Does Variation of the Inter-Domain Linker Sequence Modulate the Metal Binding Behaviour of Helix pomatia Cd-Metallothionein?
Abstract
:1. Introduction
2. Results and Discussion
2.1. The HpCdMcMT and HpCdPlMT Recombinant Peptides


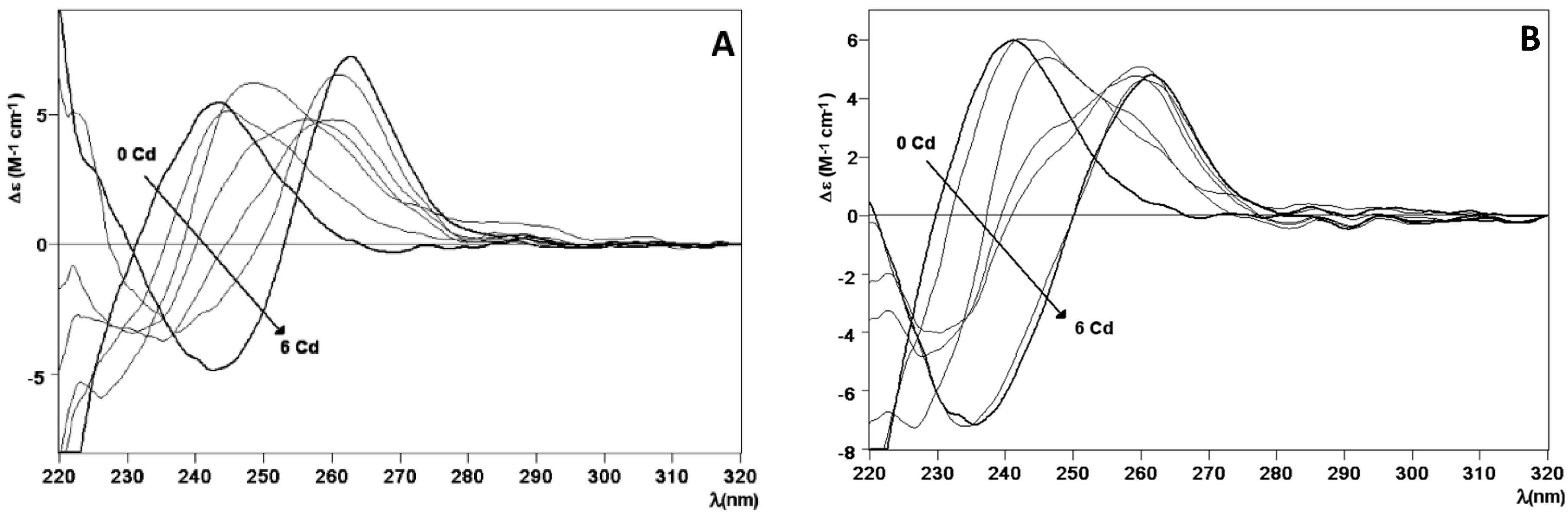
2.2. Zn and Cd Binding Abilities of HpCdMcMT and HpCdPlMT
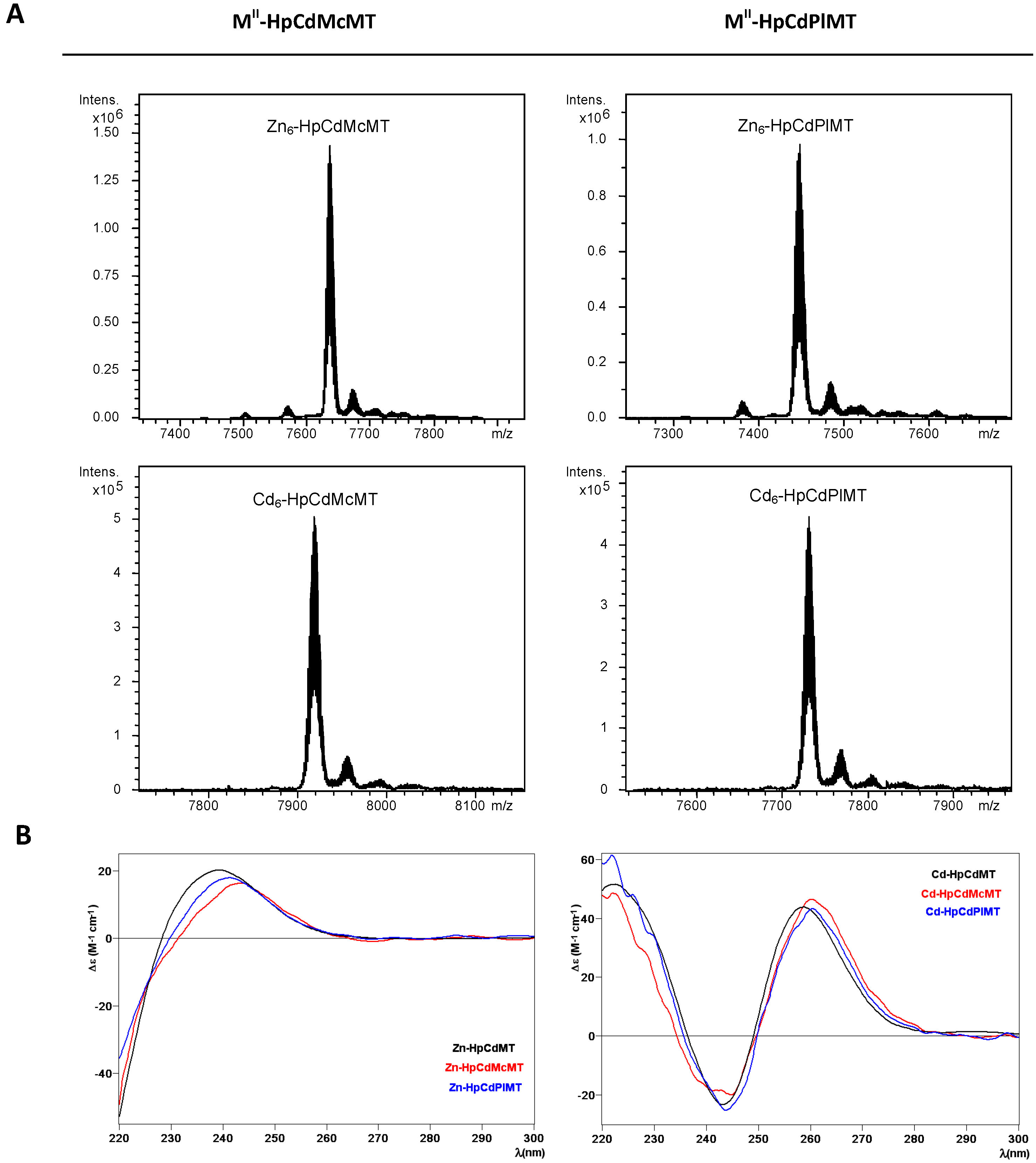
| MT | ICP-AES a | Neutral ESI-MS b | Experimental MM c | Calculated MM d |
|---|---|---|---|---|
| HpCdMT [11] | 5.8 | Zn6-MT | 7005 | 7005.6 |
| HpCdMcMT | 5.9 | Zn6-MT | 7635 | 7635.6 |
| HpCdPlMT | 6.0 | Zn6-MT | 7448 | 7448.3 |
| HpCdMT [11] | 6.2 | Cd6-MT | 7287 | 7287.8 |
| HpCdMcMT | 6.1 | Cd6-MT | 7917 | 7917.7 |
| HpCdPlMT | 6.6 | Cd6-MT | 7730 | 7730.4 |
2.3. Cu Binding Abilities of HpCdMcMT and HpCdPlMT
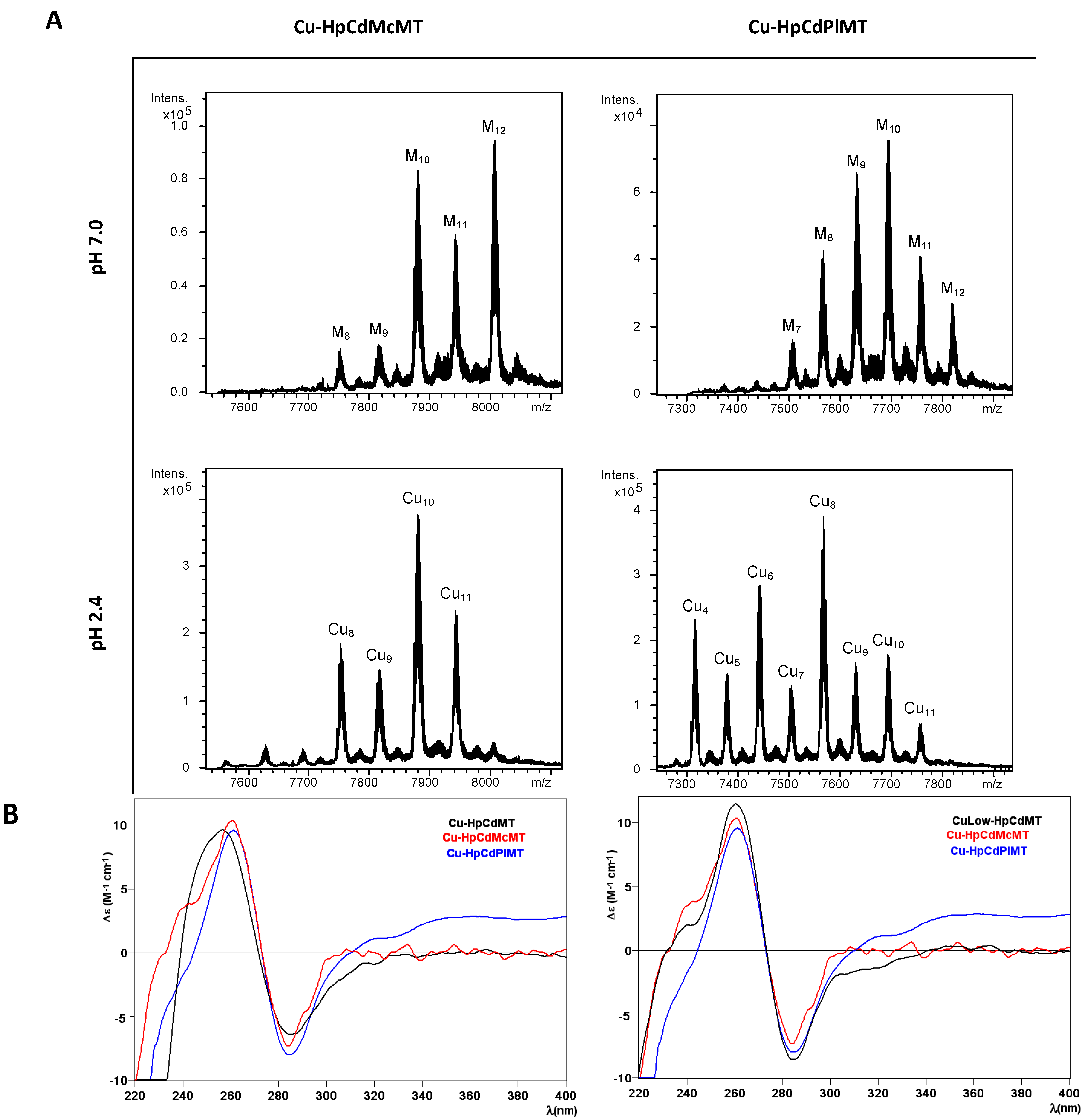
| MT | ICP-AES a | Neutral ESI-MS b | MMExp c | MMTheor d | Acidic ESI-MS b | MMExp c | MMTheor d |
|---|---|---|---|---|---|---|---|
| HpCdMT Normal aeration [15] | 2.6 Zn 1.9 Cu | M5-MT | 6932 | 6938.1 | |||
| M4-MT | 6867 | 6875.5 | apo-MT | 6623 | 6625.5 | ||
| M6-MT | 6996 | 7000.6 | Cu4-MT | 6873 | 6875.5 | ||
| M7-MT | 7062 | 7063.2 | Cu5-MT | 6930 | 6938.1 | ||
| M8-MT | 7124 | 7125.7 | |||||
| HpCdMT Low aeration [15] | 0.8 Zn 8.3 Cu | M10-MT | 7251 | 7250.8 | Cu8-MT | 7120 | 7,125.7 |
| M11-MT | 7313 | 7313.4 | Cu10-MT | 7248 | 7,250.8 | ||
| M12-MT | 7379 | 7375.9 | Cu11-MT | 7312 | 7313.4 | ||
| M8-MT | 7122 | 7125.9 | Cu9-MT | 7184 | 7188.3 | ||
| M9-MT | 7182 | 7188.3 | Cu5-MT | 6929 | 6938.1 | ||
| HpCdMcMT | 1.3 Zn 12.3 Cu | M12-MT | 8006 | 8005.8 | Cu10-MT Cu11-MT Cu8-MT Cu9-MT | 7879 7942 7753 7816 | 7880.7 7943.3 7755.6 7818.2 |
| M10-MT | 7878 | 7880.7 | |||||
| M11-MT | 7942 | 7943.3 | |||||
| M9-MT | 7816 | 7818.2 | |||||
| M8-MT | 7753 | 7755.6 | |||||
| HpCdPlMT | Cu8-MT | 7566 | 7568.3 | ||||
| M10-MT | 7692 | 7693.4 | Cu6-MT | 7442 | 7443.2 | ||
| M9-MT | 7631 | 7630.9 | Cu4-MT | 7316 | 7318.1 | ||
| 2.2 Zn | M8-MT | 7566 | 7568.3 | Cu10-MT | 7692 | 7693.4 | |
| 7.9 Cu | M11-MT | 7755 | 7755.9 | Cu9-MT | 7629 | 7630.9 | |
