Bioactive Carbohydrates and Peptides in Foods: An Overview of Sources, Downstream Processing Steps and Associated Bioactivities
Abstract
:1. Introduction
2. Bioactivities Associated with Peptides and Carbohydrates
2.1. Heart Health and Coagulation Beneficial Peptides
2.1.1. Sources and Structure of ACE-I Inhibitory Peptides
| Peptide Sequence | Observed Bioactivity | Product | Producers of Product | Product Type | Co-Product Source | Reference |
|---|---|---|---|---|---|---|
| LKPNM | Antihypertensive | PeptACE™ | Natural Factors Nutritional Products Ltd., British Columbia, Canada | Capsules | Bonito | [32] |
| LKPNM | Antihypertensive | Vasotensin® | Metagenics, USA | Tablet | Bonito | [32] |
| LKPNM | Antihypertensive | Levenorm® | Ocean Nutrition Canada Ltd., Nova Scotia, Canada | N/A | Bonito | [32] |
| LKPNM | Antihypertensive | Peptide ACE 3000 | Nippon Supplement Inc., Osaka, Japan | Capsules | Bonito | [32] |
| LKPNM | Antihypertensive | Peptide Tea | Nippon Supplement Inc., Osaka, Japan | Powder | Bonito | [33] |
| VY | Antihypertensive | Lapis Support | Tokiwa Yakuhin Co., Ltd., Tokyo, Japan | Beverage | Sardine | [33] |
| VY | Antihypertensive | Valtyron® | Senmi Ekisu Co., Ltd., Ohzu-City, Japan | Ingredient | Sardine | [33] |
| FY, VY and IY | Antihypertensive | Wakame Jelly | Riken Vitamin, Tokyo, Japan | Jelly | Undaria pinnatifida (seaweed) | [34] |
| AKYSY | Antihypertensive | Peptide Nori S | Riken Vitamin, Tokyo, Japan | Beverage | Porphyra yezoensis (seaweed) | [35] |
| AKYSY | Antihypertensive | Mainichi Kaisai Nori | Shirako Co., Ltd., Numazu City, Japan | Powder | Porphyra yezoensis (seaweed) | [35] |
| IPP and VPP | Antihypertensive | Ameal S 120 | Calpis Co., Ltd., Tokyo, Japan | Beverage | Milk | [36] |
| IPP and VPP | Antihypertensive | Ameal S | Calpis Co., Ltd., Tokyo, Japan | Tablet | Milk | [36] |
| IPP and VPP | Antihypertensive | Evolus® | Valio Ltd., Helsinki, Finland | Beverage | Milk | [36] |
| VY | Antihypertensive | Sato Marine Super P | Sato Pharmaceutical Co., Ltd., Tokyo, Japan | Tablet | Sardine | [33] |
| FFVAPFPEVFGK | Antihypertensive | Casein DP Peptio Drink | Kracie Pharmaceutical, Tokyo, Japan | Beverage | Milk | [37] |
| FFVAPFPEVFGK | Antihypertensive | C12 Peption | DMV International, Veghel, The Netherlands | Ingredient | Milk | [37] |
| LVY | Antihypertensive | Goma Pepucha | Suntory Beverage & Food Ltd., Tokyo, Japan | Beverage | Sesame | [38] |
| Numerous peptides | Antihypertensive | Bunaharitake | Yakult Health Foods Co., Ltd., Tokyo, Japan | Powder | Mushroom | [39] |
| VY, IY, IVY | Antihypertensive | StayBalance RJ | Api Co., Ltd., Gifu-City, Japan | Beverage | Royal jelly | [40] |
| VVYP | Weight management | Seishou-sabou | Moringa & Co., Ltd., Kanagawa, Japan | Beverage | Blood (bovine and porcine) | none |
| CSPHP | Cholesterol-lowering | Remake CholesterolBlock | Kyowa Hakko, Tokyo, Japan | Beverage | Soy | [41] |
| YLGYLEQLLR | Stress-relief | Lactium® | Ingredia, Arras Cedex, France | Beverage and capsules | Milk | [42] |
