Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Pathway Is Induced by Mechanical Load and Reduces the Activity of Hedgehog Signaling in Chondrogenic Micromass Cell Cultures
Abstract
:1. Introduction
2. Results
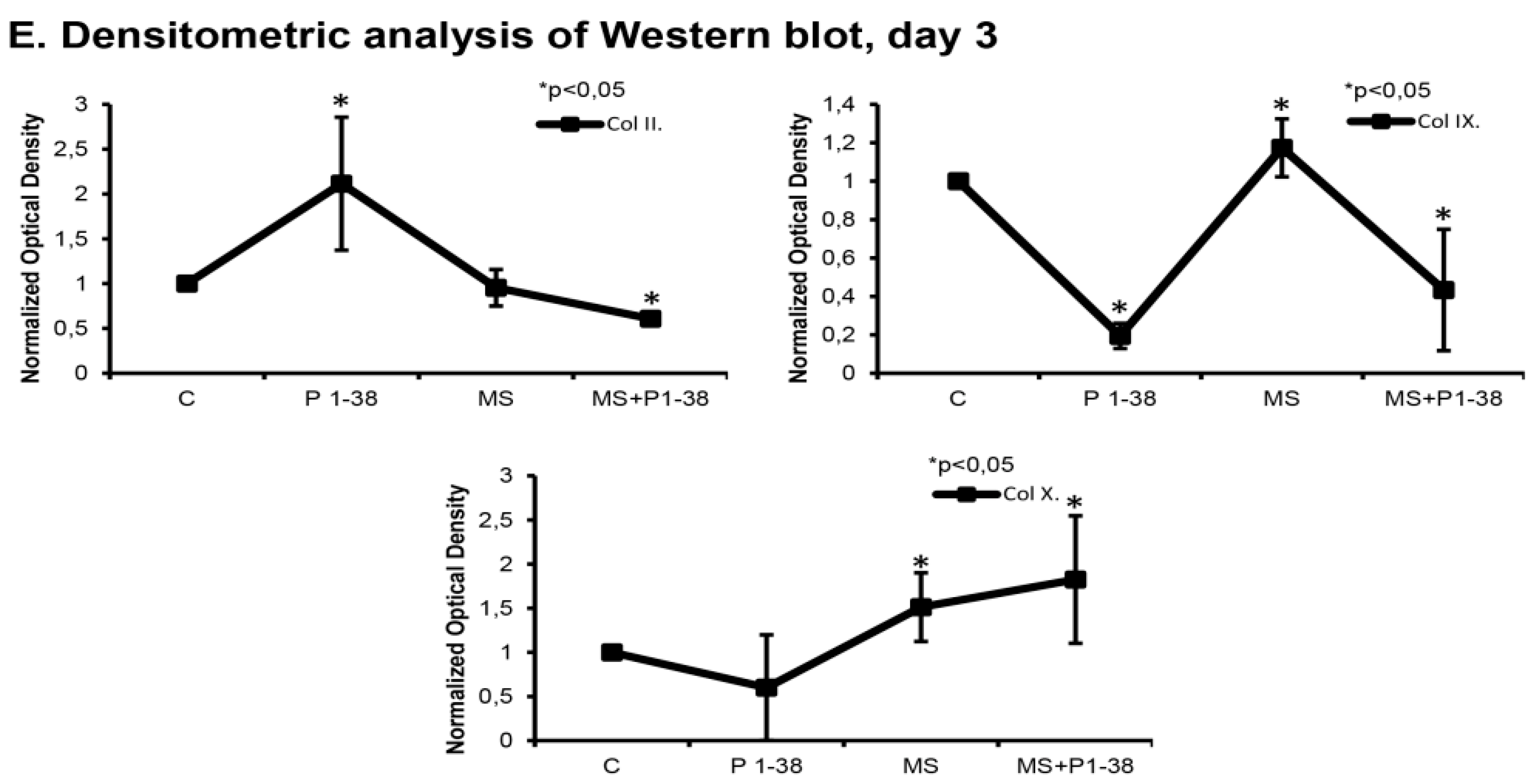
2.1. Mechanical Stimulation and PACAP (Pituitary Adenylate Cyclase Activating Polypeptide) Administration Enhance Collagen Production


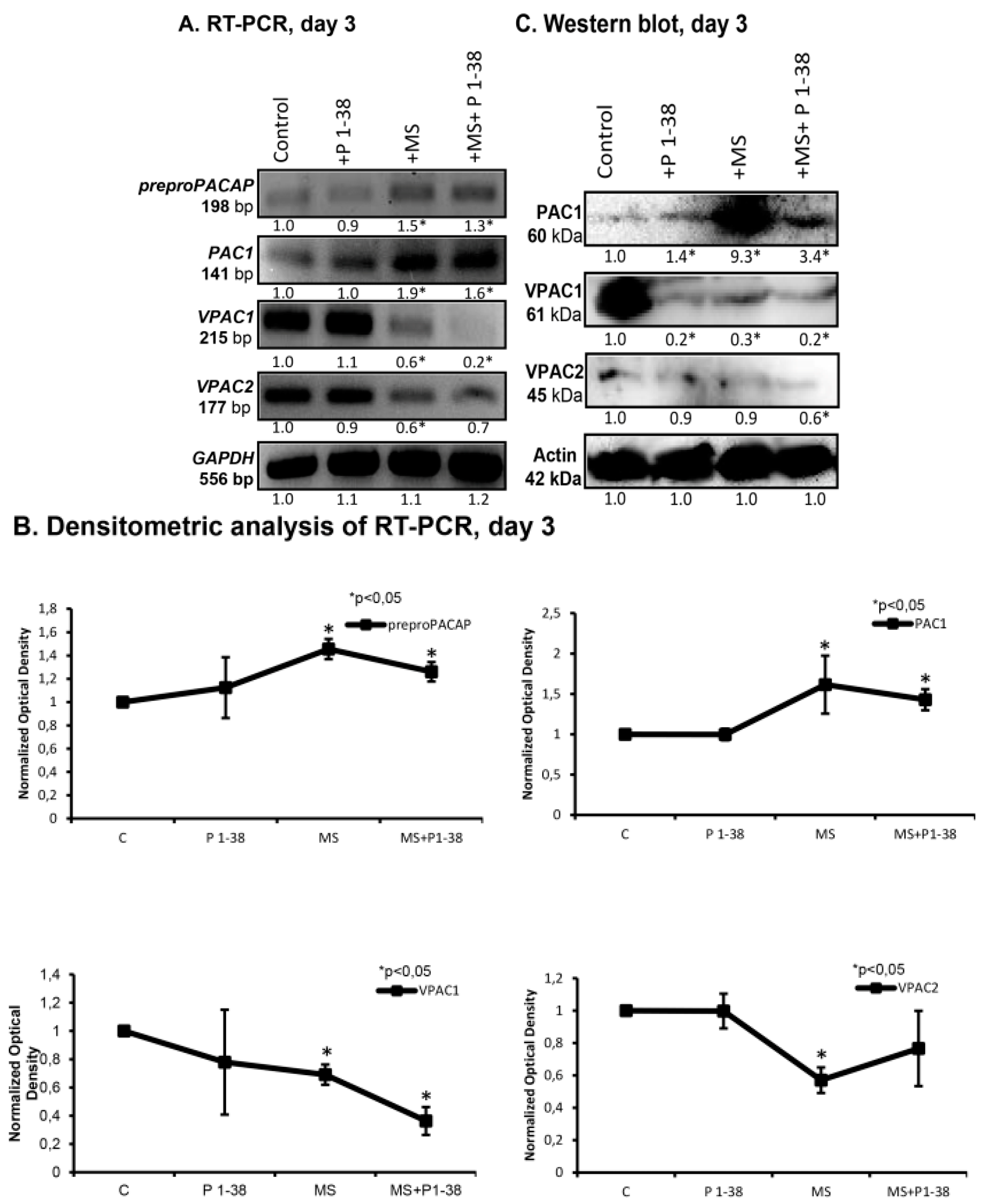
2.2. PACAP and PAC1 Receptor Expressions Are Elevated by Mechanical Loading

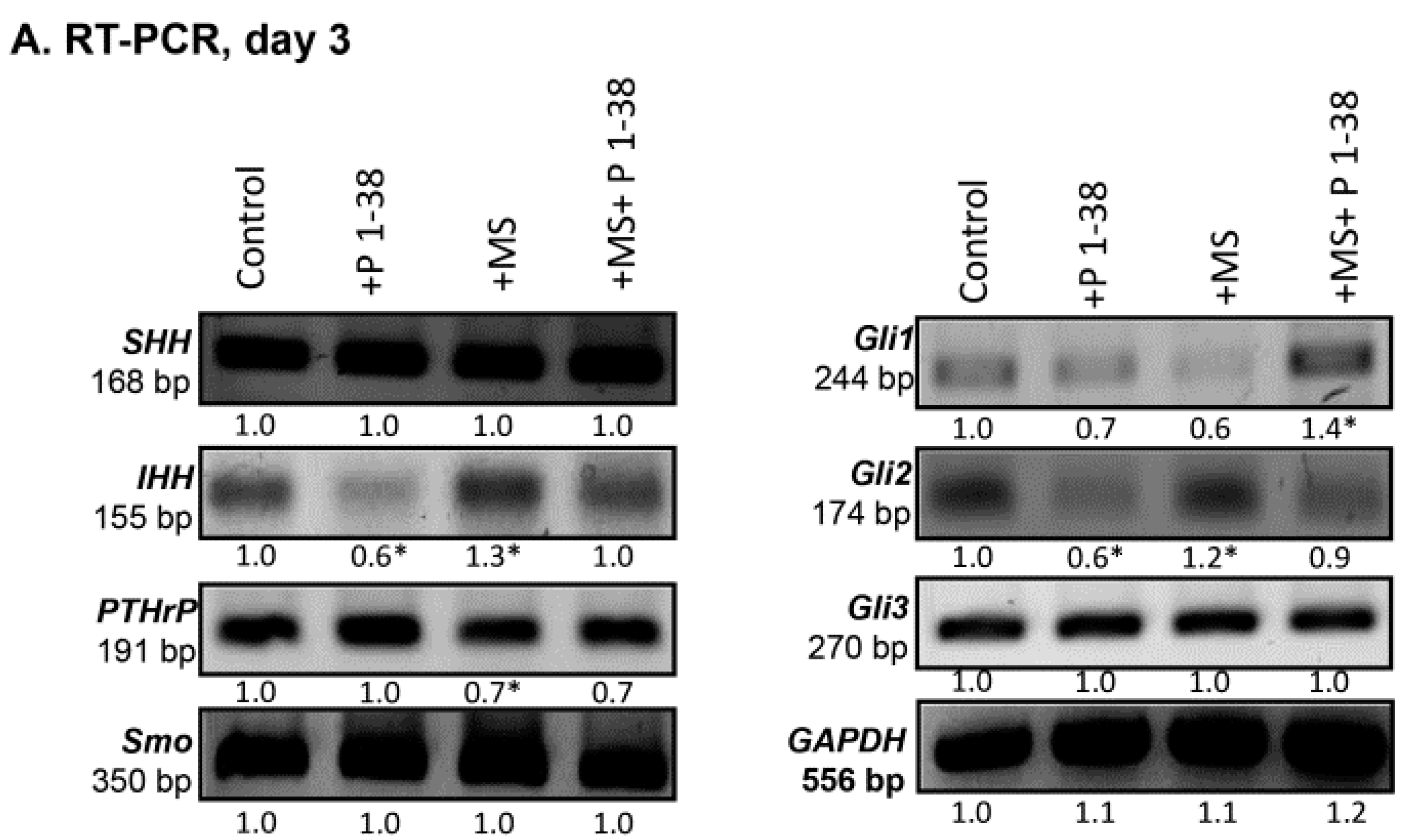
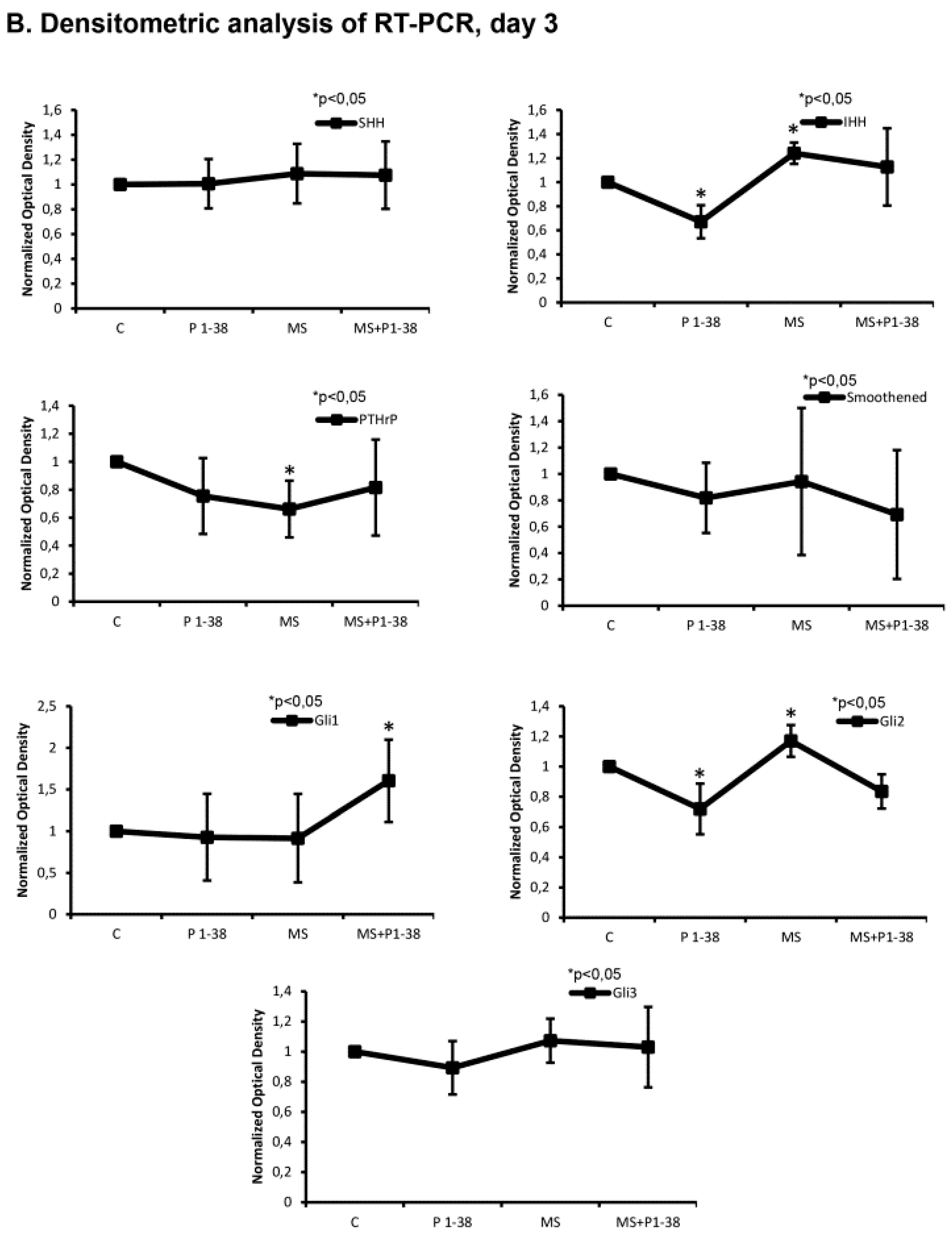
2.3. Hedgehog Signaling Pathways Become Upregulated upon Mechanical Load, While PACAP Attenuates the Activity of This Pathway

3. Discussion
4. Experimental Section
4.1. Cell Culturing
4.2. Mechanical Load and PACAP Administration
4.3. Light Microscopical Morphology
4.4. Measurement of Collagen Production with 3H-Proline Labelling
4.5. Preparation of Cell Extracts
4.6. RT-PCR Analysis
4.7. Western Blot Analysis
| Gene | Primer | Nucleotide Sequence (5′→3′) | GenBank ID | Annealing Temperature | Amplimer Size (bp) |
|---|---|---|---|---|---|
| Collagen II (Col2a1) | sense | GGA CCC AAA GGA CAG ACG G (1191–1210) | NM_204426 | 59 °C | 401 |
| antisense | TCG CCA GGA GCA CCA GTT (1573–1591) | ||||
| Collagen IX (Col9a1) | sense | GGG ACAA GAG GAA TAA ACG (1732–1750) | NM_001100911.1 | 52 °C | 163 |
| antisense | CTG GTA AAC CTG GCA ATC (1877–1894) | ||||
| Collagen X (Colxa1) | sense | TCT GGG ATG CCG CTT GTC (1681–1698) | NM_009925.4 | 56 °C | 261 |
| antisense | CGT AGG CGT GCC GTT CTT (1924–1941) | ||||
| PACAP (ADCYAP1) | sense | CTT CGC ACT ACG AGC AGG (156–163) | NM_001001291 | 52.5 °C | 198 |
| antisense | TTG ACA GCC ATT TGT TTC C (335–363) | ||||
| PAC1 (ADCYAP1R1) | sense | GTC AGA CAA CCA GGA TTA C (435–453) | NM_001098606 | 49 °C | 141 |
| antisense | TGG ATA AAG TTC CGA GTG (559–575) | ||||
| VPAC1 (VIPR1) | sense | GTT CTA TGG CAC GGT CAA (376–393) | NM_001097523 | 52 °C | 216 |
| antisense | AGC AAT GTT CGG GTT CTC (573–590) | ||||
| VPAC2 (VIPR2) | sense | TCG GAA CTA CAT CCA TCT (477–497) | NM_001014970 | 48 °C | 177 |
| antisense | TTT GCC ATA ACA CCA TAC (636–653) | ||||
| SHH (Shh) | sense | TCA GTG GCA GCG AAA TCA (787–804) | NM_204821.1 | 56 °C | 168 |
| antisense | CAT CCG GTC GAG GAA GGT (937–954) | ||||