| M12-MT | 7818 | 7818.5 | Cu5-MT | 7377 | 7380.7 | ||
| M7-MT | 7505 | 7505.8 | Cu7-MT | 7503 | 7505.8 | ||
| Cu11-MT | 7755 | 7755.9 |
3. Experimental Section
3.1. Construction and Cloning of the cDNAs Encoding the HpCdMT Linker Mutants
3.2. Synthesis and Purification of the Recombinant Zn-, Cd-, and Cu-Complexes of the HpCdMT Linker Mutants
3.3. Cd(II) Replacement Reactions with the Zn(II)-HpCdMT Mutants
3.4. Spectroscopic Analyses (ICP-AES and CD) of the Metal Complexes Formed by the HpCdMT Linker Mutants
3.5 Electrospray Ionization Time-of-Flight Mass Spectrometry (ESI-TOF MS) of the Metal Complexes Obtained from the HpCdMT Linker Mutants
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Capdevila, M.; Bofill, R.; Palacios, O.; Atrian, S. State-of-the-art of metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Blindauer, C. Metallothioneins. In RSC Metallobiology: Binding, Transport and Storage of Metal Ions in Biological Cells; Maret, W., Wedd, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2014; Volume 2, pp. 594–653. [Google Scholar]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Kägi, J.H.R.; Kojima, Y. Chemistry and biochemistry of metallothionein. In Metallothionein II; Kägi, J.H.R., Kojima, Y., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1987; pp. 25–61. [Google Scholar]
- Binz, P.A.; Kägi, J.H.R. Metallothionein: Molecular evolution and classification. In Metallothionein IV; Klassen, C.D., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 7–13. [Google Scholar]
- Palacios, O.; Atrian, S.; Capdevila, M. Zn- and Cu-thioneins: A functional classification for metallothioneins? J. Biol. Inorg. Chem. 2011, 16, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Valls, M.; Bofill, R.; González-Duarte, R.; González-Duarte, P.; Capdevila, M.; Atrian, S. A new insight into metallothionein classification and evolution. The in vivo and in vitro metal binding features of Homarus americanus recombinant MT. J. Biol. Chem. 2001, 276, 32835–32843. [Google Scholar] [CrossRef] [PubMed]
