GSPE Inhibits HMGB1 Release, Attenuating Renal IR-Induced Acute Renal Injury and Chronic Renal Fibrosis
Abstract
:1. Introduction
2. Results
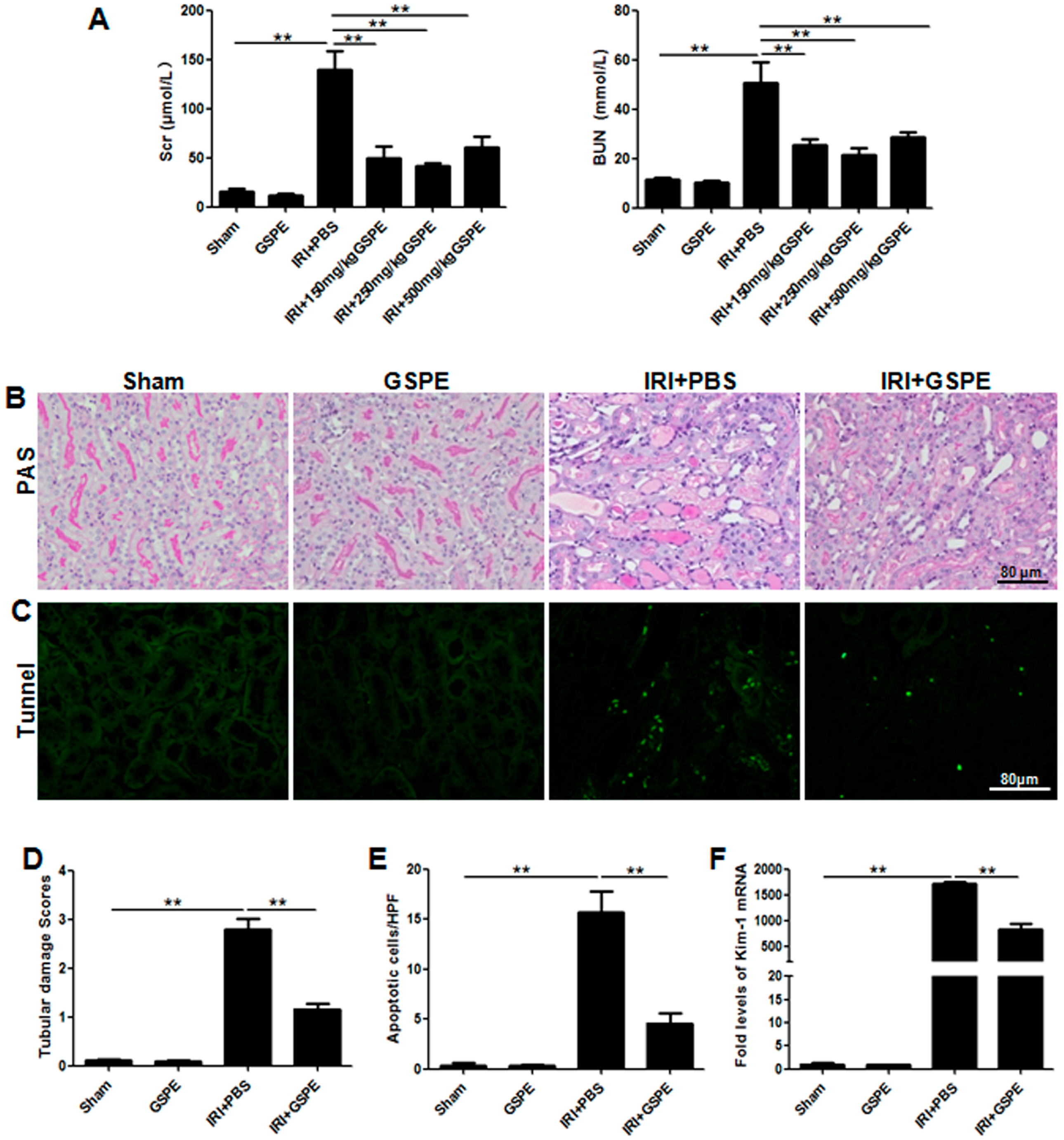
2.1. Administration of GSPE Protects Mice against Bilateral IR-Induced AKI
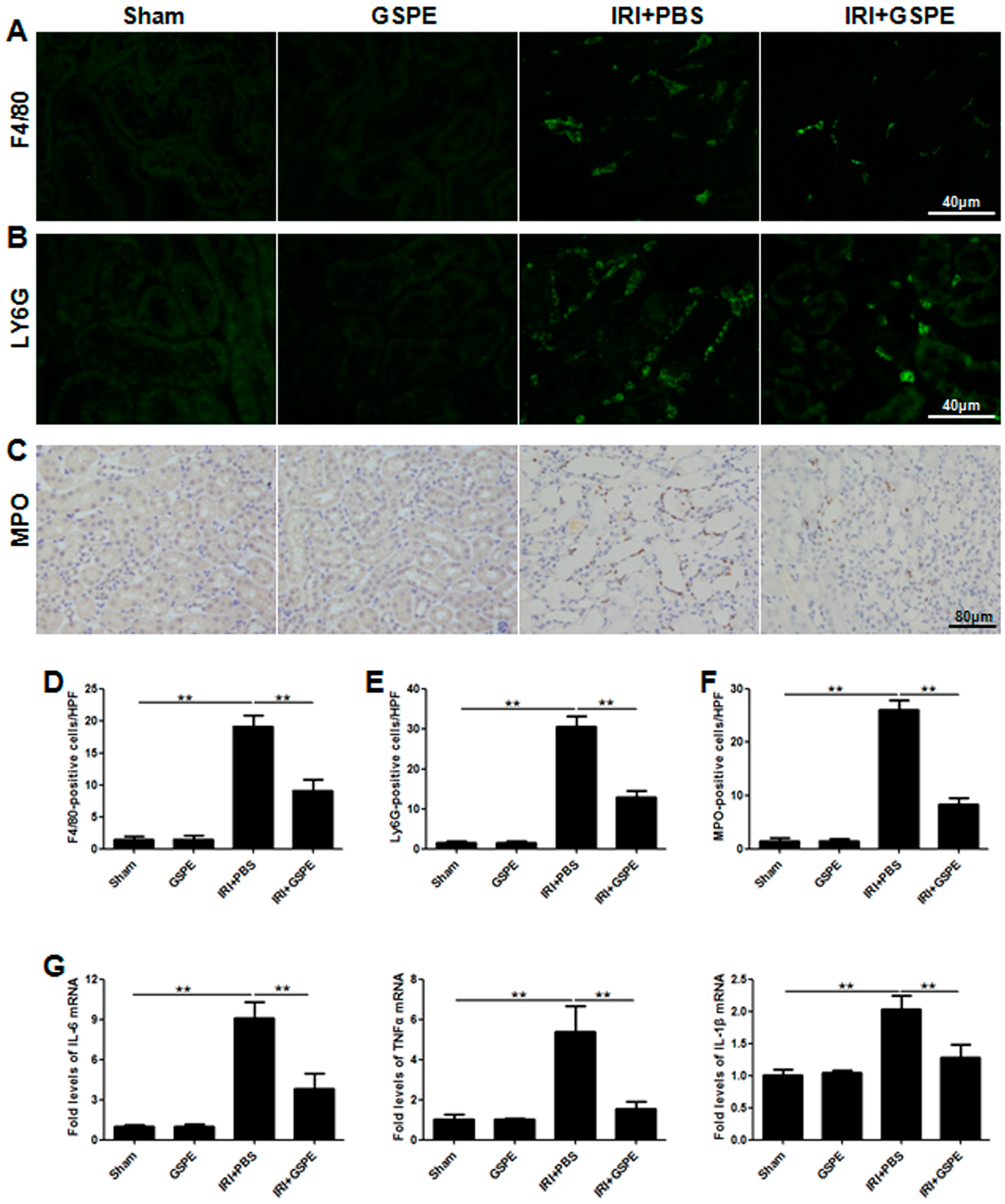
2.2. Pre-Treatment with GSPE Attenuates the IR-Induced Inflammatory Reaction
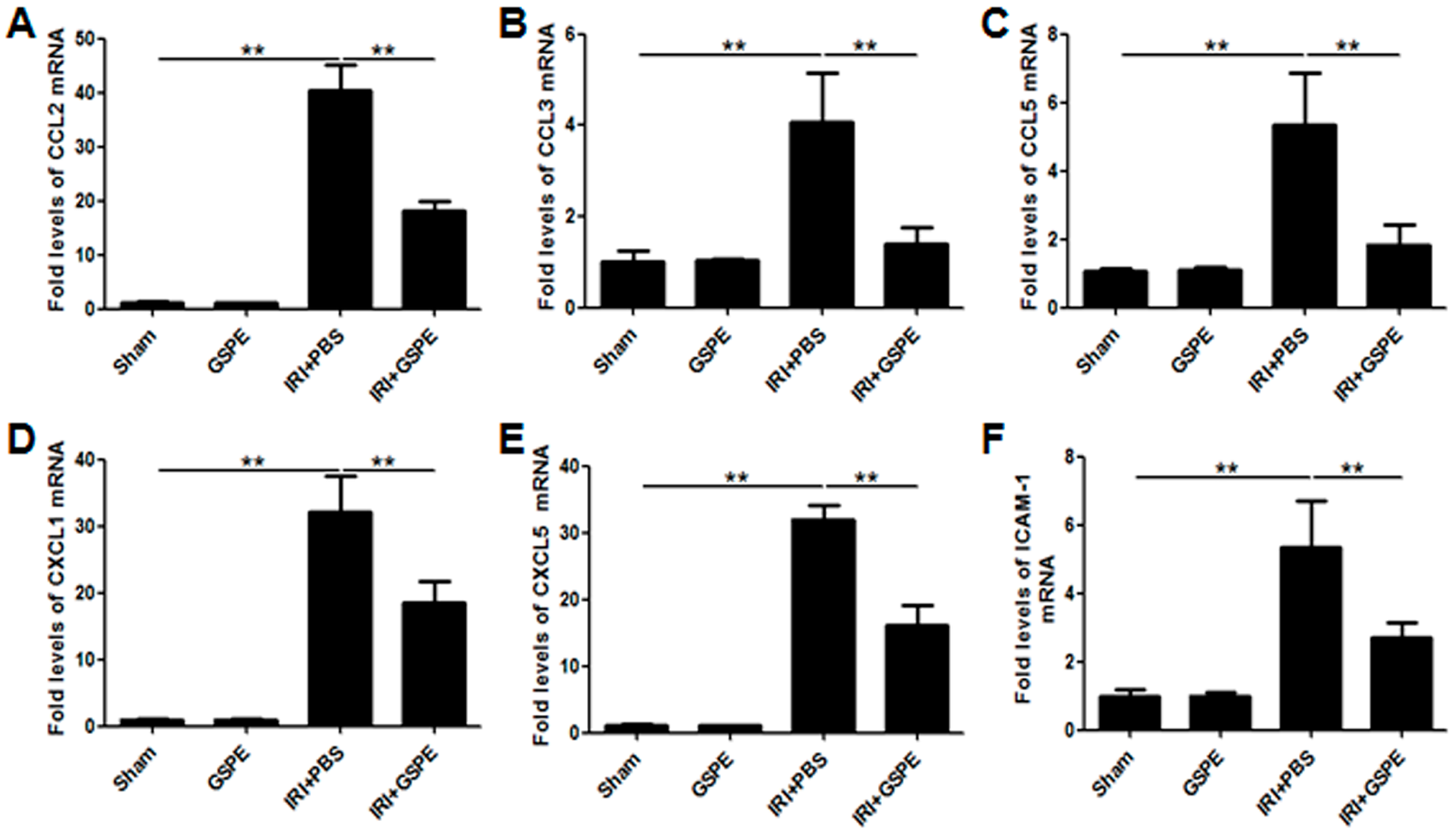
2.3. GSPE Pretreatment Inhibits the Release of Chemokines
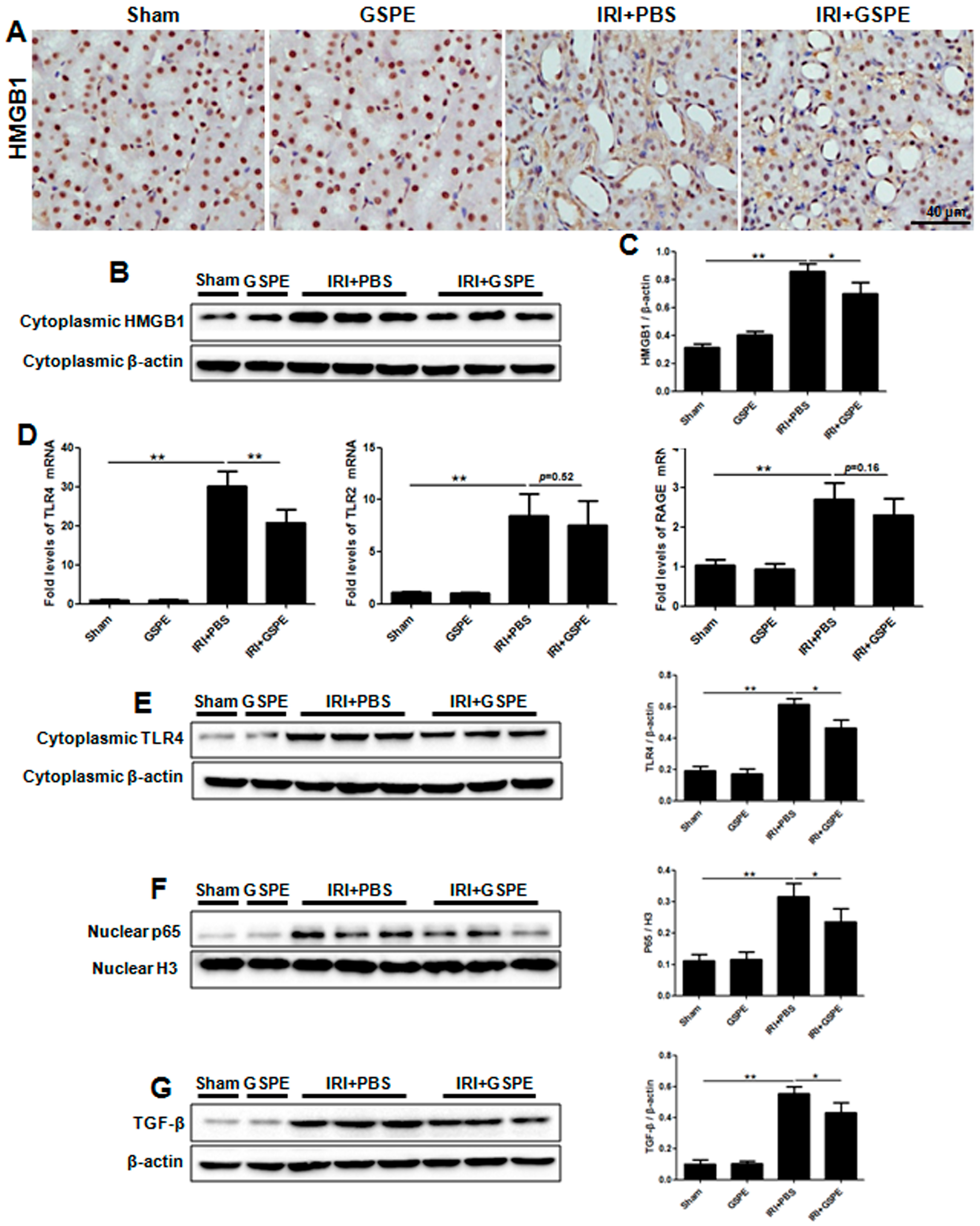
2.4. Pretreatment with GSPE Suppresses HMGB1-TLR4-p65 Activity in AKI
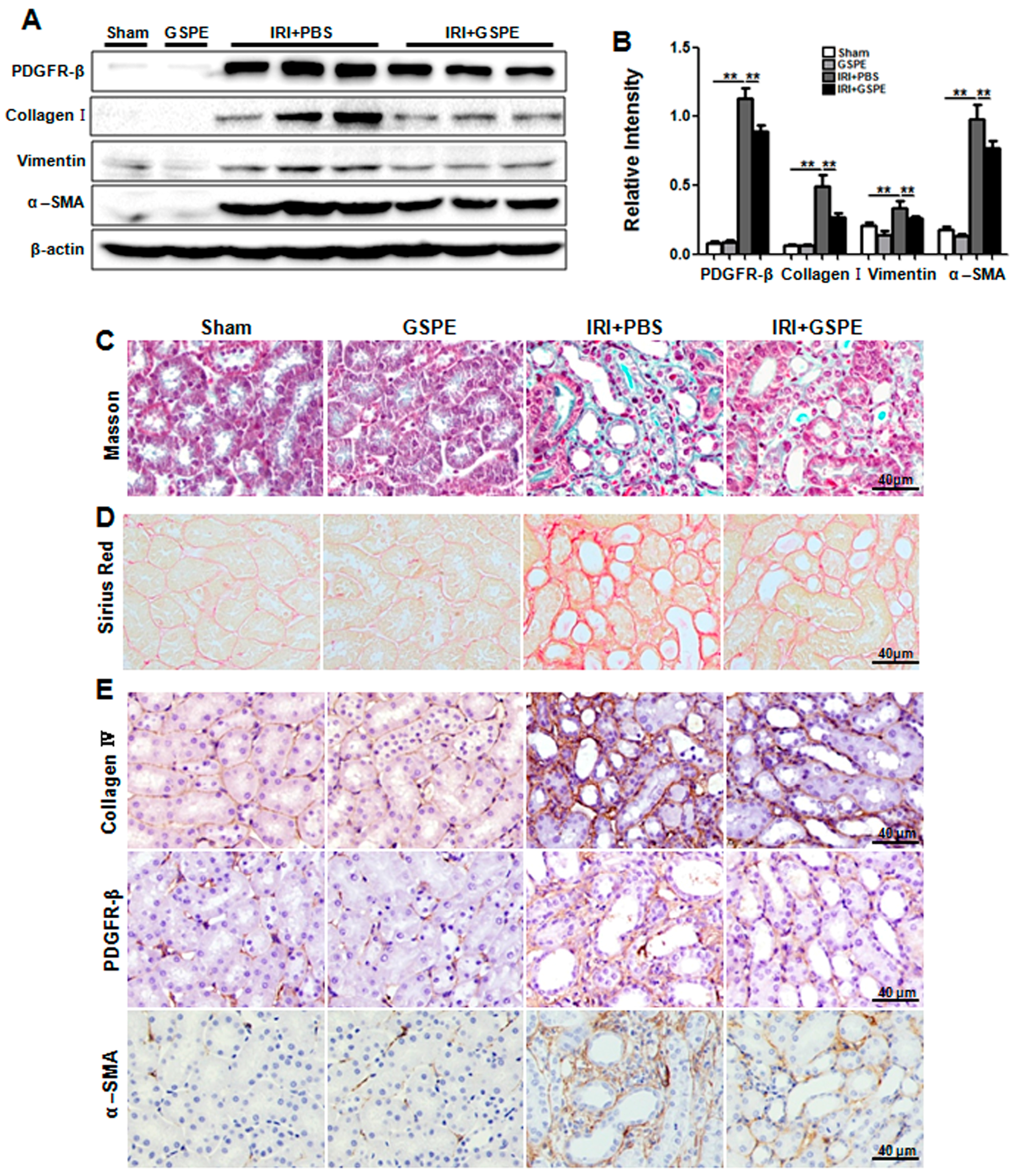
2.5. GSPE Treatment Lessens Unilateral IR-Induced Chronic Renal Fibrosis
2.6. Administration of GSPE Attenuates Unilateral IR-Induced Chronic Kidney Injury and Tubulointerstitial Inflammation
2.7. Successive Treatment with GSPE Suppresses the Activation of HMGB-TLR4-P65-TGF-β
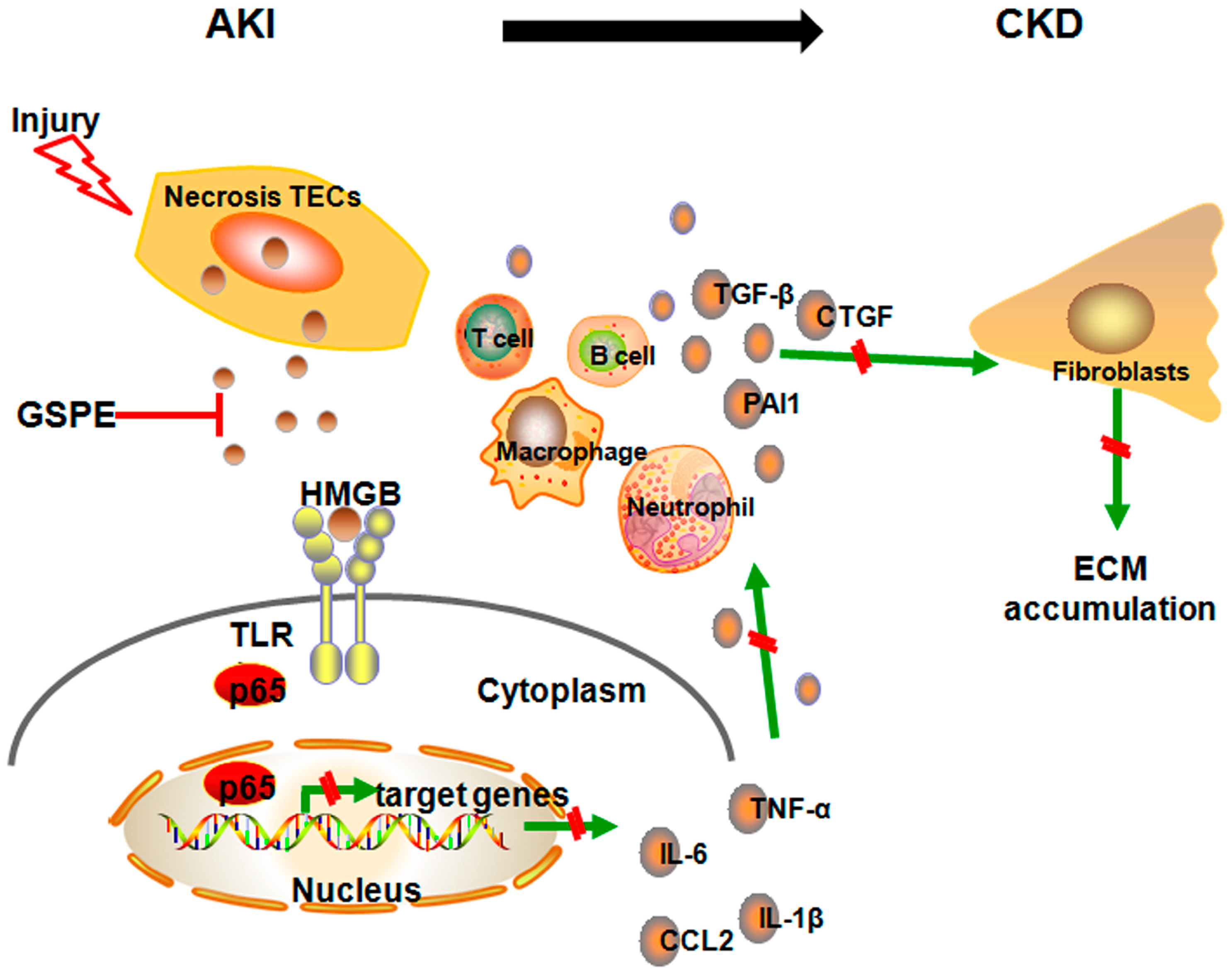
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Renal IRI Model
4.3. Assessment of Renal Function
4.4. Histological Analysis
4.5. Immunohistochemical Staining
4.6. Immunofluorescence
4.7. Apoptosis Assay