| IHH (Ihh) | sense | TCG CCT ACA AGC AGT TCA GCC (455–475) | NM_204957.1 | 60 °C | 155 |
| antisense | GCC GGT GTT CTC CTC GTC CT (590–609) | ||||
| PTHrP (Pthlh) | sense | TAC GGA AGA TCA GTA GAG G (155–173) | NM_001174106.1 | 46 °C | 191 |
| antisense | GTA GCA GGC TTA GGG TTA (328–345) | ||||
| Smoothened (SMO) | sense | TCT GCT TCG TGG GTT ACA AG (843–862) | XM_414970.4 | 56 °C | 350 |
| antisense | TGG GAT GGG TTT ATT GGT CT (1173–1192) | ||||
| Gli1 (Gli1) | sense | CTC ACC CAC CCA GCA TCA G (2604–2622) | XM_004950861.1 | 58 °C | 244 |
| antisense | AAT CCC TCC TCC ATC TCC CT (2828–2847) | ||||
| Gli2 (Gli2) | sense | TTG CTC CAA GGC TTA CTC (1553–1570) | M_001271901.1 | 50 °C | 174 |
| antisense | TTA CAG ACA TAG GGT TTC TCA T (1705–1726) | ||||
| Gli3 (Gli3) | sense | TCA CCC GTA CAT TAA CCC (579–596) | NM_001271903.1 | 52 °C | 270 |
| antisense | CTT GGA CTC GGA AAC CTG (831–848) | ||||
| GAPDH (GAPDH) | sense | GAG AAC GGG AAA CTT GTC AT (238–258) | NM_204305 | 54 °C | 556 |
| antisense | GGC AGG TCA GGT CAA CAA (775–793) |
| Antibody | Host Animal | Dilution | Distributor and Cat No. |
|---|---|---|---|
| Anti-PAC1 | rabbit, polyclonal, | 1:600 | Sigma-Aldrich, St. Louis, MO, USA; P8872 |
| Anti-VPAC1 | rabbit, polyclonal, | 1:800 | Alomone Labs., Jerusalem, Israel; AVR-001 |
| Anti-VPAC2 | rabbit, polyclonal, | 1:600 | Abcam, Camridge, UK; ab28624 |
| Anti-Coll. II. | rabbit, polyclonal, | 1:100 | Novus Biologicals, Littleton, CO, USA; NB600-844 |
| Anti-Coll. IX. | rabbit, polyclonal, | 1:800 | Abcam, Camridge, UK; ab134568 |
| Anti-Coll. X. | rabbit, polyclonal, | 1:800 | Sigma-Aldrich, St. Louis, MO, USA; C7974 |
| Anti-SHH | rabbit, polyclonal, | 1:600 | Cell Signaling, Danvers, MA, USA; C9C5 |
| Anti-IHH | rabbit, polyclonal, | 1:600 | Millipore, Billerica, MA, USA; MABF23 |
| Anti-PTHrP | mouse, monoclonal, #677939 | 1:300 | R&D Systems, Minneapolis, MN, USA; MAB6734 |
| Anti-Smoothened | mouse, monoclonal, #2D10 | 1:500 | Sigma-Aldrich, St. Louis, MO, USA; SAB1412475 |
| Anti-Gli1 | rabbit, polyclonal, | 1:600 | Cell Signaling, Danvers, MA, USA; V812 |
| Anti-Gli2 | rabbit, polyclonal, | 1:500 | Sigma-Aldrich, St. Louis, MO, USA; SAB2900411 |
| Anti-Gli3 | rabbit, polyclonal, | 1:500 | Sigma-Aldrich, St. Louis, MO, USA; HAP005534 |
| Anti-Actin | mouse, monoclonal, #AC-15 | 1:10,000 | Sigma-Aldrich, St. Louis, MO, USA; A5441 |