| N/A | Stress-relief | Stabilium® 200 | Yalacta, Caen, France | Capsules | Fish | [43] |
| N/A | Stress-relief | AntiStress 24 | Forte Pharma Laboratories, France | Capsules | Fish | [43] |
| N/A | Stress-relief | Protizen® | Copalis Sea Solutions, Boulogne-sur-mer, France | Powder | Fish | [43] |
| N/A | Joint health | CH-Alpha® | Gelita Health Products GmbH, Eberbach, Germany | Beverage | Bovine collagen | |
| N/A | Joint health | Peptan® | Rousselot SAS, Angoulême, France | Powder | Bovine collagen | [44] |
| N/A | Joint health | Collagen HM | Copalis Sea Solutions, Portel France | Powder | Fish collagen | [45] |
| N/A | Joint health | Glycollagen® | Copalis Sea Solutions, Portel, France | Powder | Skate collagen | [45] |
| N/A | Immunomodulatory | PeptiBal™ | InnoVactiv Inc., Rimouski, QC, Canada | Capsules | Shark | [46] |
| N/A | Gastrointestinal health | Seacure® | Proper Nutrition, USA | Capsules | Fish | [47] |
| N/A | Obesity and mental health | Douchi – traditional Chinese soybean product | Traditional Chinese medicine product, Hong Kong, China | N/A | N/A | [48] |
| N/A | Chinese sufu (fermented tofu) | Traditional product | Traditional Chinese medicine product, Hong Kong, China | N/A | N/A | [49] |
| Whey peptides | Blood pressure regulation and cholesterol control | BioZate®3 hydrolysed whey protein | Davisco Foods, Minnesota, MN, USA | Powder product | Whey proteins | [50] |
| Whey peptides | Blood pressure regulation | BioZate (1) hydrolysed whey protein | Davisco Foods, Minnesota, MN, USA | Powder product | Whey proteins | [51] |
| Fish collagen peptides | Skin health | Deyan, China | Deyan, Hubei, China | Powder product | Fish scale collagen peptides | [52] |
| Carnosine and Anserine | Antioxidant and anti-aging | Nivea Q-10 cream, Nivea | Nivea, France | Cream product | Meat muscle protein (beef and chicken) | [53] |
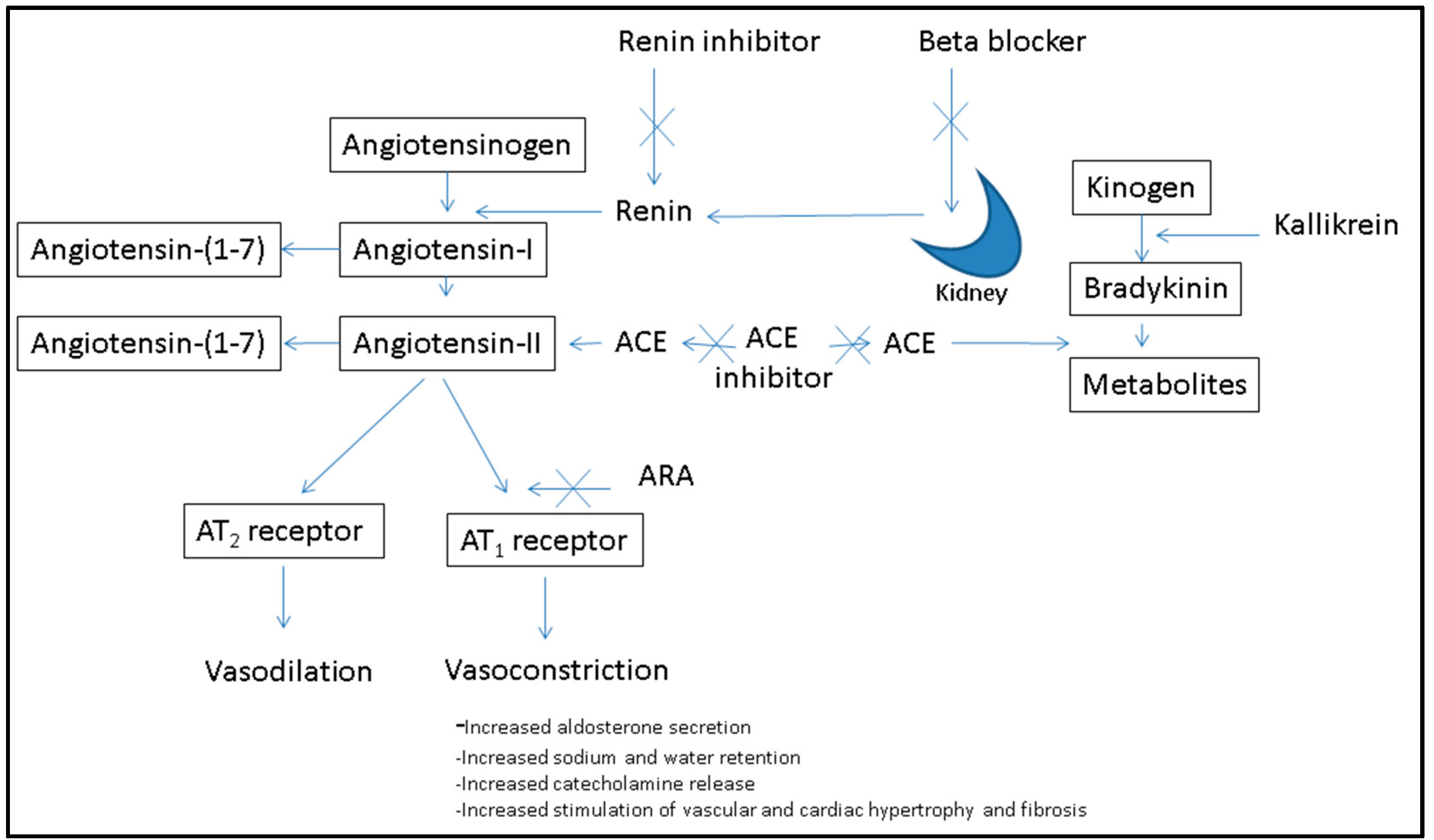
2.1.2. Sources and Structure of Renin Inhibitory Peptides
2.1.3. Sources of Platelet Activating Factor Acetylhydrolase (PAF-AH) Inhibitory Peptides
2.1.4. Sources of Dipeptidyl Dipeptidase IV (DPP-IV) Inhibitory Peptides
3. Peptides in Mental Health and Prevention of Diabetes PEP/POP Enzyme
3.1. Sources and Mechanisms of Action of Prolyl oligopeptidase (POP) or Prolyl endopeptidase (PEP) (EC 3.4.21.26) Inhibitory Peptides
3.2. Sources of DPP-IV Inhibitory Peptides
3.3. Acetylcholinesterase Inhibitory Peptides (AChE Inhibitory Peptides)
4. Bioactive Carbohydrates
4.1. Prebiotics
4.1.1. Prebiotics Carbohydrates in Brown Seaweeds
4.1.2. Prebiotic Carbohydrates in Green Seaweeds
4.1.3. Prebiotic Carbohydrates in Red Seaweeds
5. Bioactive Carbohydrates from Plant and Food Processing Co-Products
5.1. Chitin
5.2. Plant Sourced Prebiotic Oligoscaccharides, Obesity and Hypertension Prevention
6. Dairy Prebiotic Oligosaccharides
6.1. Lactulose
6.2. Galactooligosaccharides (GOS)
7. Downstream Processing
7.1. High Pressure Processing