4.8. Isolation of the Total, Cytoplasmic and Nuclear Proteins
4.9. Western Blot
4.10. Real-Time PCR
4.11. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carl, D.E.; Grossman, C.; Behnke, M.; Sessler, C.N.; Gehr, T.W. Effect of timing of dialysis on mortality in critically ill, septic patients with acute renal failure. Hemodial. Int. 2010, 14, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Kimmel, P.L. Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int. 2012, 82, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.S.; Okusa, M.D. Biology of renal recovery: molecules, mechanisms, and pathways. Nephron Clin. Pract. 2014, 127, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Javaherian, K.; Liu, J.F.; Wang, J.C. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 1978, 199, 1345–1346. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, H.; Ding, A.; Golenbock, D.T.; Latz, E.; Czura, C.J.; Fenton, M.J.; Tracey, K.J.; Yang, H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006, 26, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, R.; Andersson, A.; Mullins, G.; Ostberg, T.; Treutiger, C.J.; Arnold, B.; Nawroth, P.; Andersson, U.; Harris, R.A.; Harris, H.E. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 2005, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, Q.; Zhong, S.; Wang, L.; Guo, H.; Xiang, Y.; Ichim, T.E.; Wang, C.Y.; Chen, S.; Gong, F.; et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrol. Dial. Transplant. 2011, 26, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Nolan, S.; Slattery, C.; Feighery, R.; Ryan, M.P.; McMorrow, T. High-mobility group box protein 1: A novel mediator of inflammatory-induced renal epithelial-mesenchymal transition. Am. J. Nephrol. 2010, 32, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Liu, H.; Zhang, D.; Liu, Y.; Wang, C.; Liu, F.; Chen, J. HMGB1 Enhances the AGE-Induced Expression of CTGF and TGF-beta via RAGE-Dependent Signaling in Renal Tubular Epithelial Cells. Am. J. Nephrol. 2015, 41, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, H.W.; Park, C.W.; Yang, C.W.; Jin, D.C.; Choi, B.S. The protective effect of neutralizing high-mobility group box1 against chronic cyclosporine nephrotoxicity in mice. Transpl. Immunol. 2016, 34, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 22, 1117–1120. [Google Scholar] [PubMed]
