Inhalable Antimicrobials for Treatment of Bacterial Biofilm-Associated Sinusitis in Cystic Fibrosis Patients: Challenges and Drug Delivery Approaches
Abstract
:1. Introduction
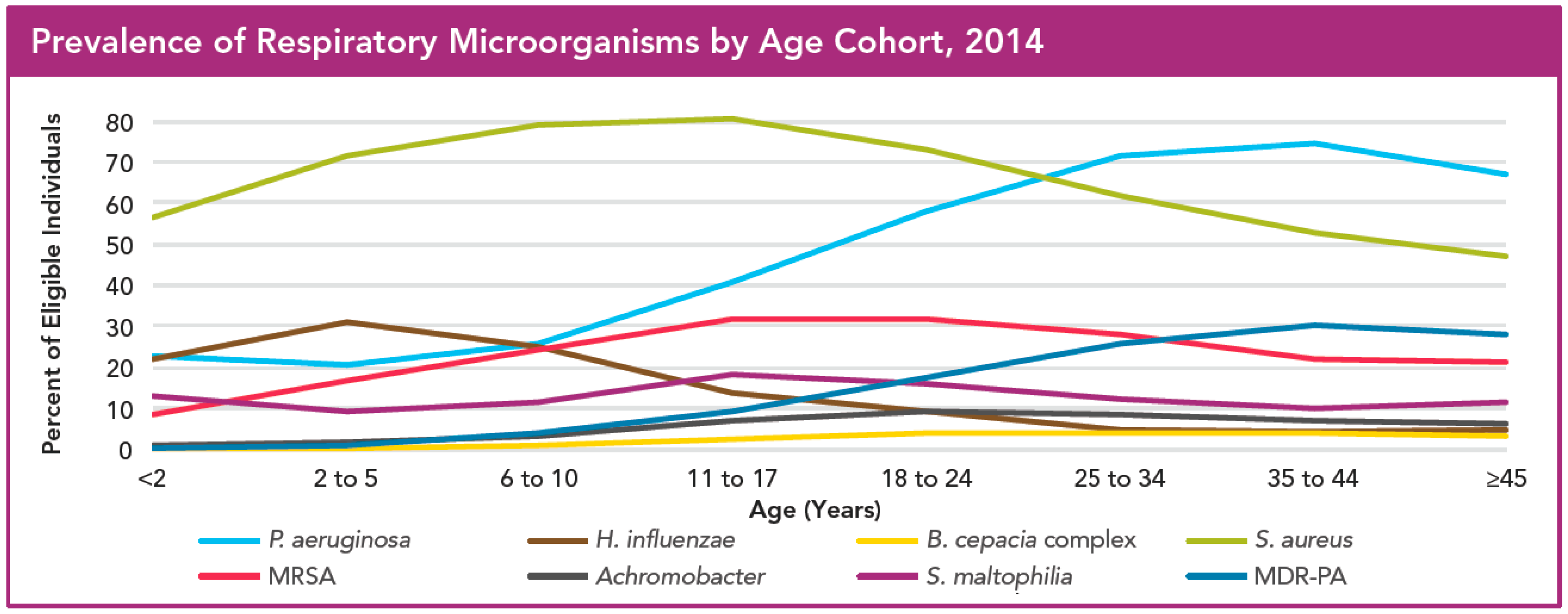
2. Pseudomonas Infections in Cystic Fibrosis Patients
3. Current Management and Treatment of Bacterial Infections in Cystic Fibrosis Patients
3.1. Antimicrobial Therapy to Treat Infections with Pseudomonas
3.2. Non-Antimicrobial Therapy to Treat Infections with Pseudomonas
3.3. Endoscopic Sinus Surgery and Preventive/Post-Operative Use of Antimicrobials
4. Antimicrobials for Inhalation: State-of-the-Art
4.1. Liquid Formulations
4.2. Dry Powders
4.3. Emergence of Resistance to Inhaled Antimicrobials and Other Adverse Effects
5. Formulation Approaches for Pulmonary Delivery of Antimicrobials
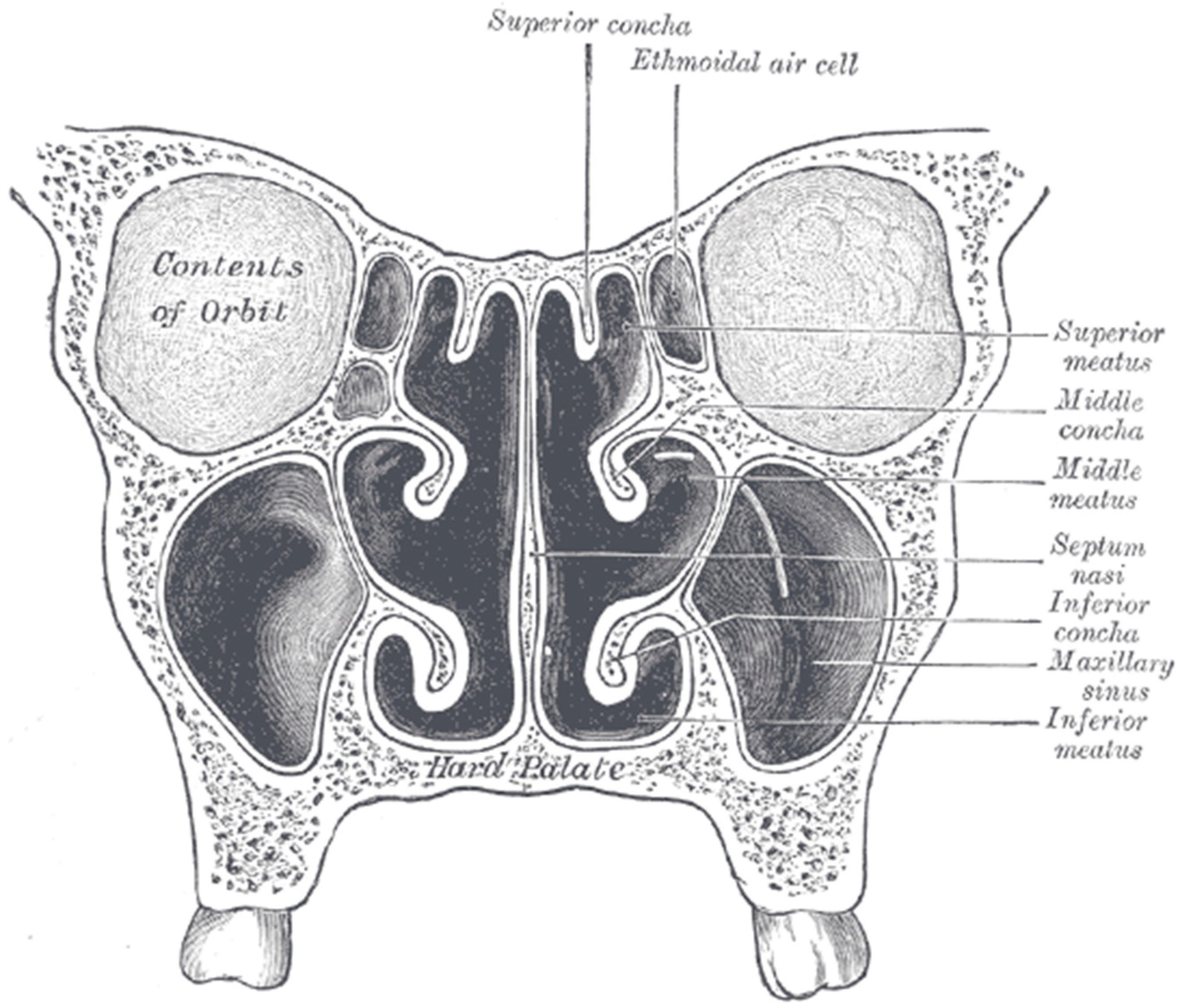
6. Delivery to Sinuses
7. Devices for Drug Delivery to the Sinuses
8. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A549 | Human lung carcinoma cell line |
| CF | Cystic fibrosis |
| CFBE41o- | Human bronchial epithelial cell line |
| CFF | Cystic Fibrosis Foundation |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CHEMS | Cholesteryl hemisuccinate |
| DCP | Dichlorophenol |
| DDAB | Didecylmethylammonium bromide |
| DMPC | Dimyristoyl-sn-glycero-3-phosphocholine |
| DMPG | Dimyristoyl phosphatidyl-glycerol |
| DNase | Deoxyribonuclease |
| DOPE | Dioleoylphosphatidylethanolamine |
| DOTAP | Dioleoyloxy-3-trimethylammonium-propane |
| DP | Dry powder |
| DPI | Dry powder inhaler |
| DPPC | Dipalmitoyl phosphatidylcholine |
| DSPC | Distearoyl phosphatidylcholine |
| eDNA | Extracellular deoxyribonucleic acid |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| ESS | Endoscopic sinus surgery |
| H441 | Human lung papillary adenocarcinoma cell line |
| HSPC | Hydrogenated soybean phosphatidylcholine |
| J774 | Murine macrophage cell line |
| LF | Liquid formulation |
| N/A | not available |
| PC | Phosphatidylcholine |
| PEG | Poly (ethylene glycol) |
| PLGA | Poly (lactic-co-glycolic) acid |
| PVA | Polyvinyl alcohol |
| TIS | Tobramycin inhalation solution |
| TIP | Tobramycin inhalation powder |
References
- Horsley, A. Genetics and pathophysiology. In Cystic Fibrosis; Horsley, A., Cunningham, S., Innes, J.A., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 1–16. [Google Scholar]
- D’Angelo, I.; Conte, C.; La Rotonda, M.I.; Miro, A.; Quaglia, F.; Ungaro, F. Improving the efficacy of inhaled drugs in cystic fibrosis: Challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev. 2014, 75, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Heijerman, H.; Westerman, E.; Conway, S.; Touw, D.; Doring, G. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: A European consensus. J. Cyst. Fibros. 2009, 8, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, Z.; Clancy, J. T100: Nebulized-concentrated tobramycin formulation for treatment of Pseudomonas aeruginosa infection in cystic fibrosis patients. Expert Opin. Orphan Drugs 2015, 3, 933–943. [Google Scholar] [CrossRef]