- Bofill, R.; Capdevila, M.; Atrian, S. Independent metal-binding features of recombinant metallothioneins convergently draw a step gradation between Zn- and Cu-thioneins. Metallomics 2009, 1, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, R.; Berger, B.; Hunziker, P.E.; Kägi, J.H.R. Metallothionein in snail Cd and Cu metabolism. Nature 1997, 388, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Hispard, F.; Schuler, D.; de Vaufleury, A.; Scheifler, R.; Badot, P.M.; Dallinger, R. Metal distribution and metallothionein induction after cadmium exposure in the terrestrial snail Helix aspersa (Gastropoda, Pulmonata). Environ. Toxicol. Chem. 2008, 27, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Pagani, A.; Pérez-Rafael, S.; Egg, M.; Höckner, M.; Brandstätter, A.; Capdevila, M.; Atrian, S.; Dallinger, R. Shaping mechanisms of metal specificity in a family of metazoan metallothioneins: Evolutionary differentiation of mollusc metallothioneins. BMC Biol. 2011, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Chabicovsky, M.; Klepal, W.; Dallinger, R. Mechanisms of cadmium toxicity in terrestrial pulmonates: Programmed cell death and metallothionein overload. Environ. Toxicol. Chem. 2004, 23, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Chabicovsky, M.; Niederstaetter, H.; Thaler, R.; Hödl, E.; Parson, W.; Rossmanith, W.; Dallinger, R. Localisation and quantification of Cd- and Cu-specific metallothionein isoform mRNA in cells and organs of the terrestrial gastropod Helix pomatia. Toxicol. Appl. Pharmacol. 2003, 190, 25–36. [Google Scholar] [CrossRef]
- Dallinger, R.; Chabicovsky, M.; Hödl, E.; Prem, C.; Hünziker, P.; Manzl, C. Copper in Helix pomatia (Gastropoda) is regulated by one single cell type: Differently responsive metal pools in rhogocytes. Am. J. Physiol. 2005, 189, R1185–R1195. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Pérez-Rafael, S.; Pagani, A.; Dallinger, R.; Atrian, S.; Capdevila, M. Cognate and noncognate metal ion coordination in metal-specific metallothioneins: The Helix pomatia system as a model. J. Biol. Inorg. Chem. 2014, 19, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Braun, W.; Vasak, M.; Robbins, A.H.; Stout, C.D.; Wagner, G.; Kagi, J.H.; Wuthrich, K. Comparison