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP response element-binding protein |
| DMMB | dimethylmethylene blue |
| ECM | extracellular matrix |
| HH | hedgehog |
| IHH | Indian hedgehog |
| MAPK | mitogen-activated protein kinase |
| NMDA | N-Methyl-d-aspartate |
| PAC1 | pituitary adenylate cyclase-activating polypeptide type I receptor |
| PACAP | pituitary adenylate cyclase polypeptide |
| PKA | protein kinase A |
| PTHrP | parathyroid hormone related peptide |
| Runx2 | Runt-related transcription factor 2 |
| SHH | Sonic hedgehog |
| TB | toluidine blue |
| TGFβ | transforming growth factor-β |
| TRPV | transient receptor potential channels for vanilloid |
| VIP | vasoactive intestinal peptide |
| VPAC | vasoactive intestinal peptide receptor |
References
- Sun, H.B. Mechanical loading, cartilage degradation, and arthritis. Ann. N. Y. Acad. Sci. 2010, 1211, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Nowlan, N.C.; Sharpe, J.; Roddy, K.A.; Prendergast, P.J.; Murphy, P. Mechanobiology of embryonic skeletal development: Insights from animal models. Birth Defects Res. Part C Embryo Today 2010, 90, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.S.; McGonnell, I.M.; Pitsillides, A.A. Mechanoadaptation of developing limbs: Shaking a leg. J. Anat. 2014, 224, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Knight, M.M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J. Orthop. Res. 2010, 28, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Orazizadeh, M.; Cartlidge, C.; Wright, M.O.; Millward-Sadler, S.J.; Nieman, J.; Halliday, B.P.; Lee, H.S.; Salter, D.M. Mechanical responses and integrin associated protein expression by human ankle chondrocytes. Biorheology 2006, 43, 249–258. [Google Scholar] [PubMed]
- Wright, M.; Jobanputra, P.; Bavington, C.; Salter, D.M.; Nuki, G. Effects of intermittent pressure-induced strain on the electrophysiology of cultured human chondrocytes: Evidence for the presence of stretch-activated membrane ion channels. Clin. Sci. 1996, 90, 61–71. [Google Scholar] [PubMed]
- Guilak, F.; Leddy, H.A.; Liedtke, W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann. N. Y. Acad. Sci. 2010, 1192, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Salter, D.M.; Wright, M.O.; Millward-Sadler, S.J. NMDA receptor expression and roles in human articular chondrocyte mechanotransduction. Biorheology 2004, 41, 273–281. [Google Scholar] [PubMed]