- Robertson, J.M.; Friedman, E.M.; Rubin, B.K. Nasal and sinus disease in cystic fibrosis. Paediatr. Respir. Rev. 2008, 9, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Linnane, B.; Kearse, L.; O’Connell, N.; Fenton, J.; Kiernan, M.G.; Dunne, C.P. A case of failed eradication of cystic fibrosis-related sinus colonization by Pseudomonas aeruginosa. BMC Pulm. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kosorok, M.R.; Farrell, P.M.; Laxova, A.; West, S.E.; Green, C.G.; Collins, J.; Rock, M.J.; Splaingard, M.L. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 2005, 293, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Frederiksen, B.; Pressler, T. Eradication of early Pseudomonas aeruginosa infection. J. Cyst. Fibros. 2005, 4 (Suppl. 2), 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Brockhaus, F.; Angyalosi, G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: A review. J. Cyst. Fibros. 2009, 8, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Taccetti, G.; Bianchini, E.; Cariani, L.; Buzzetti, A.; Costantini, D.; Trevisan, F.; Zavataro, L.; Campana, S. Early antibiotic treatment for Pseudomonas aeruginosa eradication in patients with cystic fibrosis: A randomised multicentre study comparing two different protocols. Thorax 2012, 67, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Mogayzel, P.J.; Naureckas Robinson, K.A.; Brady, C.; Guill, M.; Lahiri, T.; Lubsch, L.; Matsui, J.; Oermann, C.M.; Ratjen, F.; Rosenfeld, M.; et al. Cystic Fibrosis Foundation Pulmonary Clinical Practice Guidelines Committee. Cystic Fibrosis Foundation Pulmonary Guideline: Pharmacologic approaches to prevention and eradication of Initial Pseudomonas aeruginosa infection. Ann. ATS 2014, 11, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Munck, A.; Kho, P.; Angyalosi, G. ELITE study group. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: The ELITE trial. Thorax 2010, 65, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Doring, G.; Nikolaizik, W. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonization in patients with cystic fibrosis. Lancet 2001, 358, 983–984. [Google Scholar] [CrossRef]
- Gibson, R.L.; Emerson, J.; McNamara, S.; Burns, J.L.; Rosenfeld, M.; Yunker, A.; Hamblett, N.; Accurso, F.; Dovey, M.; Hiatt, P.; et al. Cystic Fibrosis Therapeutics Development Network Study Group. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 167, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Illing, E.A.; Woodworth, B.A. Management of the upper airway in cystic fibrosis. Curr. Opin. Pulm. Med. 2014, 20, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Chang, E. New insights into the pathogenesis of cystic fibrosis sinusitis. Int. Forum Allergy Rhinol. 2014, 4, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Aanæs, K. Bacterial sinusitis can be a focus for initial lung colonisation and chronic lung infection in patients with cystic fibrosis. J. Cyst. Fibros. 2013, 12, S1–S20. [Google Scholar] [CrossRef]