7.2. Ultrasound Processing
7.3. Microwave Assisted Extraction
7.4. Supercritical Solvent Extraction
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pimenta, D.C.; Lebrun, I. Cryptides: Buried secrets in proteins. Peptides 2007, 28, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Bioactive peptides and their potential use for the prevention of diseases associated with Alzheimers’ Disease and Mental Health Disorders: Food for Thought? Ann. Psychiatry Ment. Health 2014, 2, 1017–1025. [Google Scholar]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Stanton, C.; Ross, R.P. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic disease. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, T.; Kyprianou, T.; Martinelli, F.G.; Oppici, C.A.; Heiligers, D.; Hills, D.; Calvo, X.R.; Verhaert, P. The emergence of peptides in the pharmaceutical business: From exploration to exploitation. EuPA Open Prot. 2014, 4, 58–69. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to bonito protein peptide and maintenance of normal blood pressure (ID 1716) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1730–1744. [Google Scholar]
- Hayes, M.; Carney, B.; Slater, J.; Bruck, W. Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan; Part A extraction methods. Biotechnol. J. 2008, 3, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Carney, B.; Slater, J.; Bruck, W. Mining marine shellfish waste for bioactive molecules: Chitin and chitosan, Part B: Applications. Biotechnol. J. 2008, 3, 878–889. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R. Food-Protein derived bioactive peptides: Production, processing and potential health benefits. J. Food Sci. 2012, 71, R11–R23. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Hayes, M. Cardioprotective cryptides derived from fish and other food sources: Generation, application and future markets. J. Agric. Food Chem. 2015, 63, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, E.C.K.; Heran, B.S.; Wright, J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014, 8. [Google Scholar] [CrossRef]
- Lacourciere, Y.; Brunner, H.; Irwin, R. Effects of modulators of the renin angiotensin aldosterone system on cough. Losartan Cough Study Group. J. Hypertens. 1994, 12, 1387–1393. [Google Scholar] [PubMed]
- Stuknyté, M.; Cattaneo, S.; Masotti, F.; de Noni, I. Occurrence and fate of ACE-inhibitor peptides in cheeses and in their digestates following in vitro static gastrointestinal digestion. Food Chem. 2015, 168, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tidona, F.; Criscione, A.; Guastella, A.M.; Zuccaro, A.; Bordonaro, S.; Marletta, D. Bioactive peptides in dairy products. Ital. J. Anim. Sci. 2009, 8, 315–340. [Google Scholar]
