Revealing the Effects of Missense Mutations Causing Snyder-Robinson Syndrome on the Stability and Dimerization of Spermine Synthase
Abstract
:1. Introduction
2. Results
2.1. Effect of Missense Mutation on Monomer Stability (in Silico Modeling)
| Mutations | PoPMuSiC | DUET | FOLDX | I-Mutant 2.0 | SDM | SD | AV |
|---|---|---|---|---|---|---|---|
| M35R | −0.93 | −0.47 | −0.42 | −1.81 | −2.93 | 1.06 | −1.31 |
| G56S | −1.99 | −0.52 | −3.50 | −2.16 | −3.51 | 1.24 | −2.34 |
| F58L | −1.77 | −0.95 | 2.12 | −2.72 | −0.18 | 1.84 | −0.7 |
| G67E | −1.99 | −1.26 | −1.36 | −0.18 | −1.34 | 0.65 | −1.22 |
| P112L | −0.87 | −0.06 | −0.43 | −0.83 | −1.04 | 0.40 | −0.65 |
2.2. Effect of Missense Mutation on Monomer Stability (in Vitro Experiments)
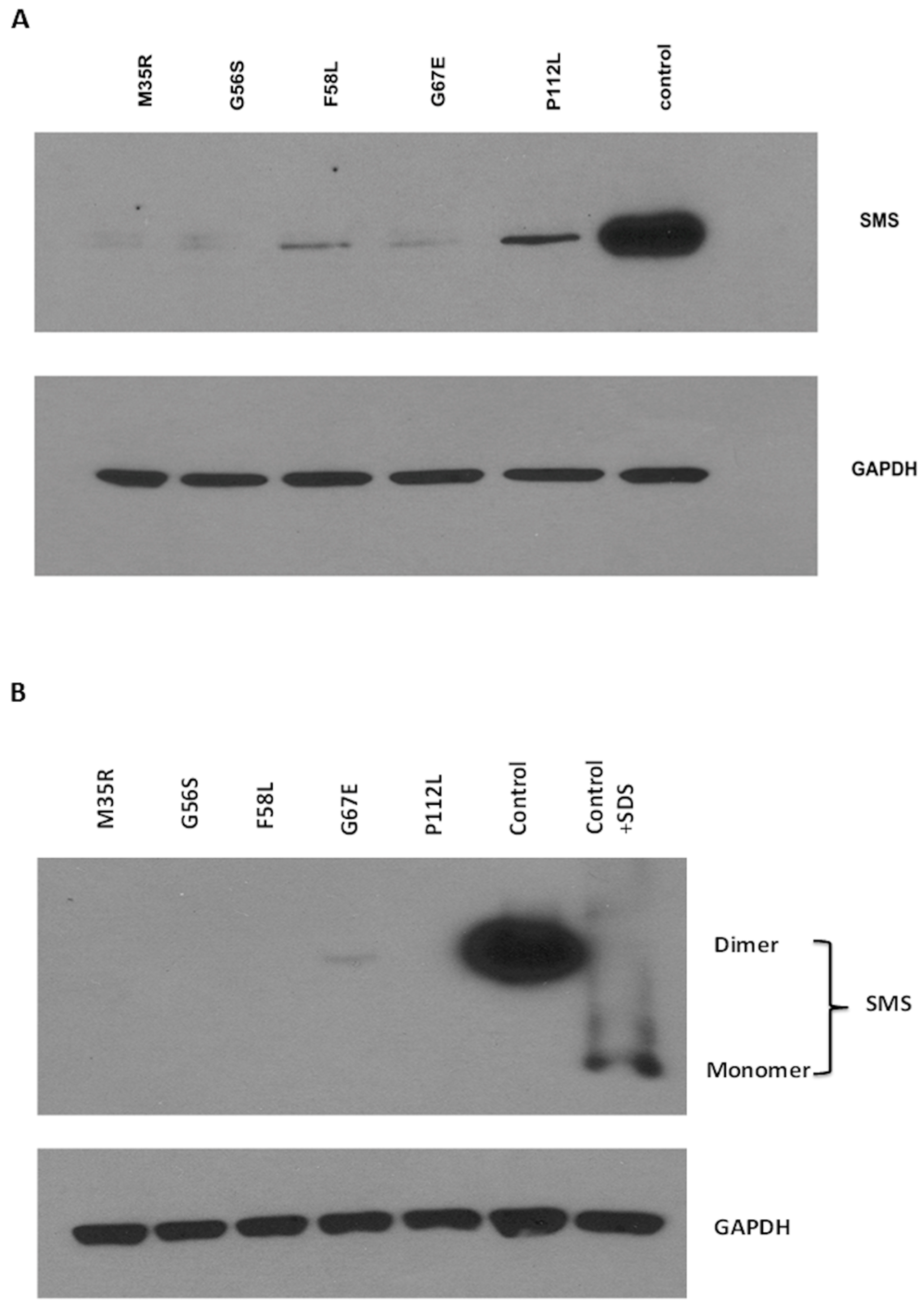
| Mutation | SMS/GAPDH Ratio | % Ctrl |
|---|---|---|
| M35R | 0.04 | 1.5 |
| G56S | 0.07 | 2.6 |
| F58L | 0.18 | 6.6 |
| G67E | 0.13 | 4.8 |
| P112L | 0.55 | 20.3 |
| Ctrl | 2.71 | 100 |
2.3. Effect of Missense Mutation on Dimer Affinity
| Mutations | BeAtMuSiC (AB) | BeAtMuSiC (CD) | Foldx (AB) | Foldx (CD) | SAAMBE (AB) | SAAMBE (CD) | SD (AB) | MEAN (AB) | SD (CD) | MEAN (CD) |
|---|---|---|---|---|---|---|---|---|---|---|
| M35R | 0.05 | 0.24 | 0.17 | −0.96 | −0.27 | −0.19 | 0.22 | 0.11 | 0.60 | −0.30 |
| G56S | −1.84 | −1.34 | −8.64 | −11.87 | 1.58 | −4.12 | 5.20 | −5.24 | 5.45 | −5.78 |
| F58L | −2.74 | −2.28 | −0.1 | −1.26 | 2.20 | 7.46 | 2.47 | −1.42 | 5.35 | 1.31 |
| G67E | −0.78 | −0.83 | 0.372 | 0.16 | −1.86 | 9.91 | 1.12 | −0.76 | 5.93 | 3.08 |
| P112L | −0.11 | −0.17 | −4.59 | −3.32 | −0.38 | 3.39 | 2.51 | −2.35 | 3.35 | −0.03 |
2.4. Result of Multiple Sequence Alignment Analysis(MSA)

3. Discussion

4. Materials and Methods
4.1. Protein Structure
4.2. Protein Binding and Folding Free Energy Prediction
4.3. Multiple Sequence Alignment
4.4. Creation of Lymphoblastoid Cell Lines
4.5. Cell Culture
4.6. Lysate Preparation
4.7. Western Blot Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. CMLS 2001, 58, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Michael, A.J. Spermine synthase. Cell. Mol. Life Sci. CMLS 2010, 67, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Matsuyama, T.; Hanzawa, Y.; Akiyama, T.; Tamaoki, M.; Saji, H.; Shirano, Y.; Kato, T.; Hayashi, H.; Shibata, D.; et al. Spermidine synthase genes are essential for survival of arabidopsis. Plant Physiol. 2004, 135, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Peron, A.; Spaccini, L.; Norris, J.; Bova, S.M.; Selicorni, A.; Weber, G.; Wood, T.; Schwartz, C.E.; Mastrangelo, M. Snyder-robinson syndrome: A novel nonsense mutation in spermine synthase and expansion of the phenotype. Am. J. Med. Genet. Part A 2013, 161, 2316–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Norris, J.; Kalscheuer, V.; Wood, T.; Wang, L.; Schwartz, C.; Alexov, E.; van Esch, H. A y328c missense mutation in spermine synthase causes a mild form of snyder-robinson syndrome. Hum. Mol. Genet. 2013, 22, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Wang, X.; Stevenson, R.E.; Pegg, A.E. Spermine synthase deficiency resulting in x-linked intellectual disability (snyder-robinson syndrome). Methods Mol. Biol. 2011, 720, 437–445. [Google Scholar] [PubMed]
