Cell Density-Dependent Upregulation of PDCD4 in Keratinocytes and Its Implications for Epidermal Homeostasis and Repair
Abstract
:1. Introduction
2. Results and Discussion
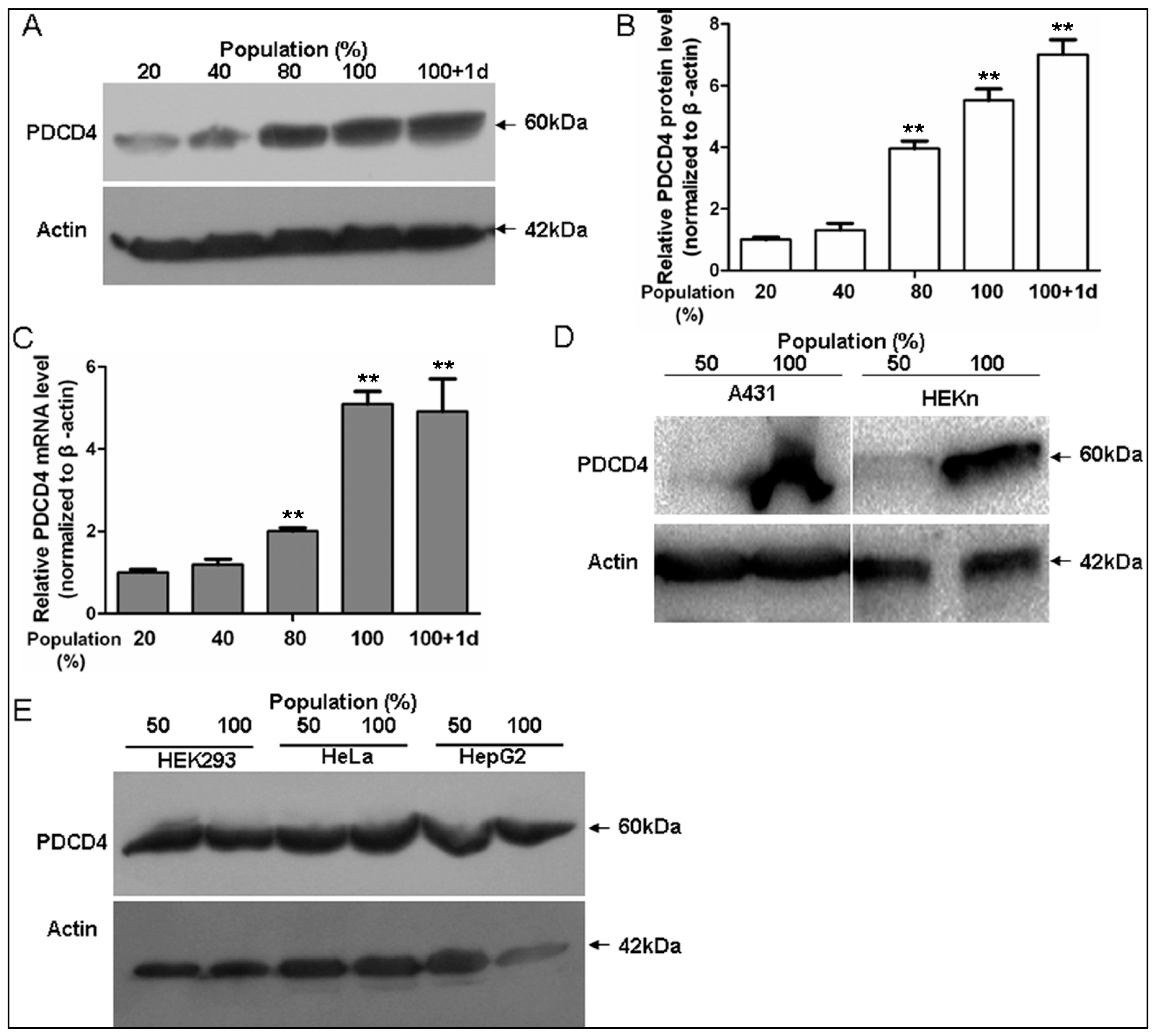
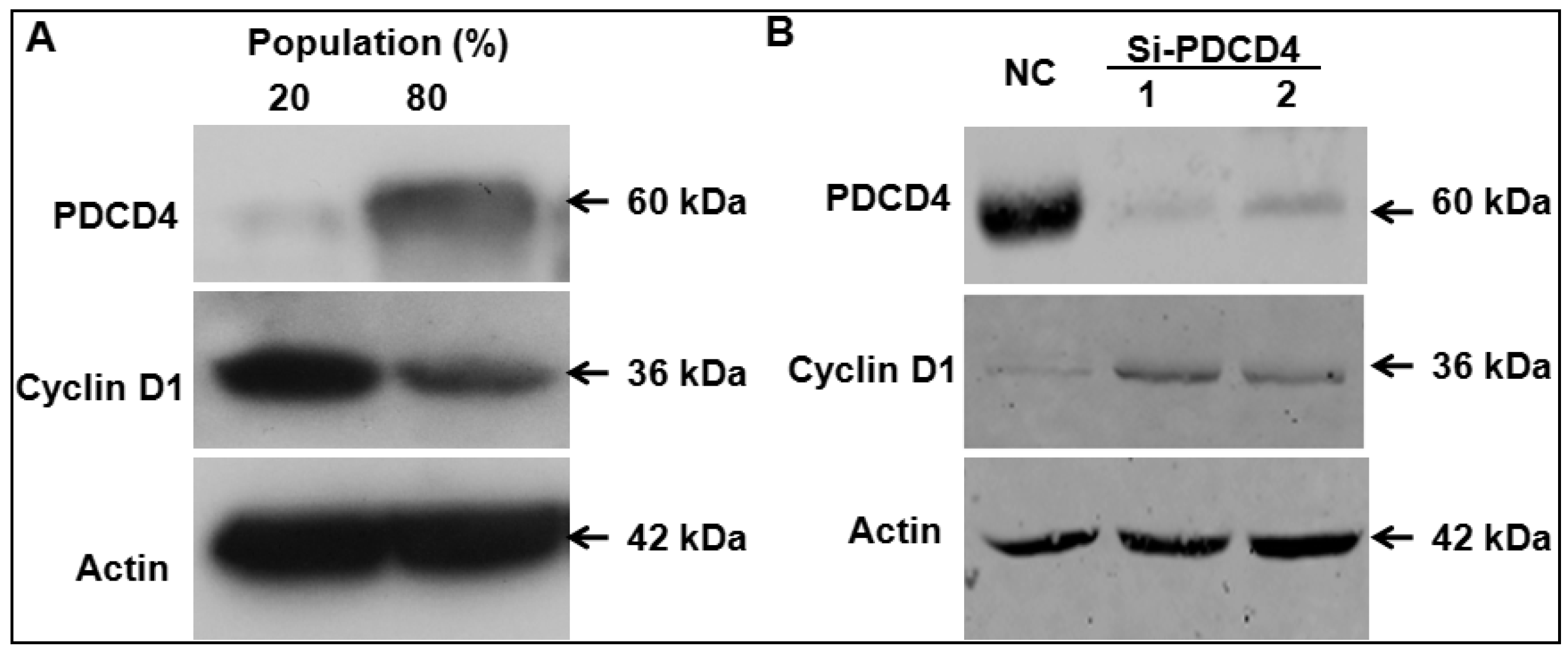
2.1. Programmed Cell Death 4 (PDCD4) Expression Depends on Keratinocytes Density
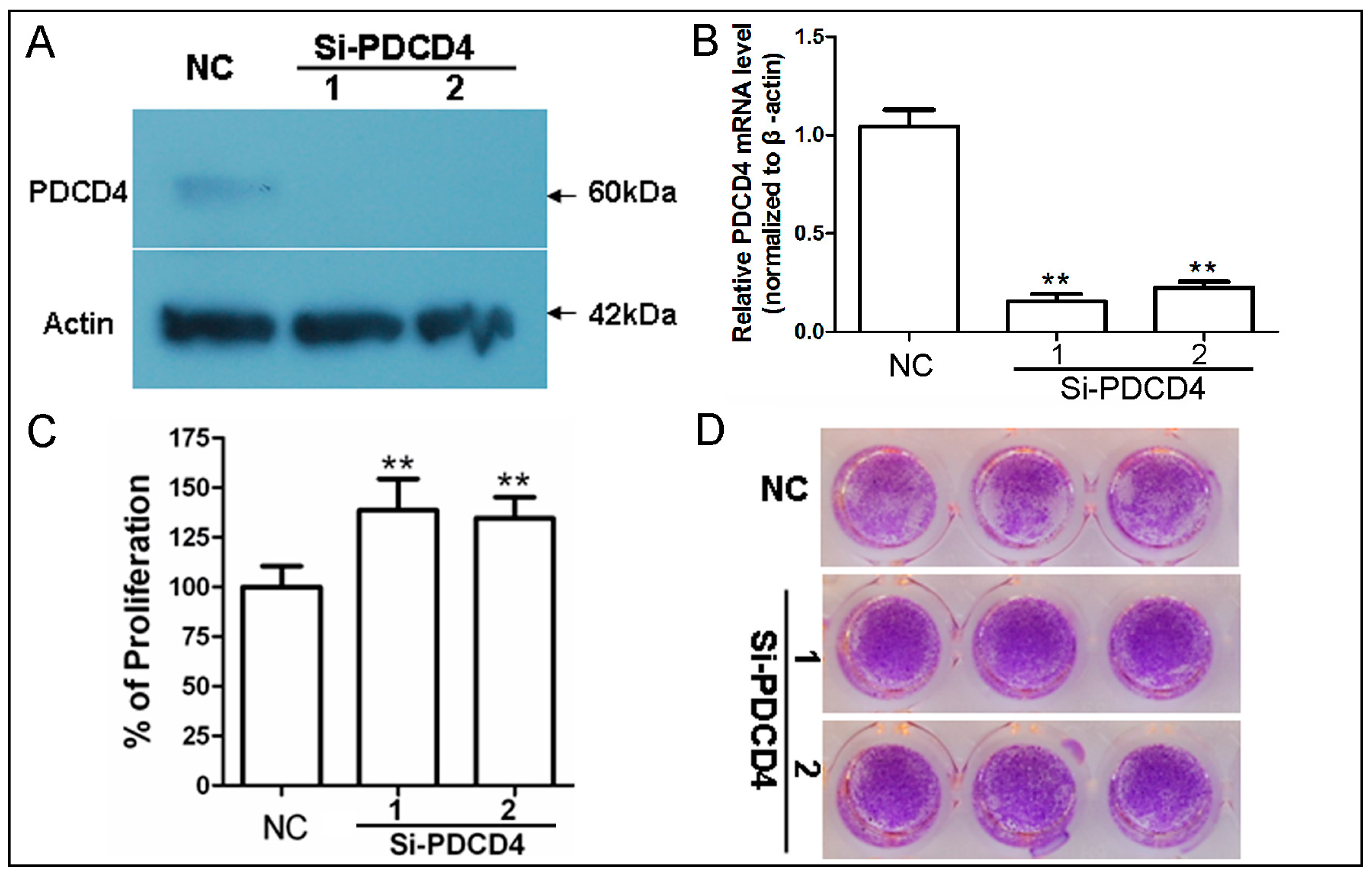
2.2. Knockdown of PDCD4 Promotes Keratinocytes Proliferation
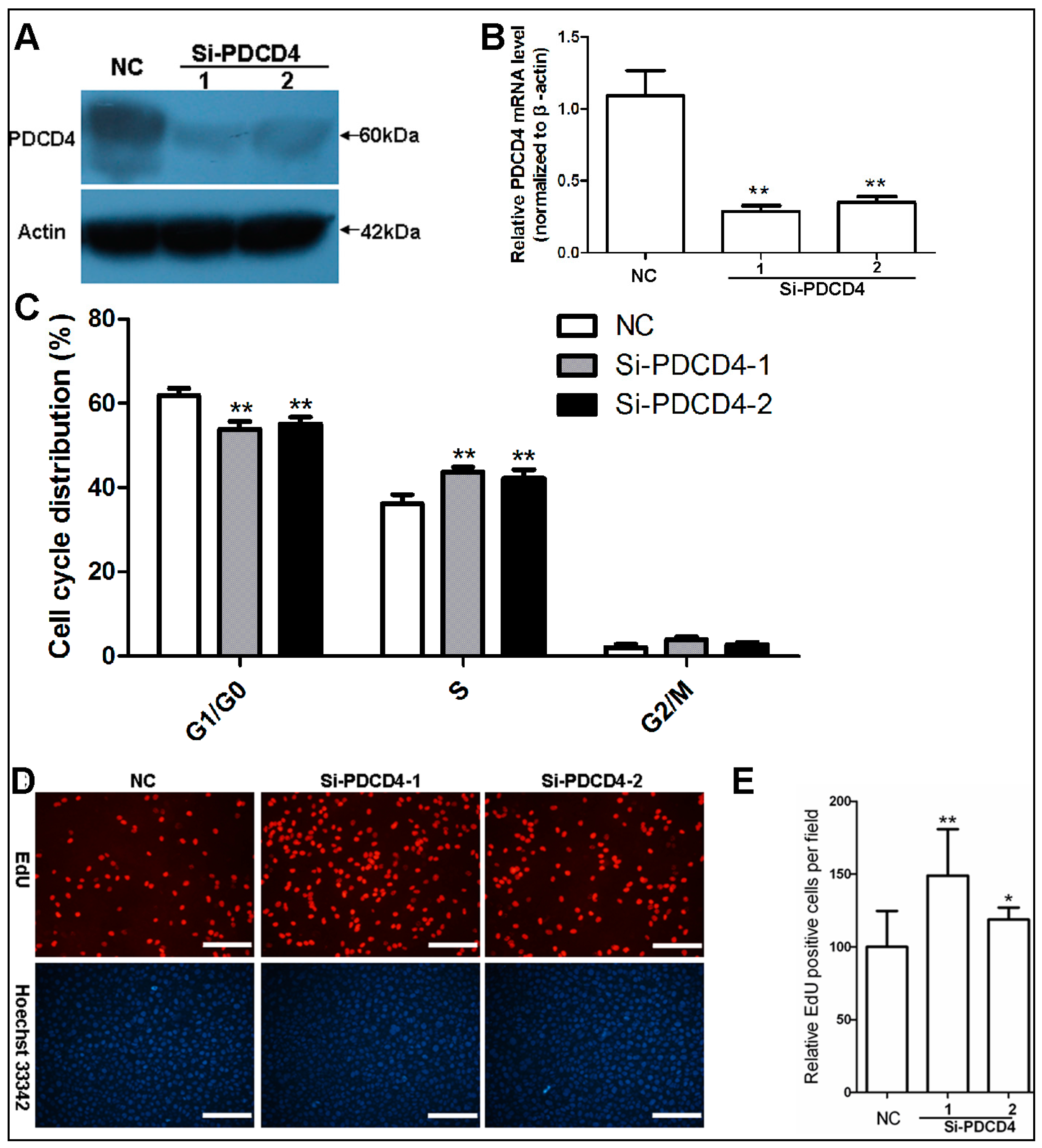
2.3. Knockdown of PDCD4 Impaires Contact Inhibition
2.4. Knockdown of PDCD4 Induces Cyclin D1 Expression
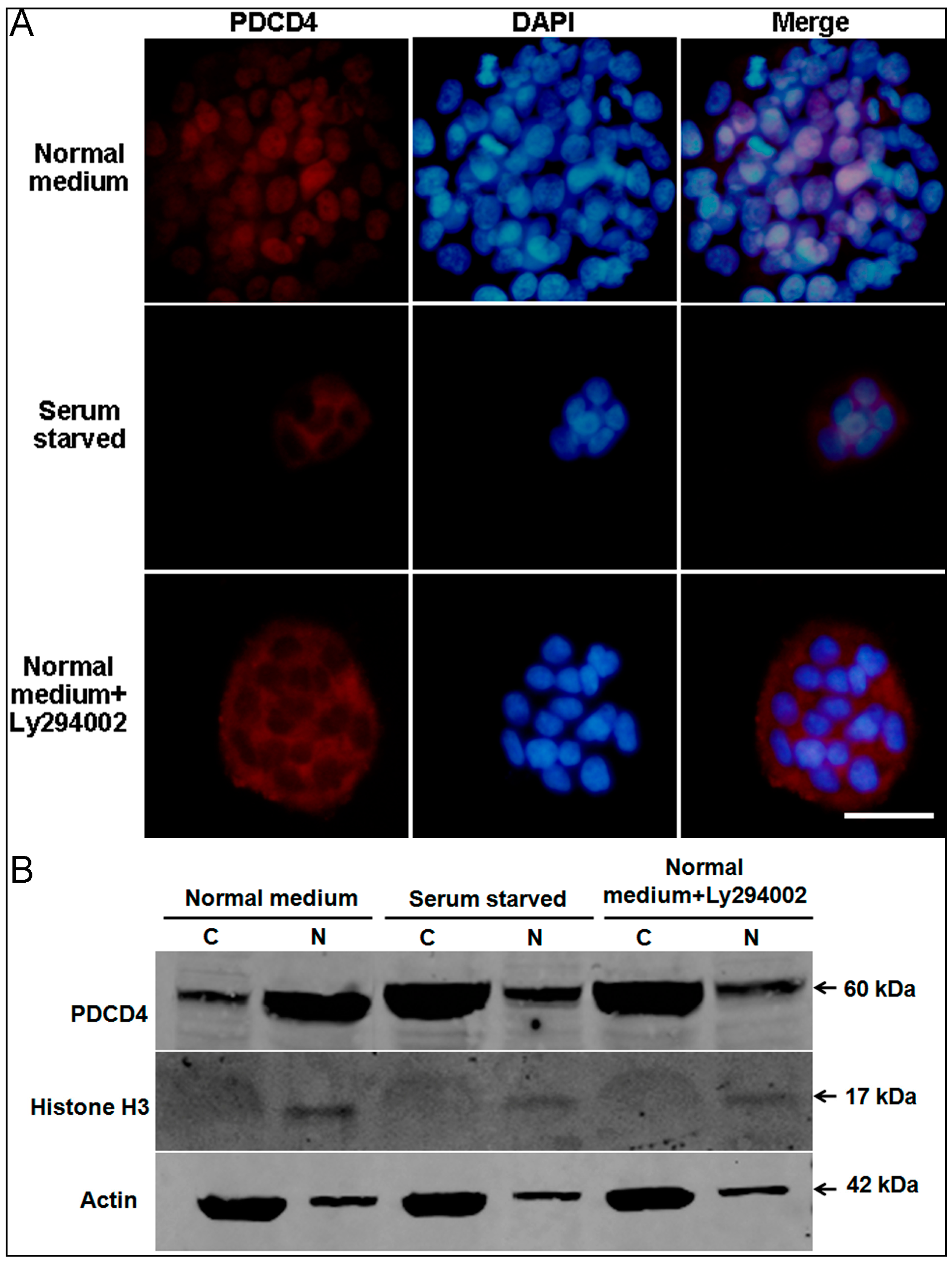
2.5. Intracellular Translocation of PDCD4
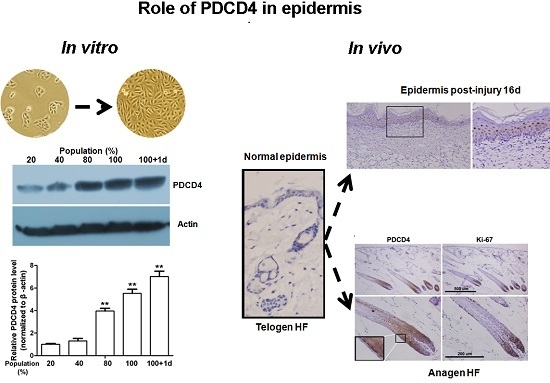
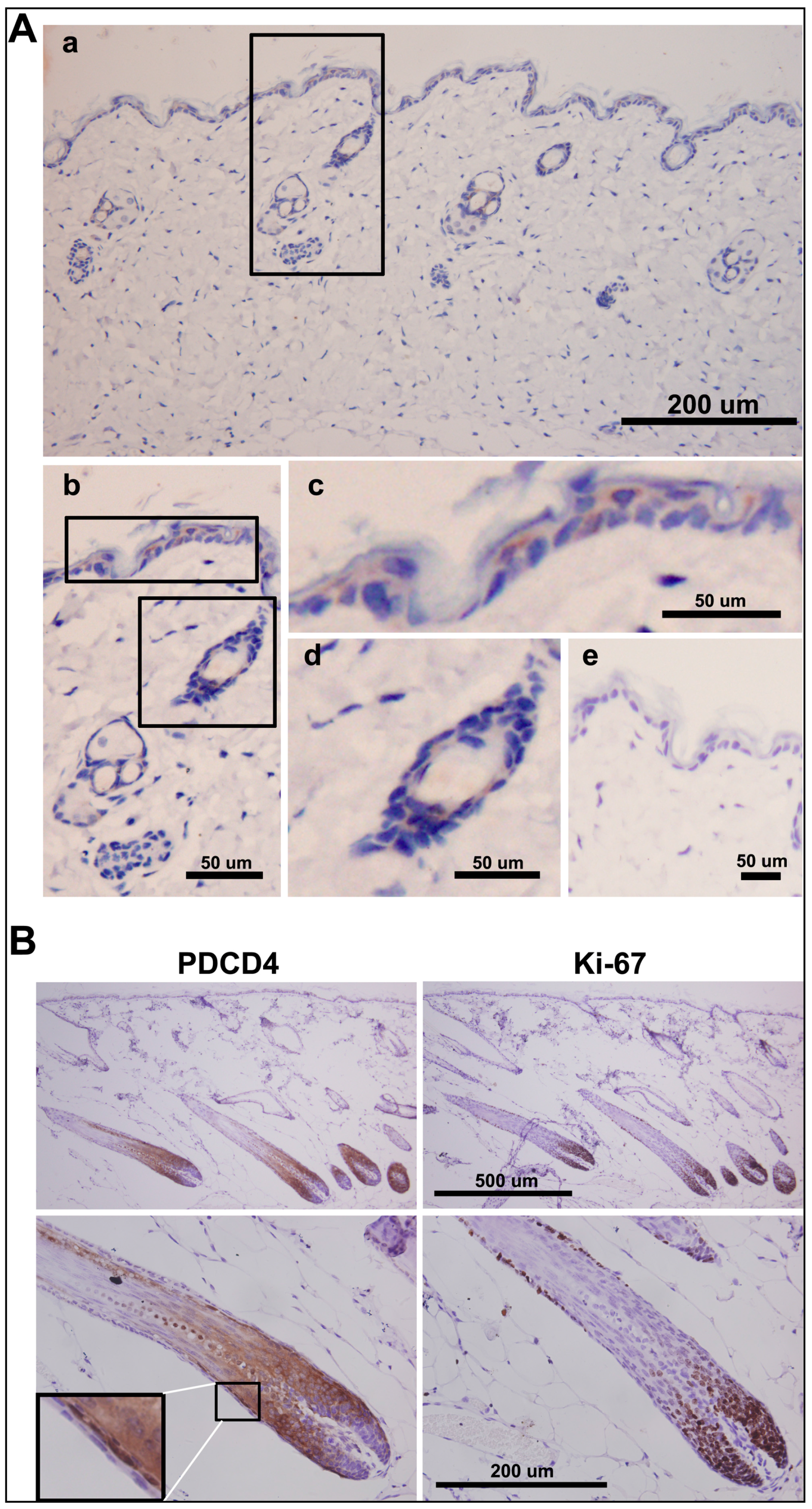
2.6. PDCD4 Is Expressed in the Epidermis and Associated with Epidermal Homeostasis
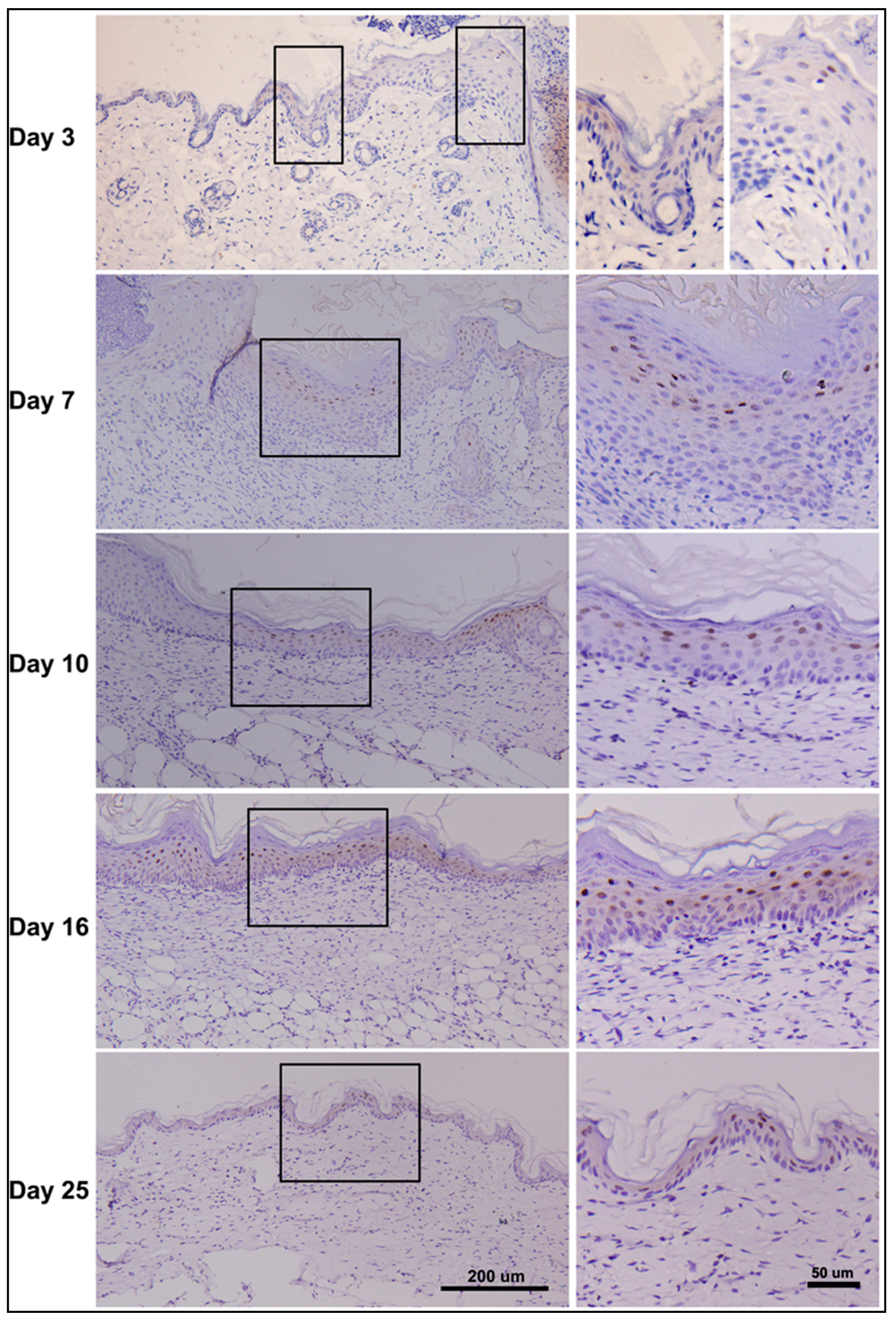
2.7. Dynamic Changes in PDCD4 Expression during Epidermal Wound Healing
2.8. Discussion
3. Materials and Methods
3.1. Cell Culture
3.2. Animals and Wound Model of Skin
3.3. Hair Cycle Induction
3.4. Western Blot Analysis
3.5. Real-Time RT-PCR
3.6. RNA Transfection
3.7. Cell Proliferation Assay
3.8. EdU Incorporation Assay
3.9. Cell Cycle Analysis
3.10. Immunofluorescence
3.11. Immnohistochemistry
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shibahara, K.; Asano, M.; Ishida, Y.; Aoki, T.; Koike, T.; Honjo, T. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene 1995, 166, 297–301. [Google Scholar] [CrossRef]