- Shrotriya, S.; Tyagi, A.; Deep, G.; Orlicky, D.J.; Wisell, J.; Wang, X.J.; Sclafani, R.A.; Agarwal, R.; Agarwal, C. Grape seed extract and resveratrol prevent 4-nitroquinoline 1-oxide induced oral tumorigenesis in mice by modulating AMPK activation and associated biological responses. Mol. Carcinog. 2015, 54, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, Y.; Gao, H.; Li, B.; Xu, L.; Cheng, M.; Jiang, B.; Ma, Y. Grape seed proanthocyanidins ameliorate diabetic nephropathy via modulation of levels of AGE, RAGE and CTGF. Nephron Exp. Nephrol. 2009, 111, e31–e41. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ding, R.; Wang, Y.; Tang, L. Grape seed proanthocyanidin extract reduces renal ischemia/reperfusion injuries in rats. Am. J. Med. Sci. 2012, 343, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, G.; Hu, Z.; Li, X.; Yang, X.; Jiang, B.; Li, X. Grape seed proanthocyanidin extract protects from cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 2014, 9, 801–807. [Google Scholar] [PubMed]
- Ulusoy, S.; Ozkan, G.; Yucesan, F.B.; Ersoz, S.; Orem, A.; Alkanat, M.; Yulug, E.; Kaynar, K.; Al, S. Anti-apoptotic and anti-oxidant effects of grape seed proanthocyanidin extract in preventing cyclosporine A-induced nephropathy. Nephrology 2012, 17, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.Y.; Song, H.S.; Park, K.U.; Mun, K.C.; Ha, E. Grape seed proanthocyanidin extract inhibits interleukin-17-induced interleukin-6 production via MAPK pathway in human pulmonary epithelial cells. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Overman, A.; Bumrungpert, A.; Kennedy, A.; Martinez, K.; Chuang, C.C.; West, T.; Dawson, B.; Jia, W.; McIntosh, M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2010, 34, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Chacon, M.R.; Ceperuelo-Mallafre, V.; Maymo-Masip, E.; Mateo-Sanz, J.M.; Arola, L.; Guitierrez, C.; Fernandez-Real, J.M.; Ardevol, A.; Simon, I.; Vendrell, J. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine 2009, 47, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Carrero, J.J.; Stenvinkel, P. Inflammation as a risk factor and target for therapy in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhong, J.; Yang, P.; Gong, F.; Wang, C.Y. HMGB1, an innate alarmin, in the pathogenesis of type 1 diabetes. Int. J. Clin. Exp. Pathol. 