- Amado, I.R.; Vázquez, J.A.; González, P.; Esteban-Fernánedz, D.; Carrera, M.; Pineiro, C. Identification of the major ACE-inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate from cuttlefish wastewater. Mar. Drugs 2014, 12, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-F.; Li, G.-Z.; Peng, H.-B.; Zhang, F.; Chen, Y.; Li, Y. Effect of marine collagen peptides on markers of metabolic nuclear receptors in Type 2 Diabetic patients with/without hypertension. Biomed. Environ. Sci. 2010, 23, 113–120. [Google Scholar] [CrossRef]
- Lafarga, T.; O’Connor, P.; Hayes, M. Identification of novel dipeptidyl peptidase IV and angiotensin I converting enzyme inhibitory peptides from meat proteins using in silico analysis. Peptides 2014, 59, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjun, G.K.G.; Gowda, L.R.; Rao, A.G.; Prakash, V. Angiotensin-I-converting enzyme inhibitory peptide derived from glycinin, the 11S globulin of soybean (Glycine max). J. Agric. Food Chem. 2006, 54, 4568–4573. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; Alashi, A.; He, R.; Malomo, S.; Aluko, R.E. Preventative treatment effects of a hemp seed (Cannabis sativa L) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2014, 53, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Souri, E.; Ali Ziai, S.; Amin, G.; Amanlou, M. Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. DARU J. Pharm. Sci. 2014, 21, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Bae, I.Y.; Lee, H.G.; Yang, C.-B. Tyr-Pro-Lys, an angiotensin I converting enzyme inhibitory peptide derived from Broccoli (Brassica oleracea Italica). Food Chem. 2006, 99, 143–148. [Google Scholar] [CrossRef]
- Shamloo, M.; Eck, P.; Beta, T. Angiotensin Converting Enzyme Inhibitory Peptides derived from cereals. J. Hum. Nutr. Food Sci. 2015, 3, 1057–1067. [Google Scholar]
- Wu, H.; Xu, N.; Sun, X.; Yu, H.; Zhou, C. Hydrolysis and purification of ACE inhibitory peptides from the marine microalga. Isochrysis Galbana. J. Appl. Phycol. 2015, 27, 351–361. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.; Baptista, J. Investigation of Azorean macroalgae for angiotensin-I converting enzyme (ACE) inhibitory peptides: Extraction, purification and antihypertensive evaluation. Planta Med. 2014, 80–82. [Google Scholar] [CrossRef]
- Li, G.-H.; Le, G.-W.; Shi, Y.-H.; Shrestha, S. Angiotensin I converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmaceutical effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Rawendra, R.D.; Aisha, C.C.I.; Hsu, J.L. A novel angiotensin converting enzyme inhibitory peptide derived from proteolytic digest of Chinese soft-shelled turtle egg white proteins. J. Proteom. 2013, 94, 359–369. [Google Scholar]
- Fujita, H.; Yoshikawa, M. LKPNM: A pro-drug type ACE inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Kawasaki, T.; Seki, E.; Osajima, K. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolysate on mild hypertensive subjects. J. Hum. Hypertens. 2000, 14, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from Wakame (Undaria pinnatifida) and their antihypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Peptide Nori S. Available online: http://en.item.rakuten.com/kenkoex/noripepu30/ (accessed on 8 October 2012).