- Juhasz, T.; Matta, C.; Somogyi, C.; Katona, E.; Takacs, R.; Soha, R.F.; Szabo, I.A.; Cserhati, C.; Szody, R.; Karacsonyi, Z.; et al. Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell Signal. 2014, 26, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, Y.; Chen, Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J. Biol. Chem. 2001, 276, 35290–35296. [Google Scholar] [CrossRef] [PubMed]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Csanaky, K.; Banki, E.; Szabadfi, K.; Reglodi, D.; Tarcai, I.; Czegledi, L.; Helyes, Z.; Ertl, T.; Gyarmati, J.; Szanto, Z.; et al. Changes in PACAP immunoreactivity in human milk and presence of PAC1 receptor in mammary gland during lactation. J. Mol. Neurosci. 2012, 48, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Koppan, M.; Varnagy, A.; Reglodi, D.; Brubel, R.; Nemeth, J.; Tamas, A.; Mark, L.; Bodis, J. Correlation between oocyte number and follicular fluid concentration of pituitary adenylate cyclase-activating polypeptide (PACAP) in women after superovulation treatment. J. Mol. Neurosci. 2012, 48, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakamachi, T.; Endo, K.; Ito, K.; Machida, T.; Oka, T.; Hori, M.; Ishizaka, K.; Shioda, S. Distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) in the human testis and in testicular germ cell tumors. Andrologia 2014, 46, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, S.; Chatenet, D.; Wurtz, O.; Doan, N.D.; Leprince, J.; Vaudry, H.; Fournier, A.; Vaudry, D. Strategies to convert PACAP from a hypophysiotropic neurohormone into a neuroprotective drug. Curr. Pharm. Des. 2011, 17, 1002–1024. [Google Scholar] [CrossRef] [PubMed]
- Gourlet, P.; Vandermeers, A.; Vertongen, P.; Rathe, J.; De, N.P.; Cnudde, J.; Waelbroeck, M.; Robberecht, P. Development of high affinity selective VIP1 receptor agonists. Peptides 1997, 18, 1539–1545. [Google Scholar] [CrossRef]
- Pavelock, K.A.; Girard, B.M.; Schutz, K.C.; Braas, K.M.; May, V. Bone morphogenetic protein down-regulation of neuronal pituitary adenylate cyclase-activating polypeptide and reciprocal effects on vasoactive intestinal peptide expression. J. Neurochem. 2007, 100, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, K.; Romanin, C.; Baumgartner, W.; Groschner, K. Intracellular Ca2+ inhibits smooth muscle L-type Ca2+ channels by activation of protein phosphatase type 2B and by direct interaction with the channel. J. Gen. Physiol. 