- Cmarik, J.L.; Min, H.; Hegamyer, G.; Zhan, S.; Kulesz-Martin, M.; Yoshinaga, H.; Matsuhashi, S.; Colburn, N.H. Differentially expressed protein PDCD4 inhibits tumor promoter-induced neoplastic transformation. Proc. Natl. Acad. Sci. USA 1999, 96, 14037–14042. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Jansen, A.P.; Baker, A.R.; Hegamyer, G.; Colburn, N.H. Translation inhibitor PDCD4 is targeted for degradation during tumor promotion. Cancer Res. 2008, 68, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.P.; Camalier, C.E.; Colburn, N.H. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005, 65, 6034–6041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Knosel, T.; Kristiansen, G.; Pietas, A.; Garber, M.E.; Matsuhashi, S.; Ozaki, I.; Petersen, I. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J. Pathol. 2003, 200, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Afonja, O.; Juste, D.; Das, S.; Matsuhashi, S.; Samuels, H.H. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene 2004, 23, 8135–8145. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Matthews, C.P.; Clair, T.; Wang, Q.; Baker, A.R.; Li, C.C.; Tan, T.H.; Colburn, N.H. Tumorigenesis suppressor PDCD4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol. Cell. Biol. 2006, 26, 1297–1306. [Google Scholar] [PubMed]