- Siltari, A.; Kivimaki, A.S.; Ehlers, P.I.; Korpela, R.; Vapaatalo, H. Effects of milk casein derived tripeptides on endothelial enzymes in vitro; a study with synthetic tripeptides. Arzneimittelforschung 2012, 62, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, S. Antihypertensive effects of tryptic hydrolysate of casein on normotensive and hypertensive volunteers. J. Jpn. Soc. Nutr. 1992, 45, 513–517. [Google Scholar] [CrossRef]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kuak, C.J.; Ikemura, K.; Takaoka, M.; Moriguchi, S.; et al. Antihypertensive effect of angiotensin-I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 2, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.C.; Abdullah, N.; Shuib, A.S. Novel angiotensin I converting enzyme inhibitory peptides derived from an edible mushroom, Pleurotus cystidiosin, O. K. Miller identified by LC-MS/MS. BMC Complement. Altern. Med. 2013, 13, 313–323. [Google Scholar] [CrossRef]
- Tokunaga, K.; Yoshida, C.; Suzuki, K.; Maruyama, H.; Futamura, Y.; Araki, Y.; Mishima, S. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol. Pharm. Bull. 2004, 27, 89–192. [Google Scholar] [CrossRef]
- Nagaoka, S.; Fujimura, W.; Morikawa, K.; Nakamura, A.; Kanamaru, Y.; Hori, G.; Yamamoto, K.; Takamura, M.; Oda, M.; Kzuo, S. Lactostatin (LLAEK) and CSPHP: New cholesterol lowering peptides derived from food proteins. In Dietary Fats and Risk of Chronic Disease; Huang, Y.S., Yanagita, T., Knap, H.R., Eds.; AOCS Press: Urbana, IL, USA, 2006. [Google Scholar]
- Nagai, T.; Suzuki, N.; Nagashima, T. Antioxidant activities and angiotensin I converting enzyme inhibitory activities of enzymatic hydrolysates from commercially available Kamaboko species. Food Sci. Technol. 2006, 12, 335–346. [Google Scholar]
- Guillerminet, F. Hydrolysed collagen improves bone metabolism and biochemical parameters in ovariectomized mice: An in vitro and an in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Bourseau, P.; Vandanjon, L.; Jaouen, P.; Chaplain-Derouiniot, M.; Massé, A.; Guérard, F.; Chabeaud, A.; Fouchereau-Péron, M.; Le Gal, Y.; Johansson, I. Fractionation of fish protein hydrolysates by ultrafiltration and nanofiltration: Impact on peptidic populations. Desalination 2009, 244, 303–320. [Google Scholar] [CrossRef]
- Boutin, Y.; Paradis, M.-E.; Couture, P.; Lamarche, B. Immunological effect of fish protein supplementation on healthy adults. J. Nat. Prod. 2012, 5, 37–44. [Google Scholar]
- Fitzgerald, A.J.; Rai, P.S.; Marchbank, T.; Taylor, G.W.; Ghosh, S.; Ritz, B.W.; Planford, R.J. Reparative properties of a commercial fish protein hydrolysate preparation. Gut 2005, 54, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wang, L.-J.; Zhu, F.-X.; Zhu, J.-Y.; Chen, X.D.; Zou, L.; Saito, M.; Li, L.-T. In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food). Food Chem. 2008, 10, 1421–1428. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Nirasawa, S.; Tatsumi, E.; Cheng, Y.; Li, L. In vivo anti-fatigue activity of sufu with fortification of isoflavone. Pharmacogn. Mag. 2014, 10, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Pins, J.; Keenan, J. The antihypertensive effects of a hydrolyzed whey protein isolate supplement (BioZate 1): A pilot study. Cardiovasc. Drugs Ther. 2002, 6, 68–69. [Google Scholar]
- Yamada, S.; Nagaoka, H.; Terajima, M.; Tsuda, N.; Hayashi, Y.; Yamauchi, M. Effect of fish collagen peptides on collagen post-translational modifications and mineralization in osteoblastic cell culture systems. Dent. Mater. J. 2013, 32, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Arihara, K. Strategies for designing novel functional meat products. Meat Sci. 2006, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Tigerstedt, R.; Bergman, P.G. Niere und Kreishaf. Skand. Arch. Physiol. 1898, 8, 223–235. [Google Scholar] [CrossRef]
