Selection and Characterization of Single Chain Antibody Fragments Specific for Hsp90 as a Potential Cancer Targeting Molecule
Abstract
:1. Introduction
2. Results
2.1. Selection of Hsp90-Specific Antibody Fragments
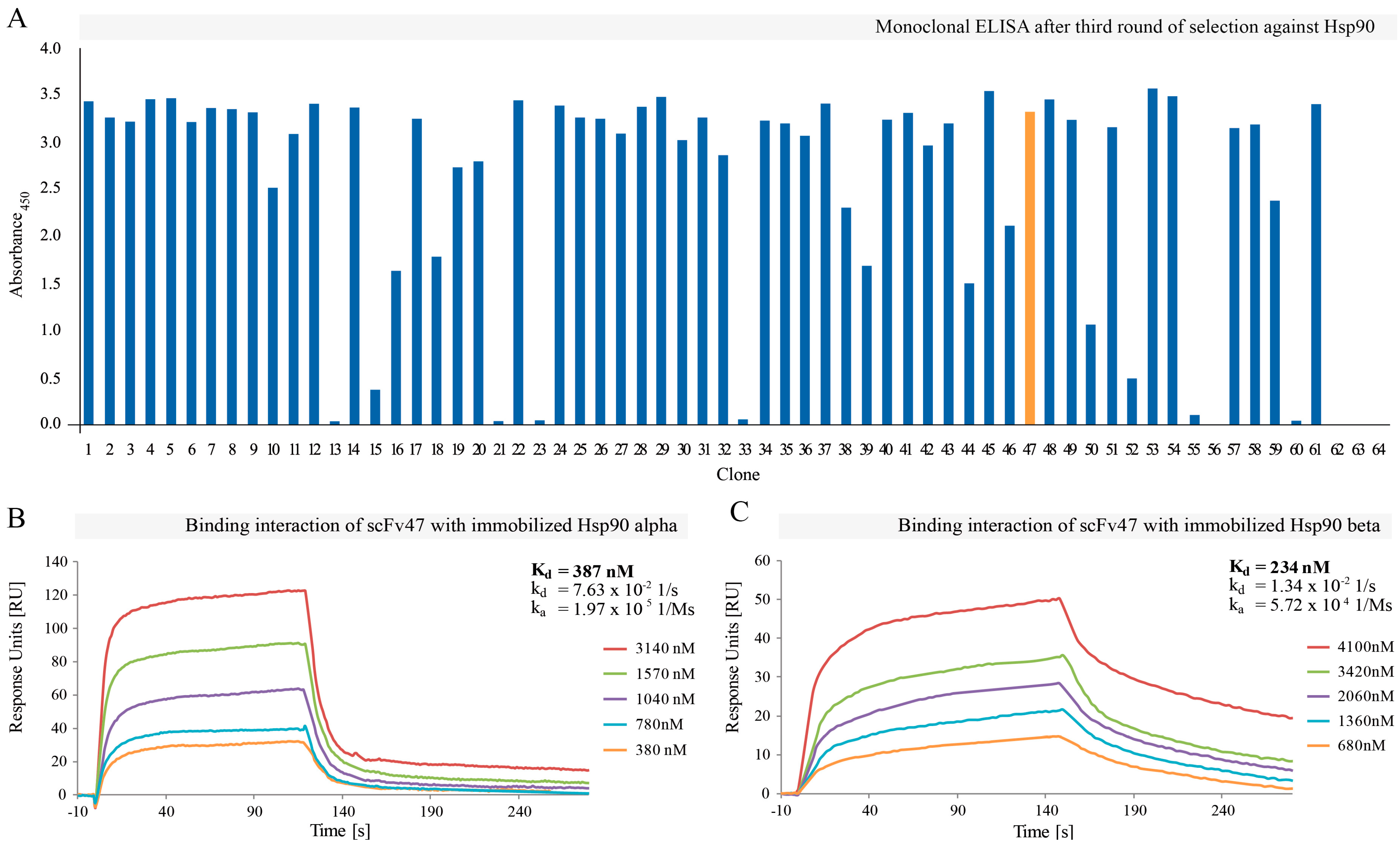
2.2. Affinity Maturation of Selected scFv47

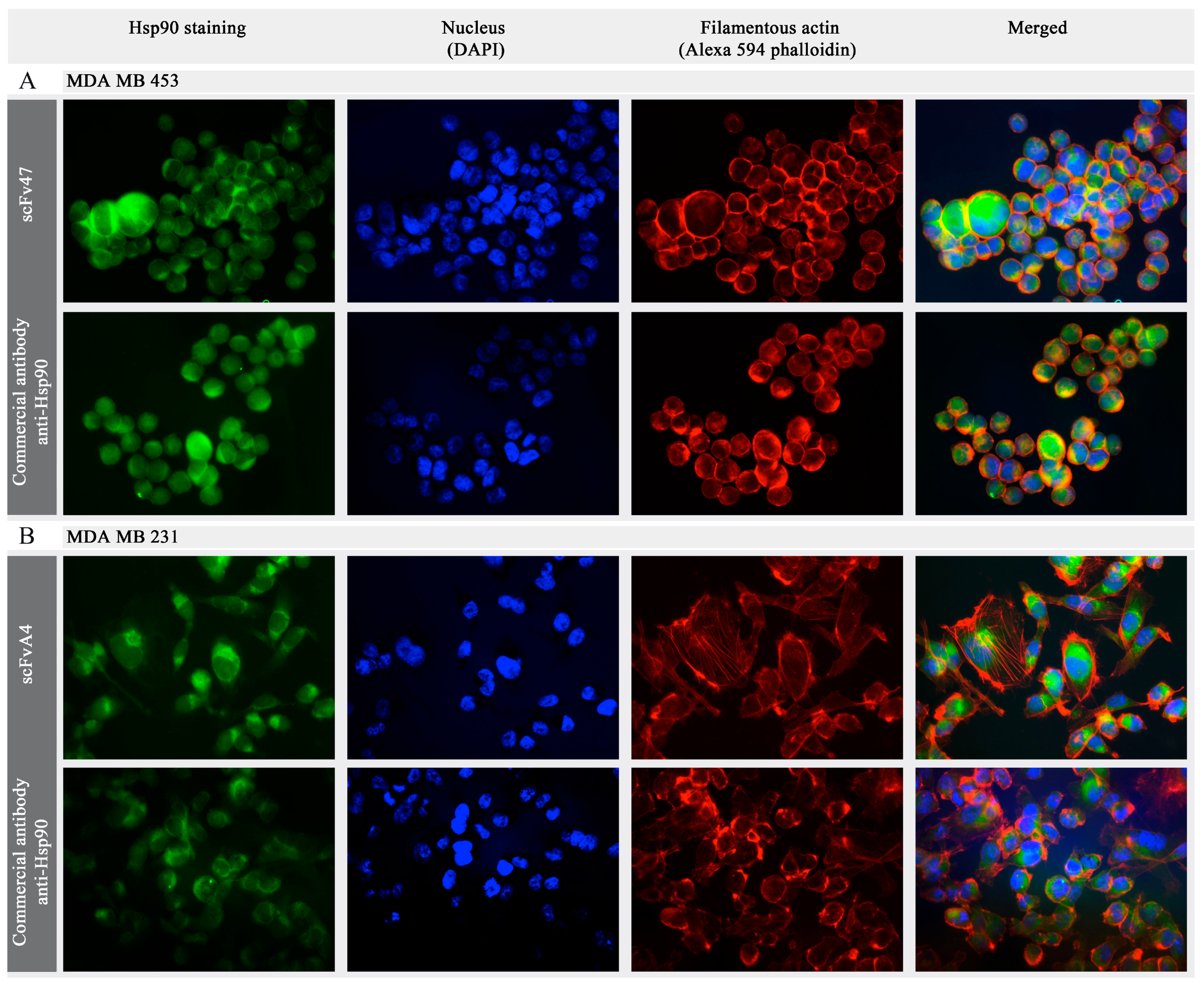
2.3. Interaction of Antibody Fragments with Hsp90 in Breast Cancer Cell Lines
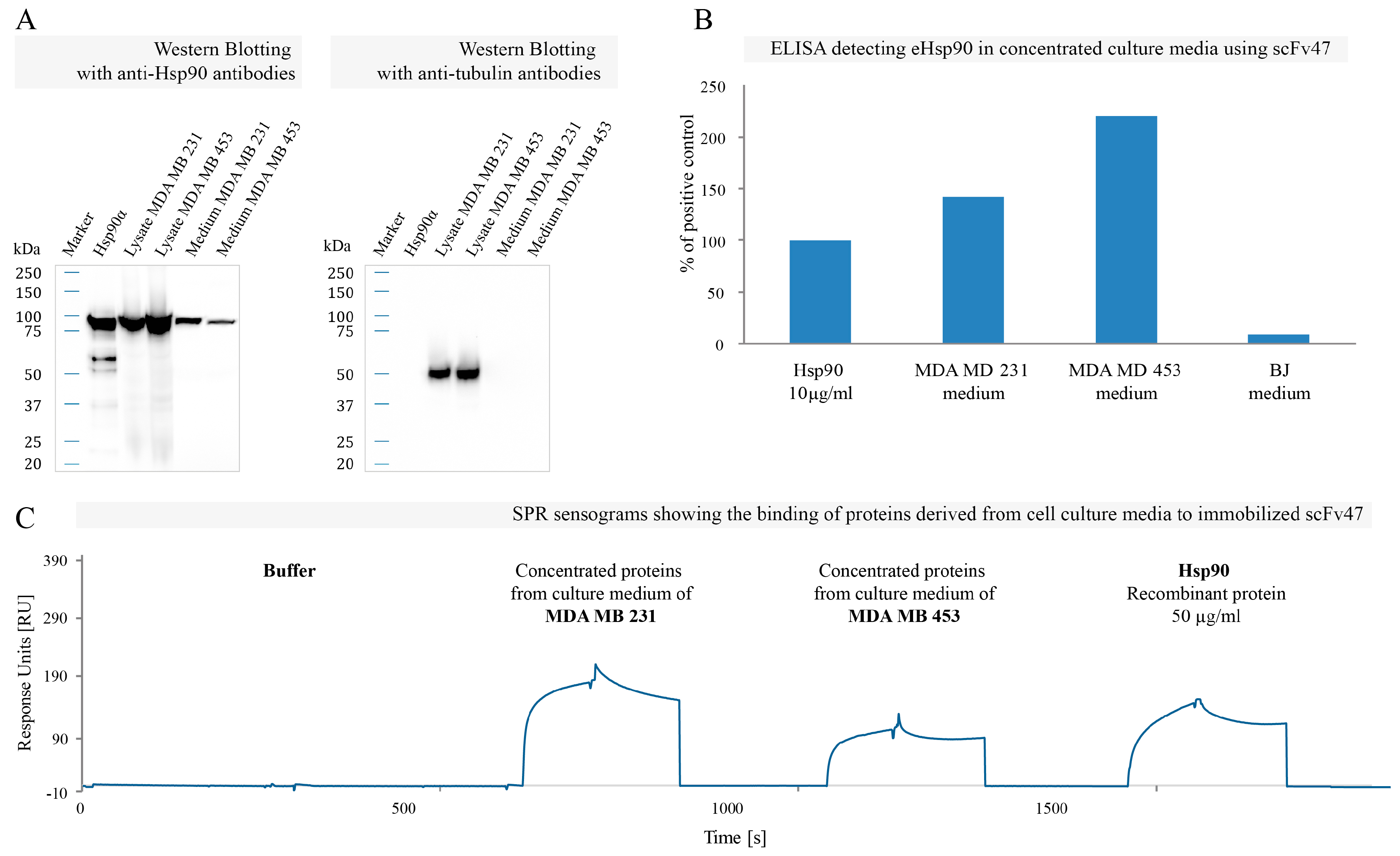
2.4. Binding of Specific scFv to Extracellular Pool of Hsp90
3. Discussion
4. Experimental Section
4.1. Selection Experiments Based on Tomlinson I and J Libraries
4.2. Affinity Maturation: Library Construction and Panning
4.3. Monoclonal ELISA of Soluble Antibody Fragments
4.4. SPR (Surface Plasmon Resonance) Screening of Selected Clones
4.5. Soluble scFv Expression and Purification
4.6. Affinity Measurements by SPR
4.7. Cell Cultures
4.8. Immunofluorescence
4.9. Preparation of Concentrated Cell Culture Media for Western Blotting and SPR Measurements
4.10. Preparation of Concentrated Cell Culture Media for ELISA
4.11. Western Blotting
4.12. ELISA for Hsp90 Detection in Cell Culture Media
4.13. SPR Measurements of Hsp90 Binding to Immobilized scFv47
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Young, J.C.; Agashe, V.R.; Siegers, K.; Hartl, F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. 2004, 5, 781–791. [Google Scholar] [CrossRef] [PubMed]
- McClellan, A.J.; Xia, Y.; Deutschbauer, A.M.; Davis, R.W.; Gerstein, M.; Frydman, J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell 2007, 131, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczuk, A.; Kiliańska, Z.M. Plejotropowa aktywność białek szoku cieplnego. Postępy Hig. Med. Dośw 2009, 63, 502–521. [Google Scholar]
- Bagatell, R.; Whitesell, L. Altered Hsp90 function in cancer: A unique therapeutic opportunity. Mol. Cancer Ther. 2004, 3, 1021–1030. [Google Scholar] [PubMed]