- Mudduluru, G.; Medved, F.; Grobholz, R.; Jost, C.; Gruber, A.; Leupold, J.H.; Post, S.; Jansen, A.; Colburn, N.H.; Allgayer, H. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 2007, 110, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Asangani, I.A.; Rasheed, S.A.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor PDCD4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008, 27, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, M.; Stribinskis, V.; Klinge, C.M.; Ramos, K.S.; Colburn, N.H.; Li, Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 2008, 27, 4373–9437. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, L.; Shi, C.; Sun, H.; Wang, J.; Li, R.; Zou, Z.; Ran, X.; Su, Y. TGF-β-induced miR-21 negatively regulates the antiproliferative activity but has no effect on EMT of TGF-β in HaCaT cells. Int. J. Biochem. Cell Biol. 2012, 44, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Dorrello, N.V.; Peschiaroli, A.; Guardavaccaro, D.; Pagano, M. S6K1- and bTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 2006, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Sawicka, K.; Siebrasse, J.P.; Brehmer-Fastnacht, A.; Peters, R.; Klempnauer, K.H. The transformation suppressor protein PDCD4 shuttles between nucleus and cytoplasm and binds RNA. Oncogene 2003, 22, 4905–4910. [Google Scholar] [CrossRef] [PubMed]
- Fassan, M.; Pizzi, M.; Giacomelli, L.; Mescoli, C.; Ludwig, K.; Pucciarelli, S.; Rugge, M. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch. 2011, 458, 413–419. [Google Scholar] [CrossRef]