- Zhang, Z.; Norris, J.; Schwartz, C.; Alexov, E. In silico and in vitro investigations of the mutability of disease-causing missense mutation sites in spermine synthase. PLoS ONE 2011, 6, e20373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Martiny, V.; Lagorce, D.; Ikeguchi, Y.; Alexov, E.; Miteva, M.A. Rational design of small-molecule stabilizers of spermine synthase dimer by virtual screening and free energy-based approach. PLoS ONE 2014, 9, e110884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teng, S.; Wang, L.; Schwartz, C.E.; Alexov, E. Computational analysis of missense mutations causing snyder-robinson syndrome. Hum. Mutat. 2010, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Min, J.; Zeng, H.; McCloskey, D.E.; Ikeguchi, Y.; Loppnau, P.; Michael, A.J.; Pegg, A.E.; Plotnikov, A.N. Crystal structure of human spermine synthase: Implications of substrate binding and catalytic mechanism. J. Biol. Chem. 2008, 283, 16135–16146. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Madej, T.; Panchenko, A.; Alexov, E. Modeling effects of human single nucleotide polymorphisms on protein-protein interactions. Biophys. J. 2009, 96, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Barenboim, M.; Masso, M.; Vaisman, I.I.; Jamison, D.C. Statistical geometry based prediction of nonsynonymous SNP functional effects using random forest and neuro-fuzzy classifiers. Proteins 2008, 71, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- De Alencastro, G.; McCloskey, D.E.; Kliemann, S.E.; Maranduba, C.M.; Pegg, A.E.; Wang, X.; Bertola, D.R.; Schwartz, C.E.; Passos-Bueno, M.R.; Sertie, A.L. New SMS mutation leads to a striking reduction in spermine synthase protein function and a severe form of snyder-robinson x-linked recessive mental retardation syndrome. J. Med. Genet. 2008, 45, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.R.; Riesch, E.; Scheurenbrand, T.; Schubach, M.; Wilhelm, C.; Steiner, I.; Hansen, J.; Courage, C.; Gallati, S.; Burki, S.; et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia 2012, 53, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. Vmd: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Dehouck, Y.; Kwasigroch, J.M.; Rooman, M.; Gilis, D. Beatmusic: Prediction of changes in protein-protein binding affinity on mutations. Nucleic Acids Res. 2013, 41, W333–W339. [Google Scholar] [CrossRef] [PubMed]