- Jego, G.; Hazoumé, A.; Seigneuric, R.; Garrido, C. Targeting heat shock proteins in cancer. Cancer Lett. 2013, 332, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. Lindquist, HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Sergentanis, T.N.; Gazouli, M.; Tsigginou, A.; Dimitrakakis, C.; Papaspyrou, I.; Eleutherakis-Papaiakovou, E.; Chrysikos, D.; Theodoropoulos, G.; Zografos, G.C.; et al. HSP90, HSPA8, HIF-1 alpha and HSP70–2 polymorphisms in breast cancer: A case-control study. Mol. Biol. Rep. 2012, 39, 10873–10879. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Sergentanis, T.N.; Nonni, A.; Papadimitriou, C.A.; Michalopoulos, N.V.; Domeyer, P.; Theodoropoulos, G.; Lazaris, A.; Patsouris, E.; Zogafos, E.; et al. Hsp90 in the continuum of breast ductal carcinogenesis: Evaluation in precursors, preinvasive and ductal carcinoma lesions. BMC Cancer 2010, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Bournakis, E.; Koutsoukos, K.; Papadimitriou, C.A. Heat shock protein 90 (hsp90) expression and breast cancer. Pharmaceuticals 2012, 5, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Piel, W.H.; Gui, L.; Bruford, E.; Monteiro, A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics 2005, 86, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Eustace, B.K.; Sakurai, T.; Stewart, J.K.; Yimlamai, D.; Unger, C.; Zehetmeier, C.; Lain, B.; Torella, C.; Henning, S.W.; Beste, G.; et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat. Cell Biol. 2004, 6, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Multhoff, G.; Farkas, B.; Wild, P.J.; Landthaler, M.; Stolz, W.; Vogt, T. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp. Dermatol. 2004, 13, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, X.; Zhuo, W.; Fu, Y.; Shi, H.; Liang, Y.; Tong, M.; Chang, G.; Luo, Y. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. USA 2009, 106, 21288–21293. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Bright, A.M.; Yerushalmi, D.; Liang, M.; Chen, M.; et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol. Cell. Biol. 2008, 28, 3344–3358. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; Samiotaki, M.; Yfanti, E.; Panayotou, G.; Patsavoudi, E. Involvement of cell surface HSP90 in cell migration reveals a novel role in the developing nervous system. J. Biol. Chem. 2004, 279, 45379–45388. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Neckers, L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007, 98, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Luo, Y. The regulatory mechanism of Hsp90alpha secretion from endothelial cells and its role in angiogenesis during wound healing. Biochem. Biophys. Res. Commun. 2010, 398, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Zhuo, W.; Shi, H.; Feng, D.; Sun, Y.; Liang, Y.; Fu, Y.; Zhou, D.; Luo, Y. The regulatory mechanism of extracellular Hsp90α on matrix metalloproteinase-2 processing and tumor angiogenesis. J. Biol. Chem. 2010, 285, 40039–40049. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sahu, D.; Tsen, F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim. Biophys. Acta 2012, 1823, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Stellas, D.; Karameris, A.; Patsavoudi, E. Monoclonal antibody 4C5 immunostains human melanomas and inhibits melanoma cell invasion and metastasis. Clin. Cancer Res. 2007, 13, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Stellas, D.; el Hamidieh, A.; Patsavoudi, E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010, 5, 11–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Hsu, Y.M.; Chen, C.C.; Chen, L.L.; Lee, C.C.; Huang, T.S. Secreted heat shock protein 90α induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression. J. Biol. Chem. 2010, 285, 25458–25466. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; Patsavoudi, E. Extracellular HSP90: Conquering the cell surface. Cell Cycle 2008, 7, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; Gaitanou, M.; Stellas, D.; Matsas, R.; Patsavoudi, E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J. Biol. Chem. 2008, 283, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Binder, R.J.; Ramalingam, T.; Srivastava, P.K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001, 14, 303–313. [Google Scholar] [CrossRef]
