Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice
Abstract
:1. Introduction
2. Results
2.1. Generation of Prl7d1 Transgenic Mice
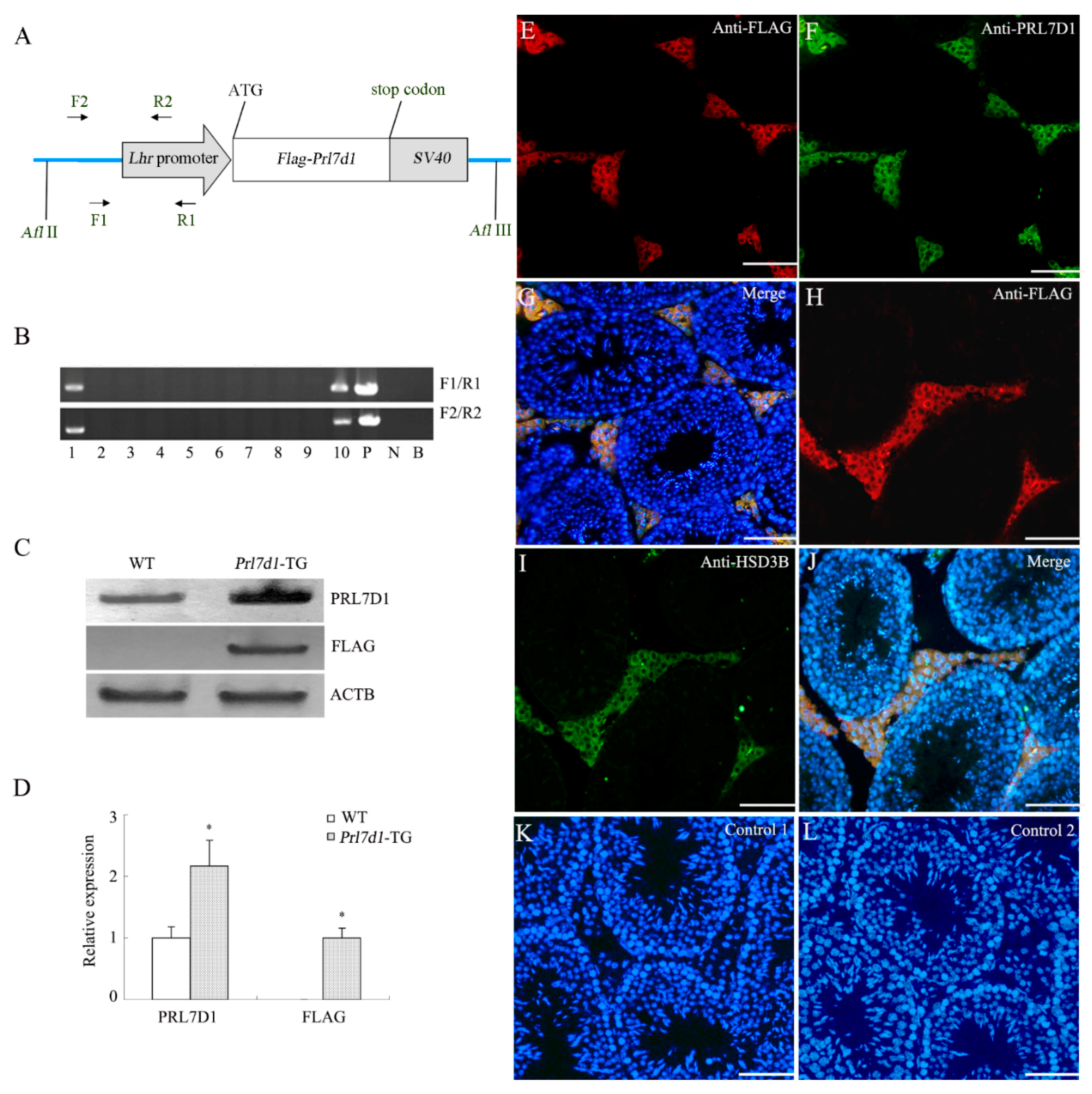
2.2. Body and Testicular Weights
2.3. Effects of Overexpressing Prl7d1 on Sperm Count and Fertility in Prl7d1 Transgenic Mice
| WT | Prl7d1-TG | |
|---|---|---|
| Pregnant females (n = 12) | 11 | 5 # |
| Litter size | 7.2 ± 0.9 | 4.2 ± 1.3 * |
| sperm count (×106) | 13.1 ± 1.5 | 7.5 ± 1.4 * |
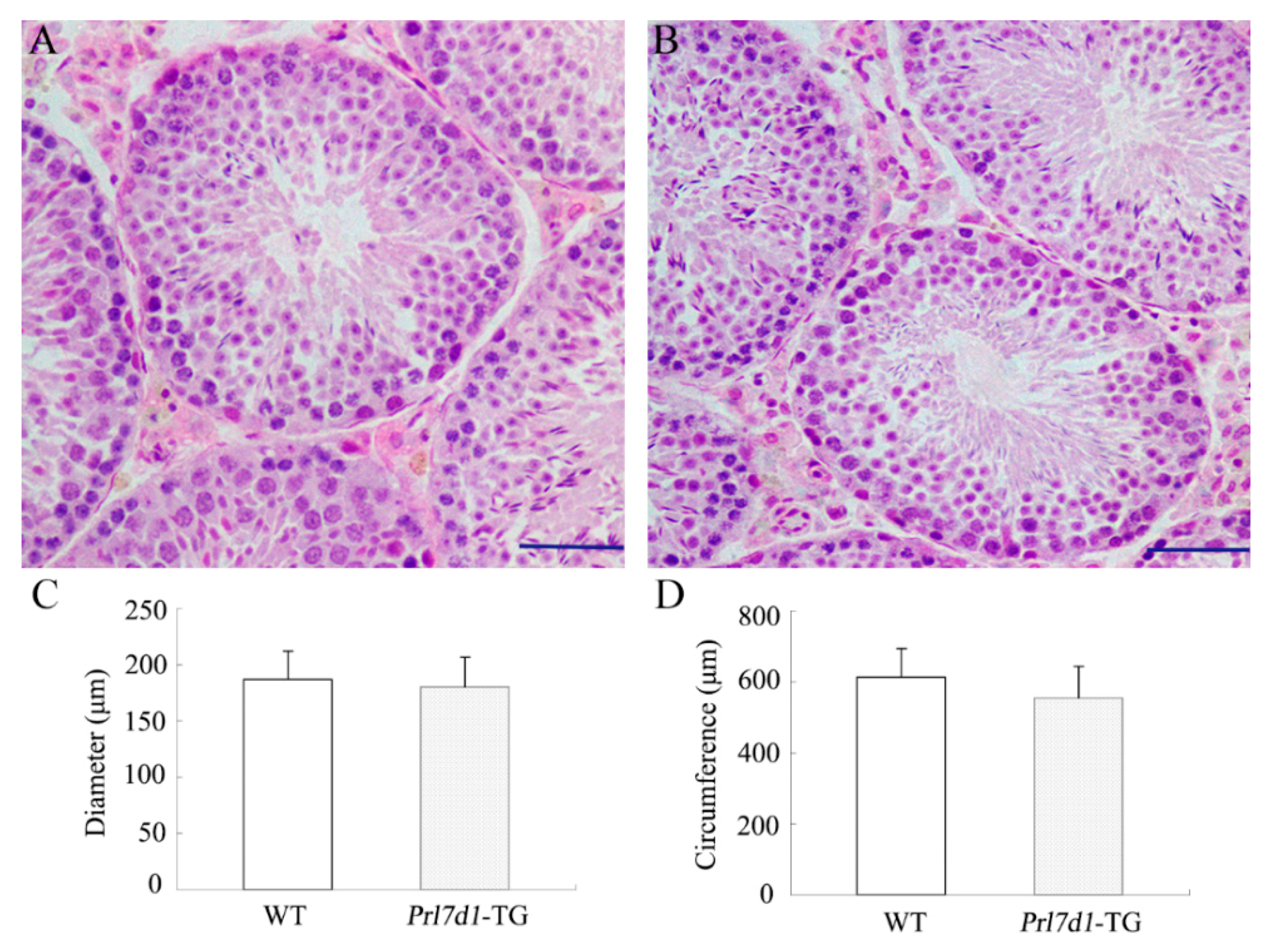
2.4. Histology of Prl7d1 Transgenic Mice Testes
2.5. Overexpression of Prl7d1 in Leydig Cells Increases Germ Cell Apoptosis and Upregulates Cleaved Caspase 3 (17 kDa) and BCL2-Associated X Protein Expression in Testes

2.6. Decreased Levels of Serum and Intra-Testicular Testosterone, but Not Gonadotropin-Releasing Hormone and Luteinizing Hormone, in Prl7d1 Transgenic Mice

2.7. Overexpression of Prl7d1 in Leydig Cells Decreases the Expression of STAR and HSD3B

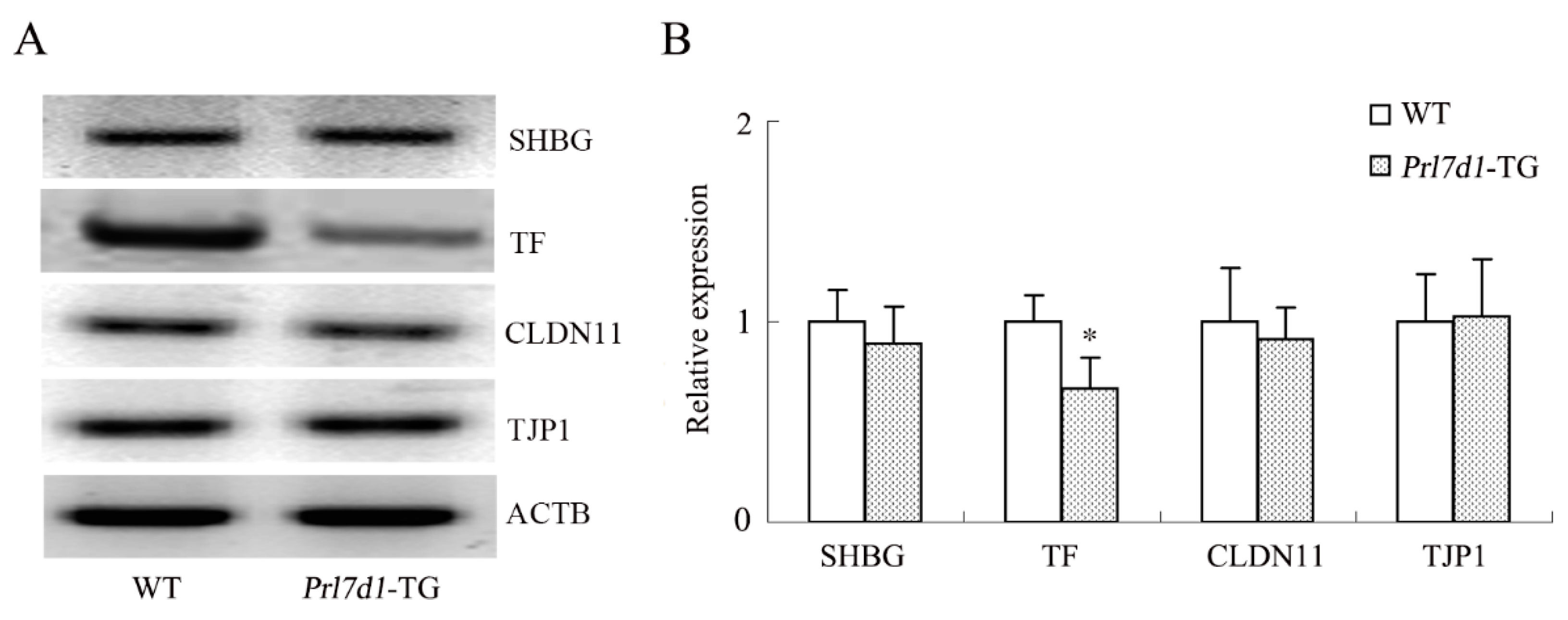
2.8. Overexpression of Prl7d1 in Leydig Cells Decreases TF Expression
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Transgene Construction
4.3. Generation of Prl7d1 Overexpressing Mice
4.4. Blood Collection and Tissue Removal
4.5. Histopathological Examination
4.6. In Situ Detection of Apoptosis
4.7. Immunofluorescent Detection of FLAG-Tagged PRL7D1 Protein
4.8. Sperm Counts
4.9. LH, GnRH and Testosterone Measurements
4.10. Assessment of Fertility
4.11. Western Blotting
| Antibody | Dilutions | Host Species | Catalog Number | Vendor |
|---|---|---|---|---|
| SHBG | 1:200 | Rabbit | bs-2410R | Bioss |
| BAX | 1:200 | Rabbit | bs-0127R | Bioss |
| BCL2 | 1:200 | Rabbit | bs-0032R | Bioss |
| CASP3 | 1:200 | Rabbit | bs-0081R | Bioss |
| CYP11A1 | 1:200 | Rabbit | bs-10099R | Bioss |
| TSPO | 1:200 | Rabbit | bs-3674R | Bioss |
| CLDN11 | 1:300 | Rabbit | 36-4500 | Life Technologies |
| TJP1 | 1:300 | Rabbit | 61-7300 | Life Technologies |
| FLAG | 1:1000 | Rabbit | 20543-1-AP | Proteintech |
| TF | 1:500 | Rabbit | 17435-1-AP | Proteintech |
| LHR | 1:500 | Rabbit | 19968-1-AP | Proteintech |
| HSD3B | 1:300 | Goat | sc-30821 | Santa Cruz Biotechnology |
| STAR | 1:300 | Rabbit | sc-25806 | Santa Cruz Biotechnology |
| PRL7D1 | 1:300 | Rabbit | sc-98474 | Santa Cruz Biotechnology |