2009, 3, 24–38. [Google Scholar] [PubMed]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Andrassy, M.; Volz, H.C.; Igwe, J.C.; Funke, B.; Eichberger, S.N.; Kaya, Z.; Buss, S.; Autschbach, F.; Pleger, S.T.; Lukic, I.K.; et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 2008, 117, 3216–3226. [Google Scholar] [CrossRef] [PubMed]
- Hori, O.; Brett, J.; Slattery, T.; Cao, R.; Zhang, J.; Chen, J.X.; Nagashima, M.; Lundh, E.R.; Vijay, S.; Nitecki, D.; et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995, 270, 25752–25761. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; He, Y.; Li, Y.Q.; Han, M.; Ma, Z.F.; Liu, X.C.; Zeng, R.; Shao, J.F.; Guo, Y.C.; He, X.Y.; et al. Telmisartan protects 5/6 Nx rats against renal injury by enhancing nNOS-derived NO generation via regulation of PPARgamma signaling. Am. J. Transl. Res. 2014, 6, 517–527. [Google Scholar] [PubMed]
- Yang, P.; Zhang, Y.; Pang, J.; Zhang, S.; Yu, Q.; He, L.; Wagner, K.U.; Zhou, Z.; Wang, C.Y. Loss of Jak2 impairs endothelial function by attenuating Raf-1/MEK1/Sp-1 signaling along with altered eNOS activities. Am. J. Pathol. 