- Römling, U.; Kader, A.; Sriramulu, D.D.; Simm, R.; Kronvall, G. Worldwide distribution of Pseudomonas aeruginosa clone C strains in the aquatic environment and cystic fibrosis patients. Environ. Microbiol. 2005, 7, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.; Van Devanter, D. Clinical applications of pulmonary delivery of antibiotics. Adv. Drug Deliv. Rev. 2015, 85, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2014 Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2014. [Google Scholar]
- Hansen, S.K.; Rau, M.H.; Johansen, H.K.; Ciofu, O.; Jelsbak, L.; Yang, L.; Folkesson, A.; Jarmer, H.Ø.; Aanæs, K.; von Buchwald, C.; et al. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J. 2012, 6, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Dosanjh, A.; Lakhani, S.; Elashoff, D.; Chin, C.; Hsu, V.; Hilman, B. A comparison of microbiologic flora of the sinuses and airway among cystic fibrosis patients with maxillary antrostomies. Pediatr. Transpl. 2000, 4, 182–185. [Google Scholar] [CrossRef]
- Wise, S.K.; Kingdom, T.T.; McKean, L.; DelGaudio, J.M.; Venkatraman, G. Presence of fungus in sinus cultures of cystic fibrosis patients. Am. J. Rhinol. 2005, 19, 47–51. [Google Scholar] [PubMed]
- Johansen, H.; Aanaes, K.; Pressler, T.; Nielsen, K.G.; Fisker, J.; Skov, M.; Høiby, N.; von Buchwald, C. Colonization and infection of the paranasal sinuses in cystic fibrosis patients is accompanied by a reduced PMN response. J. Cyst. Fibros. 2012, 11, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.; Mogayzel, P.; Robinson, K.A.; Goss, C.H.; Rosenblatt, R.L.; Kuhn, R.J.; Marshall, B.C. Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic Fibrosis Pulmonary Guidelines; Treatment of Pulmonary Exacerbations. Am. J. Respir. Crit. Care Med. 2009, 180, 602–808. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; O’Sullivan, B.P.; Robinson, K.A.; Goss, C.H.; Mogayzel, P.J.; Willey-Courand, D.B.; Bujan, J.; Finder, J.; Lester, M.; Quittell, L.; et al. Cystic fibrosis pulmonary guidelines; chronic medications for maintenance of lung heath. Am. J. Respir. Crit. Care Med. 2007, 176, 957–996. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.; Trigikidis, K.; Vardakas, K. Inhaled antibiotics beyond aminoglycosides, polymyxins and Aztreonam: A systemic review. Int. J. Antimicrob. Agents 2015, 45, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.; Singh, M.; Dwan, K. Inhaled antibiotics for long-term therapy in cystic fibrosis. Cochrane Database Syst. Rev. 2011, CD001021. [Google Scholar] [CrossRef] [PubMed]