of the NMR solution structure and the x-ray crystal structure of rat metallothionein-2. Proc. Natl. Acad. Sci. USA 1992, 89, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Ngu, T.T.; Lee, J.A.; Rushton, M.K.; Stillman, M.J. Arsenic metalation of seaweed Fucus vesiculosus metallothionein: The importance of the interdomain linker in metallothionein. Biochemistry 2009, 48, 8806–8816. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Orihuela, R.; Mir, G.; Molinas, M.; Atrian, S.; Capdevila, M. The Cd2+-binding abilities of recombinant Quercus suber metallothionein, QsMT: Bridging the gap between phytochelatins and metallothioneins. J. Biol. Inorg. Chem. 2007, 12, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Espart, A.; Gil-Moreno, S.; Palacios, P.; Capdevila, M.; Atrian, S. Understanding the internal architecture of long metallothioneins: 7-Cys building blocks in fungal (C. neoformans) MTs. Mol. Microbiol. 2015. [Google Scholar] [CrossRef]
- Dallinger, R.; Wang, Y.; Berger, B.; Mackay, E.A.; Kägi, J.H.R. Spectroscopic characterization of metallothionein from the terrestrial snail, Helix pomatia. Eur. J. Biochem. 2001, 268, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; de Rose, E.F.; Mullen, G.P.; Petering, D.H.; Shaw, C.F., III. Sequential proton resonance assignments and metal cluster topology of lobster metallothionein-1. Biochemistry 1994, 33, 8858–8865. [Google Scholar] [CrossRef] [PubMed]
- Narula, S.S.; Brouwer, M.; Hua, Y.; Armitage, I.M. Three-dimensional structure of Callinectes sapidus metallothionein-1 determined by homonuclear and heteronuclear magnetic resonance spectroscopy. Biochemistry 1995, 34, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Bofill, R.; Orihuela, R.; Romagosa, M.; Domenech, J.; Atrian, S.; Capdevila, M. C. elegans metallothionein isoform specificity: Metal binding abilities and histidine role in CeMT1 and CeMT2. FEBS J. 2009, 276, 7040–7056. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rafael, S.; Mezger, A.; Lieb, B.; Dallinger, R.; Capdevila, M.; Palacios, O.; Atrian, S. The metal binding abilities of Megathura crenulata metallothionein (McMT) in the frame of gastropoda MTs. J. Inorg. Biochem. 