4.12. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soares, M.J. The prolactin and growth hormone families: Pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod. Biol. Endocrinol. 2004, 2, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, S.M.; Ain, R.; Konno, T.; Ho-Chen, J.K.; Soares, M.J. The rat prolactin gene family locus: Species-specific gene family expansion. Mamm. Genome 2006, 17, 858–877. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A. Defining the function of a prolactin gene family member. Proc. Natl. Acad. Sci. USA 2004, 101, 16397–16398. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, A.; Binart, N. Reproductive role of prolactin. Reproduction 2007, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Haig, D. Placental growth hormone-related proteins and prolactin-related proteins. Placenta 2008, 29 (Suppl. A), S36–S41. [Google Scholar] [CrossRef] [PubMed]
- Linzer, D.I.; Nathans, D. A new member of the prolactin-growth hormone gene family expressed in mouse placenta. EMBO J. 1985, 4, 1419–1423. [Google Scholar] [PubMed]
- Jackson, D.; Volpert, O.V.; Bouck, N.; Linzer, D.I. Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science 1994, 266, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Colosi, P.; Swiergiel, J.J.; Wilder, E.L.; Oviedo, A.; Linzer, D.I. Characterization of proliferin-related protein. Mol. Endocrinol. 1988, 2, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Talamantes, F.; Wilder, E.; Linzer, D.I.; Nathans, D. Trophoblastic giant cells of the mouse placenta as the site of proliferin synthesis. Endocrinology 1988, 122, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Toft, D.J.; Linzer, D.I. Identification of three prolactin-related hormones as markers of invasive trophoblasts in the rat. Biol. Reprod. 2000, 63, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Linzer, D.I.; Fisher, S.J. The placenta and the prolactin family of hormones: Regulation of the physiology of pregnancy. Mol. Endocrinol. 1999, 13, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hao, J.; Hu, J.; Wang, Q.; Lu, Z.; Wang, L.; Yu, Q.; Wang, Y.; Li, G. Expression of proliferin-related protein in testis and the biological significance in testosterone production. Mol. Cell. Endocrinol. 2011, 343, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ascoli, M.; Fanelli, F.; Segaloff, D.L. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 2002, 23, 141–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.P.; Pakarainen, T.; Zhu, F.; Poutanen, M.; Huhtaniemi, I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology 2004, 145, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.C.; Lei, Z.; Rao Ch, V.; Beck, J.; Lamb, D.J. De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of leydig stem cells. Endocrinology 2004, 145, 4011–4015. [Google Scholar] [CrossRef] [PubMed]
- Menon, K.M.; Menon, B. Structure, function and regulation of gonadotropin receptors-a perspective. Mol. Cell. Endocrinol. 2012, 356, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.M.; Rao, C.V.; Kornyei, J.L.; Licht, P.; Hiatt, E.S. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology 1993, 132, 2262–2270. [Google Scholar] [PubMed]
- Kokk, K.; Kuuslahti, M.; Keisala, T.; Purmonen, S.; Kaipia, A.; Tammela, T.; Orro, H.; Simovart, H.E.; Pollanen, P. Expression of luteinizing hormone receptors in the mouse penis. J. Androl. 2011, 32, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen, T.; Poutanen, M.; Huhtaniemi, I. Age- and sex-specific promoter function of a 2-kilobase 5′-flanking sequence of the murine luteinizing hormone receptor gene in transgenic mice. Endocrinology 1999, 140, 5322–5329. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.J.; Russell, T.A.; Jeffs, B.; Huhtaniemi, I.; Weiss, J.; Jameson, J.L. Leydig cell-specific expression of dax1 improves fertility of the dax1-deficient mouse. Biol. Reprod. 2003, 69, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.T.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. Targeting testis-specific proteins to inhibit spermatogenesis: Lesson from endocrine disrupting chemicals. Expert Opin. Ther. Targets 2013, 17, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Sinha Hikim, A.P.; Swerdloff, R.S. Hormonal and genetic control of germ cell apoptosis in the testis. Rev. Reprod. 1999, 4, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Cunha, M.; Ferraz, L.; Silva, J.; Barros, A.; Sousa, M. Caspase-3 detection in human testicular spermatozoa from azoospermic and non-azoospermic patients. Int. J. Androl. 2011, 34, e407–e414. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Chiarini-Garcia, H.; Korsmeyer, S.J.; Knudson, C.M. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol. Reprod. 2002, 66, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Ehmcke, J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Semin. Cell Dev. Biol. 2014, 29, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K.; Bremner, W. Endocrine regulation of testicular function in men: Implications for contraceptive development. Mol. Cell. Endocrinol. 2001, 182, 175–179. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Wong, E.W.; Yan, H.H.; Mruk, D.D. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol. Cell. Endocrinol. 2010, 315, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Shirneshan, K.; Binder, S.; Bohm, D.; Wolf, S.; Sancken, U.; Meinhardt, A.; Schmid, M.; Engel, W.; Adham, I.M. Directed overexpression of insulin in leydig cells causes a progressive loss of germ cells. Mol. Cell. Endocrinol. 2008, 295, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.M.; Hikim, A.P.; Lue, Y.; Portugal, A.M.; Guo, T.B.; Hsu, S.Y.; Salameh, W.A.; Wang, C.; Hsueh, A.J.; Swerdloff, R.S. Impairment of spermatogenesis in transgenic mice with selective overexpression of bcl-2 in the somatic cells of the testis. J. Androl. 2001, 22, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.S.; Liu, Y.; Juan, H.; Yang, X. Overexpression of human spata17 protein induces germ cell apoptosis in transgenic male mice. Mol. Biol. Rep. 2013, 40, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, M.K.; Takada, S.; Kennedy, C.L.; Scott, G.; Harada, S.; Ray, M.K.; Dai, Q.; Wilhelm, D.; de Kretser, D.M.; Eddy, E.M.; et al. Sox8 is a critical regulator of adult sertoli cell function and male fertility. Dev. Biol. 2008, 316, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, Y.; Sun, F.; Saalbach, A.; Klein, C.; Miller, D.J.; Hess, R.; Nowak, R.A. Basigin null mutant male mice are sterile and exhibit impaired interactions between germ cells and sertoli cells. Dev. Biol. 2013, 380, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Zirkin, B.R.; Tenover, J.L. Aging and declining testosterone: Past, present, and hopes for the future. J. Androl. 2012, 33, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Coonce, M.M.; Rabideau, A.C.; McGee, S.; Smith, K.; Narayan, P. Impact of a constitutively active luteinizing hormone receptor on testicular gene expression and postnatal leydig cell development. Mol. Cell. Endocrinol. 