- Bock, K.D.; Gross, F. Renin and Angiotensin Tachyphylaxis. Circ. Res. 1961, 9, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Lin, Y.-S.; Hou, W.C.; Aluko, R.E. Kinetics of the inhibition of renin and ACE enzyme by flaxseed protein hydrolysate fractions. J. Funct. Foods 2009, 1, 199–207. [Google Scholar] [CrossRef]
- Li, H.; Aluko, R.E. Identification and inhibitory properties of multifunctional peptides from pea protein hydrolysate. J. Agric. Food Chem. 2010, 10, 11471–11476. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tokiwano, T.; Suzuki, N.; Kodama, I.; Yoshizawa, Y.; Gotoh, T. Renin inhibitory activity in rice and cereals. J. Biol. Macromol. 2010, 10, 83–91. [Google Scholar]
- Campbell, D.J. Renin inhibitors—Mechanisms of action. Aust. Prescr. 2009, 32, 132–135. [Google Scholar]
- Campbell, D.J.; Aggarwal, A.; Esler, M.; Kaye, D. Beta-blockers, angiotensin II and ACE inhibitors in patients with heart failure. Lancet 2001, 358, 1609–1610. [Google Scholar] [CrossRef]
- Stafforini, D.M.; Zimmerman, G.A. Unraveling the PAF-AH/Lp-PLA2 controversy. J. Lipid Res. 2014, 55, 1811–1813. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes. Expert Opin. Investig. Drugs 2003, 12, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Martinez-Alvarez, O.; Haddar, A.; Gómez-Gullén, M.C.; Momtero, M.P.; Bougatef, A. Recovery, viscoelastic and functional properties of Barbel skin gelatin: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatin polypeptides. Food Chem. 2015, 168, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food Chem. 2013, 4, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Hayes, M.; Carney, B. Angiotensin-I-Converting enzyme and prolyl endopeptidase inhibitory peptides from natural sources with a focus on marine processing by-products. Food Chem. 2011, 129, 235–244. [Google Scholar] [CrossRef]
- Sørensen, R.; Kildal, E.; Stepaniak, L.; Pripp, A.H.; Søhaug, T. Screening for peptides from fish and cheese inhibitory to prolyl endopeptidase. Food Nahrung 2004, 48, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Lawandi, J.; Gerber-Lemaire, S.; Juillerat-Jeanneret, L.; Moitessier, N. Inhibitors of prolyl endopeptidase for the therapy of human disease. J. Med. Chem. 2010, 53, 3423–3438. [Google Scholar] [CrossRef] [PubMed]
- Pripp, A.H. Quantitative structure-activity relationship of prolyl endopeptidase inhibitory peptides derived from β-casein using simple amino acid descriptors. J. Agric. Food Chem. 2006, 54, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Jang, H.J.; Lee, K.H.; Hahn, T.R.; Paik, Y.S. Prolyl endopeptidase inhibitory activity of unsaturated fatty acids. J. Agric. Food Chem. 2006, 54, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, M.; Choi, J.Y.; Uang, E.J.; Hur, J.M. Plant phenolics as prolyl endopeptidase inhibitors. Arch. Pharm. Res. 2007, 30, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Bataller, E.; Llopis, S.; Gonzalez, N.; Alvarez, B.; Montón, F. A cocoa peptide protects Caenorhabditis elegans from oxidative stress and B-amyloid peptide toxicity. PLoS ONE 2013, 1, e63283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, W.; Wallack, M.; Li, H.; Carreras, I. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioural impairment and brain amyloid pathology in murine models of Alzheimer’s disease. Mol. Psychiatry 2015, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krushner, P.; Gorrell, M. DPP-4 inhibitors in type 2 diabetes: Importance of selective enzyme inhibition and implications for clinical use. J. Fam. Pract. 2010, 59, 1–2. [Google Scholar]
- Li-Chan, E.C.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Green, B.D.; Gault, V.A.; O’Harte, F.P.; Flatt, P.F. Structurally modified analogues of glucagon-like peptide-I (GLP-1) and glucose dependent insulinotropic polypeptide (GIP) as future anti-diabetic agents. Curr. Pharm. Des. 2010, 10, 3651–3662. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Lee, K.-H.; Je, J.-Y. Enzymatic production of bioactive protein hydrolysates from tuna liver: Effect of enzymes and molecular weight on bioactivity. Int. J. Food Sci. Technol. 2010, 45, 562–568. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.S.; Mahedevan, M. Peptides derived from rice bran protect cells from obesity and Alzheimer’s disease. Int. J. Biomed. Res. 2012, 3, 131–135. [Google Scholar] [CrossRef]