- Mainz, J.; Schadlich, K.; Schien, C.; Michl, R.; Schelhorn-Neise, P.; Koitschev, A.; Koitschev, C.; Keller, P.M.; Riethmüller, J.; Wiedemann, B.; et al. Sinonasal inhalation of tobramycin vibrating aerosol in cystic fibrosis patients with upper airway Pseudomonas aeruginosa colonization: Results of a randomized, double-blind, placebo-controlled pilot study. Drug Des. Dev. Ther. 2014, 8, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, W.C.; Carvalho, G. Use of nebulized antibiotics for acute infections in chronic sinusitis. Otolaryngol. Head Neck Surg. 2002, 127, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.M.; Murphy, C.; Mitchell, M.; Smith, C.; Light, M. Management of chronic sinusitis in cystic fibrosis. Laryngoscope 1995, 105, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; King, V.V. Management of sinusitis in cystic fibrosis by endoscopic surgery and serial antimicrobial lavage. Reduction in recurrence requiring surgery. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Elkins, M.; Robinson, M.; Rose, B.R.; Elkins, M.R.; Harbour, C.; Moriarty, C.P.; Marks, G.B.; Belousova, E.G.; Xuan, W.; Bye, P.T.P. A controlled trial of long-tern inhaled hypertonic saline in patients with cystic fibrosis. N. Engl. J. Med. 2006, 354, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.; Hannan, S.A.; Badia, L.; Scadding, G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Aanaes, K.; Johansen, H.K.; Poulsen, S.S.; Pressler, T.; Buchwald, C.; Hoiby, N. Secretory IgA as a diagnostic tool for Pseudomonas aeruginosa respiratory colonization. J. Cyst. Fibros. 2013, 12, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lewis. Gray’s Anatomy, 20th ed. 1918. Available online: http://www.bartleby.com/107/illus859.html (accessed on 26 April 2016).
- Quon, B.S.; Goss, C.H.; Ramsey, B.W. Inhaled antibiotics for lower airway infections. Ann. Am. Thorac. Soc. 2014, 11, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M. MRSA patients: Proven methods to treat colonization and infection. J. Hosp. Inf. 2001, 48, S9–S14. [Google Scholar] [CrossRef]
- Elek, S.D.; Fleming, P.C. A new technique for the control of hospital cross-infection. Lancet 1960, 276, 569–572. [Google Scholar] [CrossRef]
- Barakat, H.; Kassem, M.; El-Khordagui, L.; Khalafallah, N. Vancomycin-eluting niosomes: A new approach to the inhibition of staphylococcal biofilm on abiotic surfaces. AAPS PharmSciTech 2014, 15, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Pourchez, J.; Aubert, G.; Le Guellec, S.; Navarro, L.; Forest, V.; Rusch, P.; Cottier, M. Impact of acoustic airflow nebulization on intrasinus drug deposition of a human pastinated nasal cast: New insights into the mechanisms involved. Int. J. Pharm. 2011, 421, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiddens, H.; Bos, A.; Mouton, J.; Devadson, S.; Janssens, H. Inhaled antibiotics: Dry or wet? Eur. Respir. J. 2014, 44, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Aptalis Pharma, I. Trail of Aeroquin versus Tobramycin Inhalation Solution (TIS) in Cystic Fibrosis (CF) Patients. Aptalis Pharma, Inc. Announces Results of Phase 3 Studies of Aeroquin™ (Levofloxacin Solution for Inhalation) among Patients with Cystic Fibrosis and Chronic Lung Infection NCT01270347 Study Mpex 209 and 207 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01270347 (accessed on 26 April 2016).