- Schulte, T.W.; Neckers, L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998, 42, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Taldone, T.; Gozman, A.; Maharaj, R.; Chiosis, G. Targeting Hsp90: Small-molecule inhibitors and their clinical development. Curr. Opin. Pharmcol. 2008, 8, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Chiosis, G.; Lopes, E.C.; Solit, D. Heat shock protein-90 inhibitors: A chronicle from geldanamycin to today’s agents. Curr. Opin. Investig. Drugs 2006, 7, 534–541. [Google Scholar] [PubMed]
- Yi, F.; Regan, L. Novel Class of Small Molecule Inhibitors of Hsp90. ACS Chem. Biol. 2008, 3, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Sergentanis, T.N.; Chrysikos, D.; Papadimitriou, C.A.; Dimopoulos, M.A.; Psaltopoulou, T. Hsp90 inhibitors in breast cancer: A systematic review. Breast 2013, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L; Workman, P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar]
- Eteshola, E. Isolation of scFv fragments specific for monokine induced by interferon-gamma (MIG) using phage display. J. Immunol. Methods 2010, 358, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Boulter-Bitzer, J.I.; Lee, H.; Trevors, J.T. Single chain variable fragment antibodies selected by phage display against the sporozoite surface antigen S16 of Cryptosporidium parvum. Exp. Parasitol. 2010, 125, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.P.; Ko, J.H.; Qi, Y.P. Generation and characterization of a novel single-chain antibody fragment specific against human fibrin clots from phage display antibody library. Thromb. Res. 2004, 114, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Goletz, S.; Christensen, P.A.; Kristensen, P.; Blohm, D.; Tomlinson, I.; Winter, G.; Karsten, U. Selection of large diversities of antiidiotypic antibody fragments by phage display. J. Mol. Biol. 2002, 315, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Source Bioscience-Human Single Fold scFv Libraries I + J (Tomlinson I + J). Available online: http://www.lifesciences.sourcebioscience.com (accessed on 17 July 2015).