- Rees, T.M.; Brimijoin, S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today 2003, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kihara, T.; Shimohama, S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. 2004, 64, 99–105. [Google Scholar]
- Liu, L.; Wang, L.; Cheng, Y.; Saito, M.; Yamaki, K.; Qiao, Z.; Li, L. Isoflavone content and anti-acetylcholinesterase activity in commercial Douchi a traditional Chinese, salt-fermented Soybean food. Jpn. Agric. Res. Q. 2009, 43, 301–307. [Google Scholar] [CrossRef]
- Chen, J.; Quan, M.H.; Cheng, Y.Q.; Sun, J.; Li, L.T. Acetylcholinesterase inhibitory activity of Chinese sufu (fermented tofu) ethanol-extract. Food Chem. 2012, 134, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Valverde, J. Nutraceuticals derived from marine sources: Extraction and application of bioactive carbohydrates, proteins and peptides as functional ingredients. In Nutraceutical and Functional Foods: Natural Remedy; Brar, S.K., Kaur, S., Dhillon, G.S., Eds.; INRS: Québec, QC, Canada, 2013; Chapter 13; pp. 39–49. [Google Scholar]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–319. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. 2010, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Chitin, chitosan and their derivatives from marine rest raw materials: Potential food and pharmaceutical applications. In Marine Bioactive Compounds: Sources, Characterization and Application; Hayes, M., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Wang, D.; Han, J.; Yu, Y.; Li, X.; Wang, Y.; Tim, H.; Guo, S.; Jin, S.; Luo, T.; Qin, S. Chitosan oligosaccharide decreases very low density lipoprotein tryglyceride and increases high-density lipoprotein cholesterol in high fat diet fed rats. Exp. Biol. Med. 2011, 236, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C.; Gómez, R. Raffinose family of oligosaccharides from lupin seeds as prebiotics: Application in dairy products. J. Food Prot. 2005, 68, 1246–1252. [Google Scholar] [PubMed]
- Fabek, H. Understanding the Effect of Soluble Fibres on the Hydrolysis of Starch and the Diffusion of Glucose During Simulated Human Digestion. Ph.D Thesis, The University of Guelph, Guelph, ON, Canada, 2015. [Google Scholar]
- Yeo, S.-K.; Ooi, L.-G.; Lim, T.-J.; Liong, M.-T. Antihypertensive properties of plant based prebiotics. Int. J. Mol. Sci. 2009, 10, 3517–3530. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.E.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta Glucan: Health Benefits in Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Vester Boler, B.M.; Fahey, G.C. Prebiotics of plant and microbial origin. In Direct Fed Microbials and Prebiotics for Animals: Science and Mechanisms of Action; Callaway, T.R., Ricke, S.C., Eds.; Springer: Berlin, Germany, 2011; Chapter 2; pp. 13–26. [Google Scholar]
- EFSA. Scientific Opinion on the substantiation of health claims related to galacto-oligosaccharides (GOS) and reduction of gastro-intestinal discomfort (ID 763) and decreasing potentially pathogenic microorganisms (ID 765) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Quirós, A.; Chichón, R.; Recio, I.; López-Fandiño, R. The use of high hydrostatic pressure to promote the proteolysis and release of bioactive peptides from ovalbumin. Food Chem. 2007, 117, 1734–1739. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Recio, I.; López-Fandiño, R. Influence of high hydrostatic pressure on the proteolysis of β-lactoglobulin A by trypsin. J. Dairy Res. 2006, 73, 121–128. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Chicón, R.; Belloque, J.; Recio, I.; Alonso, E.; López-Fandiño, R. Changes in the ovalbumin proteolysis profile by high pressure and its effect on IgG and IgE binding. J. Agric. Food Chem. 2008, 56, 11809–11816. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mora, P.; Penas, E.; Frias, J.; Gomez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 2, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, E.; Herrero, M.; Mendiola, J.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro Algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds; Hayes, M., Ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Mason, T.J.; Riera, E.; Vercet, A.; Lopez-Buesa, P. 13-Application of Ultrasound. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Academic Press: London, UK, 2005. [Google Scholar]