- Geller, D.E. Aerosol antibiotics in cystic fibrosis. Respir. Care 2009, 54, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C.; Swanson, J.M. Aerosolized antibacterials for the prevention and treatment of hospital-acquired pneumonia. Drugs 2007, 67, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Weiler, C.; Schiewe, J.; Friess, W. How can we bring high drug doses to the lung? Eur. J. Pharm. Biopharm. 2014, 86, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ. Microbiol. 2013, 1, 2865–2878. [Google Scholar]
- Messiaen, A.S.; Nelis, H.; Coenye, T. Investigating the role of matrix components in protection of Burkholderia cepacia complex biofilms against tobramycin. J. Cyst. Fibros. 2014, 13, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, E.; Fraidenburg, D.; Scardina, T.; Danziger, L. Inhaled antibiotics for Gram-negative respiratory infections. Clin. Microbiol. Rev. 2016, 29, 581–632. [Google Scholar] [PubMed]
- Canton, R.; Morosini, M.-I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M. New concepts in antimicrobial susceptibility testing: The mutant prevention concentration and mutant selection window approach. Vet. Dermatol. 2009, 20, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.; Pepe, M.; Quan, J.; Otto, K.; Montgomery, B.; Williams-Warren, J.; Vasiljev-K, M.; Borowitz, D.; Bowman, M.; Marshall, B.; et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N. Engl. J. Med. 1999, 340, 23–30. [Google Scholar] [CrossRef] [PubMed]
- UK Cystic Fibrosis Trust Antibiotic Working Group. Cystic Fibrosis—Our Focus. Antibiotic Treatment for Cystic Fibrosis. Available online: https://www.cysticfibrosis.org.uk/the-work-we-do/clinical-care/consensus-documents (accessed on 19 March 2016).
- Denton, M.; Kerr, K.; Mooney, L.; Keer, V.; Rajgopal, A.; Brownlee, K.; Arundel, P.; Conway, S. Transmission of colistin-resistant Pseudomonas aeruginosa between patients attending a pediatric cystic fibrosis center. Pediatr. Pulmonol. 2002, 34, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Alothman, G.A.; Ho, B.; Alsaadi, M.M.; Ho, S.L.; O’Drowsky, L.; Louca, E.; Coates, A.L. Bronchial constriction and inhaled colistin in cystic fibrosis. Chest 2005, 127, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Westerman, E.M.; Le Brun, P.P.H.; Touw, D.J.; Frijlink, H.W.; Heijerman, H.G.M. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: A pilot study. J. Cyst. Fibros. 2004, 3, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Raemdonck, K.; de Smedt, S.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, B.; Park, S.; Han, B.; Yeo, Y. A strategy to deliver genes to cystic fibrosis lungs: A battle with environment. J. Control. Release 2011, 155, 289–295. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.L.; Smyth, H.D.C. Disruption of the mucus barrier by topically applied exogenous particles. Mol. Pharm. 2010, 7, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L. Devices for aerosol delivery to treat sinusitis. J. Aerosol Med. 2007, 20, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Moller, W.; Schuschnig, U.; Celik, G.; Munzing, W.; Bartenstein, P.; Haussinger, K.; Kreyling, W.; Knoch, M.; Canis, M.; Becker, S. Topical drug delivery in chronic rhinosinusitis patients before and after sinus surgery using pulsating aerosols. PLoS ONE 2013, 8, e74991. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.; Beaulac, C.; Bouhajib, M.; Montplaisir, S.; Sharkawi, M.; Lagace, J. Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1994, 38, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, C.; Clement-Major, S.T.; Hawari, J.; Lagace, J. In vitro kinetics of drug release and pulmonary retention of microencapsulated antibiotic in liposomal formulations in relation to the lipid composition. J. Microencapsul. 1997, 14, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Halwani, M.; Herbert, S.; Suntres, Z.E.; Lafrenie, R.M.; Azghani, A.O.; Omri, A. Bismuth-thiol incorporation enhances biological activities of liposomal tobramycin against bacterial biofilm and quorum sensing molecules production by Pseudomonas aeruginosa. Int. J. Pharm. 2009, 373, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Suntres, Z.E.; Lafrenie, R.M.; Omri, A. Attenuation of Pseudomonas aeruginosa virulence factors and biofilms by co-encapsulation of bismuth-ethanedithiol with tobramycin in liposomes. J. Antimicrob. Chemother. 2010, 65, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Dorval, C.; Suntres, Z.E.; Omri, A. Bismuth-ethanedithiol incorporated in a liposome-loaded tobramycin formulation modulates the alginate levels in mucoid Pseudomonas aeruginosa. J. Pharm. Pharmacol. 2011, 63, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Pilcer, G.; Sebti, T.; Amighi, K. Formulation and characterization of lipid-coated tobramycin particles for dry powder inhalation. Pharm. Res. 2006, 23, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Pilcer, G.; Goole, J.; Van, G.B.; Blocklet, D.; Knoop, C.; Vanderbist, F.; Amighi, K. Pharmacoscintigraphic and pharmacokinetic evaluation of tobramycin DPI formulations in cystic fibrosis patients. Eur. J. Pharm. 