- Giollo, M.; Martin, A.J.; Walsh, I.; Ferrari, C.; Tosatto, S.C. Neemo: A method using residue interaction networks to improve prediction of protein stability upon mutation. BMC Genom. 2014, 15, S7. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, Y.; Grosfils, A.; Folch, B.; Gilis, D.; Bogaerts, P.; Rooman, M. Fast and accurate predictions of protein stability changes upon mutations using statistical potentials and neural networks: Popmusic-2.0. Bioinformatics 2009, 25, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, E.; Fariselli, P. Casadio R I-mutant2.0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005, 33, W306–W310. [Google Scholar] [CrossRef] [PubMed]
- Worth, C.L.; Preissner, R.; Blundell, T.L. SDM—A server for predicting effects of mutations on protein stability and malfunction. Nucleic Acids Res. 2011, 39, W215–W222. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Ascher, D.B.; Blundell, T.L. Duet: A server for predicting effects of mutations on protein stability using an integrated computational approach. Nucleic Acids Res. 2014, 42, W314–W319. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, V.; Gromiha, M.M.; Schomburg, D. Cupsat: Prediction of protein stability upon point mutations. Nucleic Acids Res. 2006, 34, W239–W242. [Google Scholar] [CrossRef] [PubMed]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The foldx web server: An online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef] [PubMed]
- FoldX 3.0–Force Field for Energy Calculations and Protein Design. Available online: http://www.mybiosoftware.com/foldx-3-0-beta3-force-field-energy-calculations-protein-design.html (accessed on 1 November 2015).
- Petukh, M.; Li, M.; Alexov, E. Predicting binding free energy change caused by point mutations with knowledge-modified MM/PBSA method. PLoS Comput. Biol. 2015, 11, e1004276. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, J.S.; Agarwala, R. Cobalt: Constraint-based alignment tool for multiple protein sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. Uniprot: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Norris, J.; Schwartz, C.; Alexov, E. Revealing the Effects of Missense Mutations Causing Snyder-Robinson Syndrome on the Stability and Dimerization of Spermine Synthase. Int. J. Mol. Sci. 2016, 17, 77. https://doi.org/10.3390/ijms17010077
Peng Y, Norris J, Schwartz C, Alexov E. Revealing the Effects of Missense Mutations Causing Snyder-Robinson Syndrome on the Stability and Dimerization of Spermine Synthase. International Journal of Molecular Sciences. 2016; 17(1):77. https://doi.org/10.3390/ijms17010077
Chicago/Turabian StylePeng, Yunhui, Joy Norris, Charles Schwartz, and Emil Alexov. 2016. "Revealing the Effects of Missense Mutations Causing Snyder-Robinson Syndrome on the Stability and Dimerization of Spermine Synthase" International Journal of Molecular Sciences 17, no. 1: 77. https://doi.org/10.3390/ijms17010077