2013, 183, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lv, J.W.; Yang, P.; Yu, Q.; Pang, J.; Wang, Z.; Guo, H.; Liu, S.; Hu, J.; Li, J.; et al. Loss of dicer exacerbates cyclophosphamide-induced bladder overactivity by enhancing purinergic signaling. Am. J. Pathol. 2012, 181, 937–946. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequence (Sense/Antisense) |
|---|---|
| Kim-1 | 5′-TAAACCAGAGATTCCCACAC-3′ |
| 5′-GATCTTGTTGAAATAGTCGTGG-3′ | |
| IL-6 | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ |
| 5′-TTGGTCCTTAGCCACTCCTTC-3′ | |
| TNF-α | 5′-CCTGTAGCCCACGTCGTAG-3′ |
| 5′-GGGAGTAGACAAGGTACAACCC-3′ | |
| IL-1β | 5′-GAAATGCCACCTTTTGACAGTG-3′ |
| 5′-TGGATGCTCTCATCAGGACAG-3′ | |
| CCL2 | 5′-TAAAAACCTGGATCGGAACCAAA-3′ |
| 5′-GCATTAGCTTCAGATTTACGGGT-3′ | |
| CCL3 | 5′-TGTACCATGACACTCTGCAAC-3′ |
| 5′-CAACGATGAATTGGCGTGGAA-3′ | |
| CCL5 | 5′-GCTGCTTTGCCTACCTCTCC-3′ |
| 5′-TCGAGTGACAAACACGACTGC-3′ | |
| CXCL1 | 5′-ACTGCACCCAAACCGAAGTC-3′ |
| 5′-TGGGGACACCTTTTAGCATCTT-3′ | |
| CXCL5 | 5′-TGCGTTGTGTTTGCTTAACCG-3′ |
| 5′-CTTCCACCGTAGGGCACTG-3′ | |
| ICAM-1 | 5′-GTGATGCTCAGGTATCCATCCA-3′ |
| 5′-CACAGTTCTCAAAGCACAGCG-3′ | |
| TLR4 | 5′-GCCTTTCAGGGAATTAAGCTCC-3′ |
| 5′-GATCAACCGATGGACGTGTAAA-3′ | |
| TLR2 | 5′-TCTAAAGTCGATCCGCGACAT-3′ |