2008, 68, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalization with dornase alfa (DNase). J. Control. Release 2015, 198, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sastre, M.; Pastora, M.; Esquisabela, A.; Sansc, E.; Viñasc, M.; Fleischerd, A.; Palominod, E.; Bachillerd, D.; Luis Pedraza, J. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2016, 498, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, C.; Sachetelli, S.; Lagace, J. In vitro bactericidal evaluation of a low phase transition temperature liposomal tobramycin formulation as a dry powder preparation against Gram-negative and Gram-positive bacteria. J. Liposome Res. 1999, 9, 301–312. [Google Scholar] [CrossRef]
- Beaulac, C.; Sachetelli, S.; Lagace, J. In vitro bactericidal efficacy of sub-MIC concentrations of liposome-encapsulated antibiotic against Gram-negative and Gram-positive bacteria. J. Antimicrob. Chemother. 1998, 41, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sachatelli, S.; Khalil, H.; Chen, T.; Beaulac, C.; Senechal, S.; Lagace, J. Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim. Biophys. Acta Biomembr. 2000, 1463, 254–266. [Google Scholar] [CrossRef]
- Beaulac, C.; Clement-Major, S.; Hawari, J.; Lagace, J. Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother. 1996, 40, 665–669. [Google Scholar] [PubMed]
- Alipour, M.; Suntres, Z.E.; Halwani, M.; Azghani, A.O.; Omri, A. Activity and interactions of liposomal antibiotics in presence of polyanions and sputum of patients with cystic fibrosis. PLoS ONE 2009, 4, e5724. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Halwani, M.; Azghani, A.O.; Lafrenie, R.M.; Omri, A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Suntres, Z.E.; Omri, A. Importance of DNase and alginate lyase for enhancing free and liposome encapsulated aminoglycoside activity against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2009, 64, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, C.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar]
- Halwani, M.; Yebio, B.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Azghani, A.O.; Omri, A. Liposome-mediated gentamicin delivery: Development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J. Antimicrob. Chemother. 2005, 55, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J.; Drulis-Kawa, Z.; Dorotkiewicz-Jach, A.; Doroszkiewicz, W.; Kozubek, A. In vitro antimicrobial activity of liposomes containing ciprofloxacin, meropenem and gentamicin against Gram-negative clinical bacterial strains. Lett. Drug Des. Discov. 2007, 4, 297–304. [Google Scholar] [CrossRef]
- Alhajlan, M.; Alhariri, M.; Omri, A. Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob. Agents Chemother. 2013, 57, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Blanchard, J.; Gonda, I. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics 2016, 8, 6. [Google Scholar]
- Baelo, A.; Levato, R.; Julián, E.; Crespo, A.; Astola, J.; Gavaldà, J.; Engel, E.; Mateos-Timoneda, M.A.; Torrents, E. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control. Release 2015, 209, 150–158. [Google Scholar] [CrossRef] [PubMed]
- DiTizio, V.; Ferguson, G.W.; Mittelman, M.W.; Khoury, A.E.; Bruce, A.W.; DiCosmo, F. A liposomal hydrogel for the prevention of bacterial adhesion to catheters. Biomaterials 1998, 19, 1877–1884. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Sousa, J.J.; Pais, A.A.; Cardoso, O.; Murtinho, D.; Serra, M.E.; Tewes, F.; Olivier, J.C. Optimization of levofloxacin-loaded crosslinked chitosan microspheres for inhaled aerosol therapy. Eur. J. Pharm. Biopharm. 2015, 96, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. The roles of lipid in anti-biofilm efficacy of lipid-polymer hybrid nanoparticles encapsulating antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 158–165. [Google Scholar] [CrossRef]
- Pastor, M.; Moreno-Sastre, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Bachiller, D.; Asensio, V.J.; Pozo, A.D.; Gainza, E.; Pedraz, J.L. Sodium colistimethate loaded lipid nanocarriers for the treatment of Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2014, 477, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sans-Serramitjana, E.; Fusté, E.; Martínez-Garriga, B.; Merlos, A.; Pastor, M.; Pedraz, J.L.; Esquisabel, A.; Bachiller, D.; Vinuesa, T.; Viñas, M. Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. J. Cyst. Fibros. 2015. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Casciaro, B.; Miro, A.; Quaglia, F.; Mangoni, M.L.; Ungaro, F. Overcoming barriers in Pseudomonas aeruginosa lung infections: Engineered nanoparticles for local delivery of a cationic antimicrobial peptide. Colloids Surf. B Biointerfaces 2015, 135, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Kolate, A.; Kore, G.; Lesimple, P.; Baradia, D.; Patil, S.; Hanrahan, J.W.; Misra, A. Polymer assisted entrapment of netilmicin in PLGA nanoparticles for sustained antibacterial activity. J. Microencapsul. 