2009, 298, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2015, 51, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Clark, B.J. Role of the steroidogenic acute regulatory protein (star) in steroidogenesis. Biochem. Pharmacol. 1996, 51, 197–205. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Aghazadeh, Y.; Fan, J.; Campioli, E.; Zirkin, B.; Midzak, A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 2015, 408, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kda): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Sokanovic, S.J.; Janjic, M.M.; Stojkov, N.J.; Baburski, A.Z.; Bjelic, M.M.; Andric, S.A.; Kostic, T.S. Age related changes of camp and mapk signaling in leydig cells of wistar rats. Exp. Gerontol. 2014, 58, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.S.; Lambright, C.R.; Furr, J.R.; Howdeshell, K.L.; Earl Gray, L., Jr. The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures. Toxicol. Lett. 2009, 186, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Saradha, B.; Vaithinathan, S.; Mathur, P.P. Single exposure to low dose of lindane causes transient decrease in testicular steroidogenesis in adult male wistar rats. Toxicology 2008, 244, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.S.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 2009, 299, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kopera, I.A.; Bilinska, B.; Cheng, C.Y.; Mruk, D.D. Sertoli-germ cell junctions in the testis: A review of recent data. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Lunenfeld, E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J. Androl. 2004, 6, 259–268. [Google Scholar] [PubMed]
- Archambeault, D.R.; Yao, H.H. Activin a, a product of fetal leydig cells, is a unique paracrine regulator of sertoli cell proliferation and fetal testis cord expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 10526–10531. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.F.; Nascimento, A.R.; Pisolato, R.; Pimenta, M.T.; Lazari, M.F.; Porto, C.S. Receptors and signaling pathways involved in proliferation and differentiation of sertoli cells. Spermatogenesis 2014, 4, e28138. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.; Skinner, M.K. E-box and cyclic adenosine monophosphate response elements are both required for follicle-stimulating hormone-induced transferrin promoter activation in sertoli cells. Endocrinology 1999, 140, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Valles, A.S.; Aveldano, M.I.; Furland, N.E. Altered lipid homeostasis in sertoli cells stressed by mild hyperthermia. PLoS ONE 2014, 9, e91127. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Ishikawa, T.; Kondo, Y.; Fujisawa, M. Cisplatin regulates sertoli cell expression of transferrin and interleukins. Mol. Cell. Endocrinol. 2008, 283, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A.; de Gendt, K.; Atanassova, N.; Walker, M.; Sharpe, R.M.; Saunders, P.T.; Denolet, E.; Verhoeven, G. The role of androgens in sertoli cell proliferation and functional maturation: Studies in mice with total or sertoli cell-selective ablation of the androgen receptor. Endocrinology 2005, 146, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Lei, Z.M. The past, present and future of nongonadal lh/hcg actions in reproductive biology and medicine. Mol. Cell. Endocrinol. 2007, 269, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Neves, E.S.; Chiarini-Garcia, H.; Franca, L.R. Comparative testis morphometry and seminiferous epithelium cycle length in donkeys and mules. Biol. Reprod. 2002, 67, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Jeon, B.; Jang, W.; Moon, C.; Kim, S. Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia 2011, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Su, X.; Hao, J.; Chen, M.; Liu, W.; Liao, X.; Li, G. Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice. Int. J. Mol. Sci. 2016, 17, 96. https://doi.org/10.3390/ijms17010096
Liu Y, Su X, Hao J, Chen M, Liu W, Liao X, Li G. Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice. International Journal of Molecular Sciences. 2016; 17(1):96. https://doi.org/10.3390/ijms17010096
Chicago/Turabian StyleLiu, Yaping, Xingyu Su, Jie Hao, Maoxin Chen, Weijia Liu, Xiaogang Liao, and Gang Li. 2016. "Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice" International Journal of Molecular Sciences 17, no. 1: 96. https://doi.org/10.3390/ijms17010096
APA StyleLiu, Y., Su, X., Hao, J., Chen, M., Liu, W., Liao, X., & Li, G. (2016). Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice. International Journal of Molecular Sciences, 17(1), 96. https://doi.org/10.3390/ijms17010096