1997, 110, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Danyadi, B.; Bognar, E.; Szabadfi, K.; Fabian, E.; Kiss, P.; Mester, L.; Manavalan, S.; Atlasz, T.; Gabriel, R.; et al. Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neurosci. Lett. 2012, 523, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shibuya, I.; Uezono, Y.; Ueta, Y.; Toyohira, Y.; Yanagihara, N.; Izumi, F.; Kanno, T.; Yamashita, H. Pituitary adenylate cyclase-activating polypeptide causes Ca2+ release from ryanodine/caffeine stores through a novel pathway independent of both inositol trisphosphates and cyclic AMP in bovine adrenal medullary cells. J. Neurochem. 1998, 70, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, T.; Matta, C.; Katona, E.; Somogyi, C.; Takacs, R.; Gergely, P.; Csernoch, L.; Panyi, G.; Toth, G.; Reglodi, D.; et al. Pituitary adenylate cyclase activating polypeptide (PACAP) signalling exerts chondrogenesis promoting and protecting effects: Implication of calcineurin as a downstream target. PLoS ONE 2014, 9, e91541. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, T.; Matta, C.; Katona, E.; Somogyi, C.; Takacs, R.; Hajdu, T.; Helgadottir, S.L.; Fodor, J.; Csernoch, L.; Toth, G.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Signalling Enhances Osteogenesis in UMR-106 Cell Line. J. Mol. Neurosci. 2014, 54, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Racz, B.; Gallyas, F.; Tamas, A.; Gaal, V.; Marton, Z.; Gabriel, R.; Reglodi, D. Pituitary adenylate cyclase activating polypeptide in the retina: Focus on the retinoprotective effects. Ann. N. Y. Acad. Sci. 2010, 1200, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Banki, E.; Kovacs, K.; Nagy, D.; Juhasz, T.; Degrell, P.; Csanaky, K.; Kiss, P.; Jancso, G.; Toth, G.; Tamas, A.; et al. Molecular mechanisms underlying the Nephroprotective effects of PACAP in diabetes. J. Mol. Neurosci. 2014, 54, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botz, B.; Bolcskei, K.; Kereskai, L.; Kovacs, M.; Nemeth, T.; Szigeti, K.; Horvath, I.; Mathe, D.; Kovacs, N.; Hashimoto, H.; et al. Differential regulatory role of pituitary adenylate cyclase-activating polypeptide in the serum-transfer arthritis model. Arthritis Rheumatol. 2014, 66, 2739–2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimesaat, M.M.; Dunay, I.R.; Schulze, S.; Fischer, A.; Grundmann, U.; Alutis, M.; Kuhl, A.A.; Tamas, A.; Toth, G.; Dunay, M.P.; et al. Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS ONE 2014, 9, e108389. [Google Scholar] [CrossRef] [PubMed]