- Palamarchuk, A.; Efanov, A.; Maximov, V.; Aqeilan, R.I.; Croce, C.M.; Pekarsky, Y. Akt phosphorylates and regulates PDCD4 tumor suppressor protein. Cancer Res. 2005, 65, 11282–11286. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Knies, J.L.; Stark, C.; Colburn, N.H. PDCD4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene 2003, 22, 3712–3720. [Google Scholar] [CrossRef] [PubMed]
- Bitomsky, N.; Wethkamp, N.; Marikkannu, R.; Klempnauer, K.H. siRNA-mediated knockdown of PDCD4 expression causes upregulation of p21(WAF1/Cip1) expression. Oncogene 2008, 27, 4820–4829. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Jansen, A.P.; Komar, A.A.; Zheng, X.; Merrick, W.C.; Costes, S.; Lockett, S.J.; Sonenberg, N.; Colburn, N.H. The transformation suppressor PDCD4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol. Cell. Biol. 2003, 23, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Cho, M.H.; Zakowicz, H.; Hegamyer, G.; Sonenberg, N.; Colburn, N.H. A novel function of the MA-3 domains in transformation and translation suppressor PDCD4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol. Cell. Biol. 2004, 24, 3894–3906. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Wang, Q.; Yang, H.S. AKT Activation by PDCD4 knockdown up-regulates cyclin D1 expression and promotes cell proliferation. Genes Cancer 2011, 2, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Akar, U.; Steiner, M.; Zorrilla-Calancha, I.; Tirado-Gomez, M.; Colburn, N.; Danilenko, M.; Kornblau, S.; Berestein, G.L. Programmed cell death-4 tumor suppressor protein contributes to retinoic acid-induced terminal granulocytic differentiation of human myeloid leukemia cells. Mol. Cancer Res. 2007, 5, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ozaki, I.; Mizuta, T.; Hamajima, H.; Matsuhashi, S. Involvement of programmed cell death 4 in transforming growth factor-β1-induced apoptosis in human hepatocellular carcinoma. Oncogene 2006, 25, 6101–6112. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, A.N.; Baker, A.R.; Hegamyer, G.; Kirschmann, D.A.; Colburn, N.H. PDCD4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene 2010, 29, 3921–3932. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, S.; Narisawa, Y.; Ozaki, I.; Mizuta, T. Expression patterns of programmed cell death 4 protein in normal human skin and some representative skin lesions. Exp. Dermatol. 2007, 16, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Bae, J.M.; Chun, Y.S.; Chung, J.H.; Jeon, Y.K.; Kim, I.S.; Kim, M.S.; Park, J.W. HIF-1α controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1). Biochim. Biophys. Acta 2008, 1783, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Swat, A.; Dolado, I.; Rojas, J.M.; Nebreda, A.R. Cell density-dependent inhibition of epidermal growth factor receptor signaling by p38α mitogen-activated protein kinase via Sprouty2 downregulation. Mol. Cell. Biol. 2009, 29, 3332–3343. [Google Scholar] [CrossRef] [PubMed]