- Shirsath, S.; Sonawane, S.; Gogate, P. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Proc. 2012, 53, 10–13. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Effect of ultrasound pre-treatment on the drying kinetics of brown seaweed Ascophyllum nodosum. Ultrason. Sonochem. 2015, 23, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Manasseh, R.; Mawson, R.; Ashokkumar, M. Ultrasonic recovery and modification of food ingredients. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G., Weiss, J., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Rastogi, N.K. Opportunities and challenges in application of ultrasound in food processing. Crit. Rev. Food Sci. Nutr. 2011, 51, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Zill, E.H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Lin, Y.; Zhang, H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014, 150, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbite moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Bashari, M.; Eibaid, A.; Wang, J.; Tian, Y.; Xu, X.; Jin, Z. Influence of low ultrasound intensity on the degradation of dextran catalyzed by dextranase. Ultrason. Sonochem. 2013, 20, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Guiseppi-Elie, A.; Choi, S.H.; Geckeler, K.E. Ultrasonic processing of enzymes: Effect on enzymatic activity of glucose oxidase. J. Mol. Catal. B: Enzym. 2009, 58, 118–123. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Cooke, P.H.; Yadav, M.P.; Hotchkiss, A.T. Physico-chemical characterization of alkaline soluble polysaccharides from sugar beet pulp. Food Hydrocoll. 2009, 23, 1554–1562. [Google Scholar] [CrossRef]
- Zeng, W.-C.; Zhang, Z.; Gao, H.; Jia, L.-R.; Chen, W.-Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012, 89, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Marcus, S.L.; Li, L. Microwave-assisted acid hydrolysis of proteins combined with liquid chromatography MALDI MS/MS for protein identification. J. Am. Soc. Mass Spectrom. 2005, 16, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.T. Supercritical fluid chromatography for the 21st century. J. Supercrit. Fluids 2009, 47, 566–573. [Google Scholar] [CrossRef]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. Trends Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Matute, A.I.; Ramos, L.; Martínez-Castro, I.; Sanz, M. Fractionation of honey carbohydrates using pressurized liquid extraction with activated charcoal. J. Agric. Food Chem. 2008, 56, 8309–8313. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J. Mol. Catal. B: Enzym. 2014, 101, 108–114. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, M.; Tiwari, B.K. Bioactive Carbohydrates and Peptides in Foods: An Overview of Sources, Downstream Processing Steps and Associated Bioactivities. Int. J. Mol. Sci. 2015, 16, 22485-22508. https://doi.org/10.3390/ijms160922485
Hayes M, Tiwari BK. Bioactive Carbohydrates and Peptides in Foods: An Overview of Sources, Downstream Processing Steps and Associated Bioactivities. International Journal of Molecular Sciences. 2015; 16(9):22485-22508. https://doi.org/10.3390/ijms160922485
Chicago/Turabian StyleHayes, Maria, and Brijesh K. Tiwari. 2015. "Bioactive Carbohydrates and Peptides in Foods: An Overview of Sources, Downstream Processing Steps and Associated Bioactivities" International Journal of Molecular Sciences 16, no. 9: 22485-22508. https://doi.org/10.3390/ijms160922485