2015, 32, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Solleti, V.S.; Alhariri, M.; Halwani, M.; Omri, A. Antimicrobial properties of liposomal azithromycin for Pseudomonas infections in cystic fibrosis patients. J. Antimicrob. Chemother. 2015, 70, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, D.; Scalia, M.; Nicolosi, V.M.; Pignatello, R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Citardi, M.; Leong, J.-L. Topical antimicrobials in the management of chronic rhinosinusitis: A systematic review. Am. J. Rhinol. 2008, 22, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Vecellio, L.; de Gersem, R.; Le Guellec, S.; Reychler, G.; Pitance, L.; Le Pennec, D.; Diot, P.; Chantrel, G.; Bonfils, P.; Jamar, F. Deposition of aerosols delivered by nasal route with jet and mesh nebulizers. Int. J. Pharm. 2011, 407, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Suman, J.D.; Laube, B.L.; Dalby, R. Comparison of nasal deposition and clearance of aerosol generated by nebulizer and an aqueous spray pump. Pharm. Res. 1999, 16, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.H.; Woo, R.J.; Fong, K.J. Intranasal deposition of nebulized saline: A radionuclide distribution study. Am. J. Rhinol. 2006, 20, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, R.; Badre, R.; Flottes, L.; Riu, R.; Fey, A. A new method of aerosol penetration into the sinuses. Presse Med. 1959, 30, 1097–1098. [Google Scholar]
- Maniscalco, M.; Sofia, M.; Weitzberg, E.; Lundberg, J.O. Sounding airflow enhances aerosol delivery into the paranasal sinuses. Eur. J. Clin. Investig. 2006, 36, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Möller, W.; Schuschnig, U.; Meyer, G.; Mentzel, H.; Keller, M. Ventilation and drug delivery to the paranasal sinuses: Studies in a nasal cast using pulsating airflow. Rhinology 2008, 46, 213–220. [Google Scholar] [PubMed]
- Durand, M.; Rusch, P.; Granjon, D.; Chantrel, G.; Prades, J.M.; Dubois, F.; Esteve, D.; Pouget, J.F.; Martin, C. Preliminary study of the deposition of aerosol in the maxillary sinuses using a plastinated model. J. Aerosol Med. 2001, 14, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.J.; Athanasiadis, T.; Thwin, M.; Singhal, D.; Weitzel, E.K.; Wormald, P.J. A prospective controlled trial of pulsed nasal nebulizer in maximally dissected cadavers. Am. J. Rhinol. 2008, 22, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Möller, W.; Schuschnig, U.; Meyer, G.; Häussinger, K.; Keller, M.; Junge-Hülsing, B.; Mentzel, H. Ventilation and aerosolized drug delivery to the paranasal sinuses using pulsating airflow—A preliminary study. Rhinology 2009, 47, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Draft Guidance for Industry: Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action (5383DFT); Food and Drug Administration (FDA): Taipei, Taiwan, 2003.
| Drug | Product Name | Development Status | Formulation |
|---|---|---|---|
| Tobramycin | Tobi®, Bramitob® | Marketed | LF, DP |
| Levofloxacin | Aeroquin® | Phase III (NCT01270347, NCT01180634) * | LF |
| Aztreonam lysine | Cayston® | Marketed | LF |
| Colistimethate sodium | Promixin®, Colobreathe® | Marketed | LF, DP |
| Azithromycin | Zithromax® | Marketed | |
| Amikacin | Arikayce™ | Phase III (NCT01315678) * | LF |
| Ciprofloxacin | Cipro Inhale® | Phase II (NCT00645788) * | DP |
| Antimicrobial | Composition | Form | In Vitro/In Vivo Model | Improved Antimicrobial Effect? | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Tobramycin | DSPC/DMPG (10:1 molar ratio) | LF | Intratracheal administration in healthy rats or rats chronically infected with P. aeruginosa in agar beads | Yes | Increased residence time of tobramycin in lungs | [63] |
| Tobramycin | DPPC/DMPG (10:1 to 15:1 molar ratio) | LF | Intratracheal administration in rats chronically infected with P. aeruginosa in agar beads | Yes | Increased residence time of tobramycin in lungs | [64] |
| Tobramycin bismuth-thiol | DSPC/cholesterol (2:1 molar ratio) | LF | Clinical strains of P. aeruginosa from CF sputum | Yes | Disturbs bacterial membrane integrity and protects against binding to eDNA | [65,66,67] |
| Tobramycin | Cholesterol/lecithin | DP | Pilot study in CF patients receiving dose via a breath-actuated DPI | N/A | Improved lung deposition | [68,69] |
| Tobramycin | Alginate/chitosan, DNase | LF | CF patient sputum Galleria mellonella PA01 infection model | Yes, complete eradication | Prolonged residence time at infection site, improved penetration through CF sputum | [70] |
| Tobramycin | Precirol® ATO 5 50:50 Compritol® 888 ATO: Precirol® ATO 5/Miglyol1812 | LF | Clinical strains of P. aeruginosa from CF sputum Artificial CF sputum model Cytotoxicity in H441 and A549 cells biodistribution study in mice | Yes | Sustained release of antimicrobial, fusion with bacterial membrane | [71] |