2012, 108, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Peroza, E.A.; Schmucki, R.; Güntert, P.; Freisinger, E.; Zerbe, O. The β(E)-domain of wheat E(c)-1 metallothionein: A metal-binding domain with a distinctive structure. J. Mol. Biol. 2009, 387, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Villarreal, L.; Capdevila, M.; Atrian, S. The Saccharomyces cerevisiae Crs5 metallothionein metal-binding abilities and its role in the response to zinc overload. Mol. Microbiol. 2007, 63, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; Domenech, J.; Bofill, R.; You, C.; Mackay, E.A.; Kägi, J.H.R.; Capdevila, M.; Atrian, S. The metal-binding features of the recombinant mussel Mytilus edulis MT-10-IV metallothionein. J. Biol. Inorg. Chem. 2008, 13, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Espart, A.; Espín, J.; Ding, C.; Thiele, D.J.; Atrian, S.; Capdevila, M. Full characterization of the Cu-, Zn- and Cd-binding properties of CnMT1 and CnMT2, two metallothioneins of the pathogenic fungus Cryptococcus neoformans acting as virulence factors. Metallomics 2014, 6, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Cols, N.; Romero-Isart, N.; Gonzalez-Duarte, R.; Atrian, S.; Gonzalez-Duarte, P. Recombinant synthesis of mouse Zn3-β and Zn4-α metallothionein 1 domains and characterization of their cadmium(II) binding capacity. Cell. Mol. Life Sci. 1997, 53, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Bongers, J.; Walton, C.D.; Richardson, D.E.; Bell, J.U. Micromolar protein concentrations and metalloprotein stoichiometries obtained by inductively coupled plasma. Atomic emission spectrometric determination of sulfur. Anal. Chem. 1988, 60, 2683–2686. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Domenech, J.; Pagani, A.; Tio, L.; Villarreal, L.; Atrian, S. Zn- and Cd-metallothionein recombinant species from the most diverse phyla may contain sulfide (S2−) ligands. Angew. Chem. Int. Ed. Engl. 2005, 44, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Fabris, D.; Zaia, J.; Hathout, Y.; Fenselau, C. Retention of thiol protons in two classes of protein zinc ion coordination centers. J. Am. Chem. Soc. 1996, 118, 12242–12243. [Google Scholar] [CrossRef]