- Belso, N.; Szell, M.; Pivarcsi, A.; Kis, K.; Kormos, B.; Kenderessy, A.S.; Dobozy, A.; Kemeny, L.; Bata-Csorgo, Z. Differential expression of D-type cyclins in HaCaT keratinocytes and in psoriasis. J. Investig. Dermatol. 2008, 128, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Lankat-Buttgereit, B.; Goke, R. The tumour suppressor PDCD4: Recent advances in the elucidation of function and regulation. Biol. Cell 2009, 101, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Liu, S.S.; Chan, K.K.; Ngan, H.Y. Tumour suppressive function and modulation of programmed cell death 4 (PDCD4) in ovarian cancer. PLoS ONE 2012, 7, e30311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.H.; Simons, B.D.; Watt, F.M. Sic transit gloria: Farewell to the epidermal transit amplifying cell? Cell Stem Cell 2007, 1, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Leupold, J.H.; Asangani, I.A.; Mudduluru, G.; Allgayer, H. Promoter cloning and characterization of the human programmed cell death protein 4 (PDCD4) gene: Evidence for ZBP-89 and Sp-binding motifs as essential PDCD4 regulators. Biosci. Rep. 2012, 32, 2812–2897. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Y.; Yang, J.; Krall, T.J.; Huo, Y.; Zhang, C. An essential role of PDCD4 in vascular smooth muscle cell apoptosis and proliferation: Implications for vascular disease. Am. J. Physiol. Cell Physiol. 2010, 298, C1481–C1488. [Google Scholar] [CrossRef] [PubMed]
