26.16 | 8.29 | 13.40 | 62.56 | 7.70 | 14.44 | 0.66 | 0.41 | 0.15 | 0.05 | |
| Purification factor (Y2) | p-value | 0.002 a | 0.028 a | 0.170 | 0.003 a | 0.005 a | 0.030 a | 0.870 | 0.000 a | 0.016 a | 0.670 | 0.040 a | 0.022 a | 0.025 a | 0.197 |
| F-ratio | 14.00 | 6.11 | 2.12 | 13.35 | 11.65 | 5.91 | 0.03 | 59.96 | 7.61 | 0.19 | 5.20 | 6.73 | 6.39 | 1.85 | |
| Yield %(Y3) | p-value | 0.037 a | 0.141 | 0.019 a | 0.313 | 0.000 a | 0.196 | 0.062 | 0.136 | 0.016 a | 0.022 a | 0.011 a | 0.865 | 0.246 | 0.030 a |
| F-ratio | 5.39 | 2.45 | 7.13 | 1.10 | 66.90 | 1.86 | 4.18 | 2.53 | 7.71 | 6.78 | 8.70 | 0.03 | 1.48 | 5.90 | |
| Reagent | Relative Activity (% ± SD) a | |
|---|---|---|
| Concentration 5 mM | Concentration 10 mM | |
| Without component | 100 ± 0.00 d | 100 ± 0.00 b |
| NaCl | 136 ± 1.7 a | 112 ± 2.5 a |
| ZnCl2 | 122 ± 2.5 b | 92 ± 2.0 c |
| KCl | 112 ± 2.0 c | 85 ± 3.0 d |
| MnCl2 | 110 ± 2.0 c | 98 ± 2.0 b |
| MgCl2 | 44 ± 2.6 f | 31 ± 1.5 e |
| CaCl2 | 34 ± 2.0 g | 23 ± 1.5 f |
| FeCl3 | 25 ± 3.00 h | 0.00 ± 0.00 g |
| Sodium dodecyl sulfate (SDS) | 54 ± 3.5 e | 33 ± 2.5 e |
| Ethylenediaminetetraacetate (EDTA) | 15 ± 2.0 i | 7 ± 2.0 h |
| PMSF (Phenylmethanesulfonylflouride) | 100 ± 1.3 d | 100 ± 1.7 b |
| Run Order | Independent Variable | ||||
|---|---|---|---|---|---|
| Block | PEG Molecular Mass (g/mol,X1) | PEG Concentration (w/w, X2) | Citrate Concentration (w/w, X3) | NaCl Concentration (w/w, X4) | |
| 1 | 3 | 6000 | 20 | 12 | 5 |
| 2c | 3 | 6000 | 14.5 | 12 | 5 |
| 3 | 3 | 1500 | 14.5 | 12 | 5 |
| 4 | 3 | 6000 | 14.5 | 16 | 5 |
| 5 | 3 | 10,000 | 14.5 | 12 | 5 |
| 6 | 3 | 6000 | 9 | 12 | 5 |
| 7 | 3 | 6000 | 14.5 | 12 | 10 |
| 8 | 3 | 6000 | 14.5 | 12 | 0 |
| 9 | 3 | 6000 | 14.5 | 8 | 5 |
| 10 c | 3 | 6000 | 14.5 | 12 | 5 |
| 11 c | 1 | 6000 | 14.5 | 12 | 5 |
| 12 c | 1 | 6000 | 14.5 | 12 | 5 |
| 13 | 1 | 4000 | 17.25 | 14 | 7.5 |
| 14 | 1 | 8000 | 17.25 | 10 | 7.5 |
| 15 | 1 | 8000 | 11.75 | 10 | 2.5 |
| 16 | 1 | 8000 | 11.75 | 14 | 7.5 |
| 17 | 1 | 8000 | 17.25 | 14 | 2.5 |
| 18 | 1 | 4000 | 11.75 | 10 | 7.5 |
| 19 | 1 | 4000 | 11.75 | 14 | 2.5 |
| 20 | 1 | 4000 | 17.25 | 10 | 2.5 |
| 21 c | 2 | 6000 | 14.5 | 12 | 5 |
| 22 | 2 | 4000 | 17.25 | 10 | 7.5 |
| 23 | 2 | 8000 | 17.25 | 10 | 2.5 |
| 24 | 2 | 8000 | 11.75 | 14 | 2.5 |
| 25 | 2 | 8000 | 17.25 | 14 | 7.5 |
| 26 | 2 | 4000 | 11.75 | 14 | 7.5 |
| 27 | 2 | 4000 | 11.75 | 10 | 2.5 |
| 28 | 2 | 8000 | 11.75 | 10 | 7.5 |
| 29 | 2 | 4000 | 17.25 | 14 | 2.5 |
| 30 c | 2 | 6000 | 14.5 | 12 | 5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhelli, A.M.; Abdul Manap, M.Y.; Mohammed, A.S.; Mirhosseini, H.; Suliman, E.; Shad, Z.; Mohammed, N.K.; Meor Hussin, A.S. Response Surface Methodology Modelling of an Aqueous Two-Phase System for Purification of Protease from Penicillium candidum (PCA 1/TT031) under Solid State Fermentation and Its Biochemical Characterization. Int. J. Mol. Sci. 2016, 17, 1872. https://doi.org/10.3390/ijms17111872
Alhelli AM, Abdul Manap MY, Mohammed AS, Mirhosseini H, Suliman E, Shad Z, Mohammed NK, Meor Hussin AS. Response Surface Methodology Modelling of an Aqueous Two-Phase System for Purification of Protease from Penicillium candidum (PCA 1/TT031) under Solid State Fermentation and Its Biochemical Characterization. International Journal of Molecular Sciences. 2016; 17(11):1872. https://doi.org/10.3390/ijms17111872
Chicago/Turabian StyleAlhelli, Amaal M., Mohd Yazid Abdul Manap, Abdulkarim Sabo Mohammed, Hamed Mirhosseini, Eilaf Suliman, Zahra Shad, Nameer Khairulla Mohammed, and Anis Shobirin Meor Hussin. 2016. "Response Surface Methodology Modelling of an Aqueous Two-Phase System for Purification of Protease from Penicillium candidum (PCA 1/TT031) under Solid State Fermentation and Its Biochemical Characterization" International Journal of Molecular Sciences 17, no. 11: 1872. https://doi.org/10.3390/ijms17111872