- Kamal, A.; Thao, L.; Sensintaffar, J.; Zhang, L.; Boehm, M.F.; Fritz, L.C.; Burrows, F.J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003, 425, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tsen, F.; Sahu, D.; Bhatia, A.; Chen, M.; Multhoff, G.; Woodley, D.T. Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: Intentionally or unintentionally. Int. Rev. Cell Mol. Biol. 2013, 303, 203–235. [Google Scholar] [PubMed]
- Sahu, D.; Zhao, Z.; Tsen, F.; Cheng, C.F.; Park, R.; Situ, A.J.; Dai, J.; Eginli, A.; Shams, S.; Chen, M.; et al. A potentially common peptide target in secreted heat shock protein-90α for hypoxia-inducible factor-1α-positive tumors. Mol. Biol. Cell 2012, 23, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; el Hamidieh, A.; Mamalaki, A.; Patsavoudi, E. The 4C5 cell-impermeable anti-HSP90 antibody with anti-cancer activity, is composed of a single light chain dimer. PLoS ONE 2011, 6, e23906. [Google Scholar] [CrossRef] [PubMed]
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Banerji, U. Heat shock protein 90 as a drug target: Some like it hot. Clin. Cancer Res. 2009, 15, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Banerji, U.; Tavana, B.; George, G.C.; Aaron, J.; Kurzrock, R. Targeting the molecular chaperone heat shock protein 90 (HSP90): Lessons learned and future directions. Cancer Treat. Rev. 2013, 39, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Carnero, A.; Paz-Ares, L. Inhibition of HSP90 molecular chaperones: Moving into the clinic. Lancet Oncol. 2013, 14, 358–369. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Scroggins, B.; Koga, F.; Lee, M.J.; Trepel, J.; Felts, S.; Carreras, C.; Neckers, L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 2008, 27, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Trachsel, E.; Kaspar, M.; Schliemann, C.; Sommavilla, R.; Rybak, J.N.; Rosli, C.; Borsi, L.; Neri, D. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int. J. Cancer 2008, 122, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petters, E.; Sokolowska-Wedzina, A.; Otlewski, J. Selection and Characterization of Single Chain Antibody Fragments Specific for Hsp90 as a Potential Cancer Targeting Molecule. Int. J. Mol. Sci. 2015, 16, 19920-19935. https://doi.org/10.3390/ijms160819920
Petters E, Sokolowska-Wedzina A, Otlewski J. Selection and Characterization of Single Chain Antibody Fragments Specific for Hsp90 as a Potential Cancer Targeting Molecule. International Journal of Molecular Sciences. 2015; 16(8):19920-19935. https://doi.org/10.3390/ijms160819920
Chicago/Turabian StylePetters, Edyta, Aleksandra Sokolowska-Wedzina, and Jacek Otlewski. 2015. "Selection and Characterization of Single Chain Antibody Fragments Specific for Hsp90 as a Potential Cancer Targeting Molecule" International Journal of Molecular Sciences 16, no. 8: 19920-19935. https://doi.org/10.3390/ijms160819920
APA StylePetters, E., Sokolowska-Wedzina, A., & Otlewski, J. (2015). Selection and Characterization of Single Chain Antibody Fragments Specific for Hsp90 as a Potential Cancer Targeting Molecule. International Journal of Molecular Sciences, 16(8), 19920-19935. https://doi.org/10.3390/ijms160819920