| 5′-CTACGGGCAGTGGTGAAAACT-3′ | |
| RAGE | 5′-ACTACCGAGTCCGAGTCTACC-3′ |
| 5′-CCCACCTTATTAGGGACACTGG-3′ | |
| β-actin | 5′-GGCTGTATTCCCCTCCATCG-3′ |
| 5′-CCAGTTGGTAACAATGCCATGT-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Wang, K.; Zhang, C.; Zhang, C.; Li, Y.; Zhang, Y.; Chang, X.; Zhou, Q.; Yao, Y.; Liu, Y.; et al. GSPE Inhibits HMGB1 Release, Attenuating Renal IR-Induced Acute Renal Injury and Chronic Renal Fibrosis. Int. J. Mol. Sci. 2016, 17, 1647. https://doi.org/10.3390/ijms17101647
Zhan J, Wang K, Zhang C, Zhang C, Li Y, Zhang Y, Chang X, Zhou Q, Yao Y, Liu Y, et al. GSPE Inhibits HMGB1 Release, Attenuating Renal IR-Induced Acute Renal Injury and Chronic Renal Fibrosis. International Journal of Molecular Sciences. 2016; 17(10):1647. https://doi.org/10.3390/ijms17101647
Chicago/Turabian StyleZhan, Juan, Kun Wang, Conghui Zhang, Chunxiu Zhang, Yueqiang Li, Ying Zhang, Xiaoyan Chang, Qiaodan Zhou, Ying Yao, Yanyan Liu, and et al. 2016. "GSPE Inhibits HMGB1 Release, Attenuating Renal IR-Induced Acute Renal Injury and Chronic Renal Fibrosis" International Journal of Molecular Sciences 17, no. 10: 1647. https://doi.org/10.3390/ijms17101647