- Somogyvari-Vigh, A.; Reglodi, D. Pituitary adenylate cyclase activating polypeptide: A potential neuroprotective peptide. Curr. Pharm. Des. 2004, 10, 2861–2889. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, T.; Helgadottir, S.L.; Tamas, A.; Reglodi, D.; Zakany, R. PACAP and VIP signaling in chondrogenesis and osteogenesis. Peptides 2015, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Persson, E.; Lerner, U.H. The neuropeptide VIP potentiates IL-6 production induced by proinflammatory osteotropic cytokines in calvarial osteoblasts and the osteoblastic cell line MC3T3-E1. Biochem. Biophys. Res. Commun. 2005, 335, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Fodor, J.; Szijgyarto, Z.; Juhasz, T.; Gergely, P.; Csernoch, L.; Zakany, R. Cytosolic free Ca2+ concentration exhibits a characteristic temporal pattern during in vitro cartilage differentiation: A possible regulatory role of calcineurin in Ca-signalling of chondrogenic cells. Cell Calcium 2008, 44, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A.; Cicco-Bloom, E.; Nicot, A.; Lelievre, V. Hedgehog signaling: New targets for GPCRs coupled to cAMP and protein kinase A. Ann. N. Y. Acad. Sci. 2006, 1070, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martin, M.C.; Mallo, M.; Ros, M.A. Long bone development requires a threshold of Hox function. Dev. Biol. 2014, 392, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Reiter, J.F. A central region of Gli2 regulates its localization to the primary cilium and transcriptional activity. J. Cell Sci. 2014, 127, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Nowlan, N.C.; Dumas, G.; Tajbakhsh, S.; Prendergast, P.J.; Murphy, P. Biophysical stimuli induced by passive movements compensate for lack of skeletal muscle during embryonic skeletogenesis. Biomech. Model. Mechanobiol. 2012, 11, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, P.A.; Suchyna, T.M.; Ostrow, L.W.; Sachs, F. Mechanosensitive ion channels as drug targets. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.C.; Lopez-Zapata, D.F.; Wilkins, R.J. TRPV4 channels activity in bovine articular chondrocytes: Regulation by obesity-associated mediators. Cell Calcium 2014, 56, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Mobasheri, A. Beta1-integrins co-localize with Na, K-ATPase, epithelial sodium channels (ENaC) and voltage activated calcium channels (VACC) in mechanoreceptor complexes of mouse limb-bud chondrocytes. Histol. Histopathol. 2003, 18, 343–351. [Google Scholar] [PubMed]
- Karimi, T.; Barati, D.; Karaman, O.; Moeinzadeh, S.; Jabbari, E. A developmentally inspired combined mechanical and biochemical signaling approach on zonal lineage commitment of mesenchymal stem cells in articular cartilage regeneration. Integr. Biol. 2015, 7, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Khoshgoftar, M.; Wilson, W.; Ito, K.; van Donkelaar, C.C. Influence of the temporal deposition of extracellular matrix on the mechanical properties of tissue-engineered cartilage. Tissue Eng. Part A 2014, 20, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, L.; Wang, F.; Zhu, Q.; Yang, L. Contribution of PTHrP to mechanical strain-induced fibrochondrogenic differentiation in entheses of Achilles tendon of miniature pigs. J. Biomech. 2014, 47, 2406–2414. [Google Scholar] [CrossRef] [PubMed]
- Brifault, C.; Gras, M.; Liot, D.; May, V.; Vaudry, D.; Wurtz, O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing M2 microglia/macrophage polarization. Stroke 2015, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Szabo, A.; Kiss, P.; Reglodi, D.; Setalo, G., Jr.; Kovacs, K.; Tamas, A.; Toth, G.; Gabriel, R.; et al. PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem. Int. 2014, 64, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef] [PubMed]
- Winding, B.; Wiltink, A.; Foged, N.T. Pituitary adenylyl cyclase-activating polypeptides and vasoactive intestinal peptide inhibit bone resorption by isolated rabbit osteoclasts. Exp. Physiol. 1997, 82, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.M.; Kwag, J.H.; Kim, K.H.; Kim, C.H. Effects of neuropeptides and mechanical loading on bone cell resorption in vitro. Int. J. Mol. Sci. 2014, 15, 5874–5883. [Google Scholar] [CrossRef] [PubMed]