- Perez-Rafael, S.; Kurz, A.; Guirola, M.; Capdevila, M.; Palacios, O.; Atrian, S. Is MtnE, the fifth Drosophila metallothionein, functionally distinct from the other members of this polymorphic protein family? Metallomics 2012, 4, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rafael, S.; Monteiro, F.; Dallinger, R.; Atrian, S.; Palacios, O.; Capdevila, M. Cantareus aspersus metallothionein metal binding abilities: The unspecific CaCd/CuMT isoform provides hints about the metal preference determinants in metallothioneins. Biochim. Biophys. Acta 2014, 1884, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Moreno, S.; Jiménez-Martí, E.; Palacios, Ò.; Zerbe, O.; Dallinger, R.; Capdevila, M.; Atrian, S. Does Variation of the Inter-Domain Linker Sequence Modulate the Metal Binding Behaviour of Helix pomatia Cd-Metallothionein? Int. J. Mol. Sci. 2016, 17, 6. https://doi.org/10.3390/ijms17010006
Gil-Moreno S, Jiménez-Martí E, Palacios Ò, Zerbe O, Dallinger R, Capdevila M, Atrian S. Does Variation of the Inter-Domain Linker Sequence Modulate the Metal Binding Behaviour of Helix pomatia Cd-Metallothionein? International Journal of Molecular Sciences. 2016; 17(1):6. https://doi.org/10.3390/ijms17010006
Chicago/Turabian StyleGil-Moreno, Selene, Elena Jiménez-Martí, Òscar Palacios, Oliver Zerbe, Reinhard Dallinger, Mercè Capdevila, and Sílvia Atrian. 2016. "Does Variation of the Inter-Domain Linker Sequence Modulate the Metal Binding Behaviour of Helix pomatia Cd-Metallothionein?" International Journal of Molecular Sciences 17, no. 1: 6. https://doi.org/10.3390/ijms17010006
APA StyleGil-Moreno, S., Jiménez-Martí, E., Palacios, Ò., Zerbe, O., Dallinger, R., Capdevila, M., & Atrian, S. (2016). Does Variation of the Inter-Domain Linker Sequence Modulate the Metal Binding Behaviour of Helix pomatia Cd-Metallothionein? International Journal of Molecular Sciences, 17(1), 6. https://doi.org/10.3390/ijms17010006