- Pankow, S.; Bamberger, C.; Klippel, A.; Werner, S. Regulation of epidermal homeostasis and repair by phosphoinositide 3-kinase. J. Cell Sci. 2006, 119 Pt 19, 4033–4046. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Fowlis, D.J.; Cousins, F.M.; Duffie, E.; Bryson, S.; Balmain, A.; Akhurst, R.J. Concerted action of TGF-β1 and its type II receptor in control of epidermal homeostasis in transgenic mice. Genes Dev. 1995, 9, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [PubMed]
- Schafer, M.; Werner, S. Cancer as an overhealing wound: An old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 2008, 9, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [PubMed]
- Pedersen, T.X.; Leethanakul, C.; Patel, V.; Mitola, D.; Lund, L.R.; Dano, K.; Johnsen, M.; Gutkind, J.S.; Bugge, T.H. Laser capture microdissection-based in vivo genomic profiling of wound keratinocytes identifies similarities and differences to squamous cell carcinoma. Oncogene 2003, 22, 3964–3976. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Feng, Y.; Sun, H.; Zhang, L.; Hao, L.; Shi, C.; Wang, J.; Li, R.; Ran, X.; Su, Y.; et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am. J. Pathol. 2012, 181, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Reiter, J.F. Wounding mobilizes hair follicle stem cells to form tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 4093–4098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Z.; Yang, H.S. Downregulation of tumor suppressor PDCD4 promotes invasion and activates both β-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene 2008, 27, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Stenn, K.S.; Link, R.E. Telogen skin contains an inhibitor of hair growth. Br. J. Dermatol. 1990, 122, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmuller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Long, S.; Zhao, N.; Wang, Y.; Sun, H.; Zou, Z.; Wang, J.; Ran, X.; Su, Y. Cell Density-Dependent Upregulation of PDCD4 in Keratinocytes and Its Implications for Epidermal Homeostasis and Repair. Int. J. Mol. Sci. 2016, 17, 8. https://doi.org/10.3390/ijms17010008
Wang T, Long S, Zhao N, Wang Y, Sun H, Zou Z, Wang J, Ran X, Su Y. Cell Density-Dependent Upregulation of PDCD4 in Keratinocytes and Its Implications for Epidermal Homeostasis and Repair. International Journal of Molecular Sciences. 2016; 17(1):8. https://doi.org/10.3390/ijms17010008
Chicago/Turabian StyleWang, Tao, Shuang Long, Na Zhao, Yu Wang, Huiqin Sun, Zhongmin Zou, Junping Wang, Xinze Ran, and Yongping Su. 2016. "Cell Density-Dependent Upregulation of PDCD4 in Keratinocytes and Its Implications for Epidermal Homeostasis and Repair" International Journal of Molecular Sciences 17, no. 1: 8. https://doi.org/10.3390/ijms17010008