| Tobramycin | DPPC/DMPG | DP | Clinical strains of P. aeruginosa | Yes | Fusion with bacterial membrane | [72,73,74] |
| Tobramycin | DPPC/DMPG DSPC/DMPC | LF | Intratracheal administration rats chronically infected with P. aeruginosa in agar beads | Yes, complete eradication | Fusion with bacterial membrane (in vivo study) | [75] |
| Tobramycin Polymyxin B | DMPC/cholesterol DPPC/cholesterol | LF | Clinical strains of P. aeruginosa in CF sputum | Yes Yes | Protection against binding to eDNA and degradation | [76] |
| Tobramycin Amikacin Gentamicin | DPPC/cholesterol | LF | Laboratory strains of P. aeruginosa | Yes, complete eradication | Fusion with bacterial membrane | [77] |
| Tobramycin Gentamicin Amikacin | DMPC/cholesterol | LF | Clinical strains of P. aeruginosa from CF sputum | Not in the presence of mucin and CF sputum | Binding of the liposomes to mucin, alginate or sputum components | [78] |
| Gentamicin | PLGA | LF | Laboratory strains of P. aeruginosa 96-h peritoneal murine infection model | Yes | Controlled/ sustained release of antimicrobial | [79] |
| Gentamicin | DPPC/DMPG (ratio 10:1) | LF | Clinical strains of P. aeruginosa from CF sputum Cytotoxicity in A549 cells | Yes | Quorum sensing reduction, reduced binding to eDNA | [80] |
| Gentamicin | DMPC/cholesterol DPPC/cholesterol DSPC/cholesterol | LF | Clinical strains of P. aeruginosa | Yes | Protection of antimicrobial against degradation or fusion with bacterial membrane | [81] |
| Gentamicin Ciprofloxacin | PC/cholesterol/DOTAP PC/DOPE/DOTAP | LF | Clinical and laboratory strains of P. aeruginosa | No | Reduced binding to non-target materials | [82] |
| Clarithromycin | DDAB/DPPC/cholesterol DCP/DPPC/cholesterol DPPC/cholesterol | LF | Clinical strains of P. aeruginosa | Yes, complete eradication | Electrostatic attraction possibly followed by fusion, protection of the antimicrobial | [83] |
| Ciprofloxacin | HSPC/cholesterol | DP | Phase II clinical trials (Lipoquin®) for non-CF infections with P. aeruginosa | Yes | Sustained release at site of infection | [84] |
| Ciprofloxacin | PLGA, poly(lysine), DNase | LF | P. aeruginosa planktonic and biofilms Cytotoxicity in J774 cells | Yes | Biofilm formation prevention, improved penetration through biofilm | [85] |
| Ciprofloxacin | PEG/gelatin | LF | Clinical strains of P. aeruginosa | Yes | Sustained release of antimicrobial | [86] |
| Levofloxacin | Chitosan | DP | Clinical strains of P. aeruginosa | No | Immediate release at site | [87] |
| Levofloxacin | PLGA PLGA/PC | LF | P. aeruginosa planktonic and in biofilm | Yes, but to a lower extent | PLGA/PC particles could enhance antimicrobial | [88] |
| Colistimethate sodium | Precirol® ATO 5 | LF | Clinical strains of P. aeruginosa from CF sputum Cytotoxicity in H441 and A549 cells | Yes | Sustained release of antimicrobial | [89] |
| Colistimethate sodium | Precirol® ATO 5/Miglyol® 812 | LF | Clinical strains of P. aeruginosa from CF sputum Cytotoxicity in H441 and A549, biodistribution study in mice | Yes | Sustained release of antimicrobial | [89] |
| Colistimethate sodium | Precirol® ATO 5/Miglyol® 812 | LF | Clinical strains of P. aeruginosa from CF sputum | Yes, but only in biofilms | Reduced binding to non-target materials, improved delivery to proximity of bacteria in biofilm | [90] |
| Colistin | PLGA/PVA/chitosan | DP | Artificial mucus Laboratory strains of P. aeruginosa | Yes | Sustained release of antimicrobial | [91] |
| Netilmicin | PLGA/dextran sulfate | LF | Cytotoxicity in CFBE 41o- cells, Laboratory strains of P. aeruginosa | No | Protection against binding to eDNA | [92] |
| Azithromycin | DPPC/cholesterol (6:1 molar ratio) | LF | Clinical strains of P. aeruginosa Cytotoxicity on erythrocytes and A549 cells | Yes | Attenuated production of virulence factors and reduced bacterial mobility | [93] |
| Vancomycin | DPPC/cholesterol DPPC/DOPE/CHEMS | LF | Clinical strains of P. aeruginosa | Yes | Fusion with bacterial membrane | [94] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłodzińska, S.N.; Priemel, P.A.; Rades, T.; Mørck Nielsen, H. Inhalable Antimicrobials for Treatment of Bacterial Biofilm-Associated Sinusitis in Cystic Fibrosis Patients: Challenges and Drug Delivery Approaches. Int. J. Mol. Sci. 2016, 17, 1688. https://doi.org/10.3390/ijms17101688
Kłodzińska SN, Priemel PA, Rades T, Mørck Nielsen H. Inhalable Antimicrobials for Treatment of Bacterial Biofilm-Associated Sinusitis in Cystic Fibrosis Patients: Challenges and Drug Delivery Approaches. International Journal of Molecular Sciences. 2016; 17(10):1688. https://doi.org/10.3390/ijms17101688
Chicago/Turabian StyleKłodzińska, Sylvia Natalie, Petra Alexandra Priemel, Thomas Rades, and Hanne Mørck Nielsen. 2016. "Inhalable Antimicrobials for Treatment of Bacterial Biofilm-Associated Sinusitis in Cystic Fibrosis Patients: Challenges and Drug Delivery Approaches" International Journal of Molecular Sciences 17, no. 10: 1688. https://doi.org/10.3390/ijms17101688