- Burdan, F.; Szumilo, J.; Korobowicz, A.; Farooquee, R.; Patel, S.; Patel, A.; Dave, A.; Szumilo, M.; Solecki, M.; Klepacz, R.; et al. Morphology and physiology of the epiphyseal growth plate. Folia Histochem. Cytobiol. 2009, 47, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Demers, C.N.; Girard-Lauriault, P.L.; Stachura, D.; Wertheimer, M.R.; Antoniou, J.; Mwale, F. Effect of nitrogen-rich cell culture surfaces on type X collagen expression by bovine growth plate chondrocytes. Biomed. Eng. Online 2011, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Thomas, A.; Dudhia, J.; Bayliss, M.T.; Kadler, K.E.; Duance, V.C. Modification of the composition of articular cartilage collagen fibrils with increasing age. Connect. Tissue Res. 2008, 49, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Duan, L.; Zhu, W.; Xiong, J.; Wang, D. Extracellular matrix production in vitro in cartilage tissue engineering. J. Transl. Med. 2014, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.W.; Friel, N.A.; Williams, J.M.; Cole, B.J.; Wimmer, M.A. Effect of impaction sequence on osteochondral graft damage: The role of repeated and varying loads. Am. J. Sports Med. 2010, 38, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Bries, A.D.; Weiner, D.S.; Jacquet, R.; Adamczyk, M.J.; Morscher, M.A.; Lowder, E.; Askew, M.J.; Steiner, R.P.; Horne, W.I.; Landis, W.J. A study in vivo of the effects of a static compressive load on the proximal tibial physis in rabbits. J. Bone Jt. Surg. Am. 2012, 94. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Juhasz, T.; Matta, C.; Fodor, J.; Katona, E.; Bartok, A.; Olah, T.; Sebe, A.; Csernoch, L.; Panyi, G.; et al. Switch of voltage-gated K+ channel expression in the plasma membrane of chondrogenic cells affects cytosolic Ca2+-oscillations and cartilage formation. PLoS ONE 2011, 6, e27957. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H.; Persson, E. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J. Musculoskelet. Neuronal. Interact. 2008, 8, 154–165. [Google Scholar] [PubMed]
- Shioda, S.; Ohtaki, H.; Nakamachi, T.; Dohi, K.; Watanabe, J.; Nakajo, S.; Arata, S.; Kitamura, S.; Okuda, H.; Takenoya, F.; et al. Pleiotropic functions of PACAP in the CNS: Neuroprotection and neurodevelopment. Ann. N. Y. Acad. Sci. 2006, 1070, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Ehlen, H.W.; Buelens, L.A.; Vortkamp, A. Hedgehog signaling in skeletal development. Birth Defects Res. C. Embryo Today 2006, 78, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Niewiadomski, P.; Tse, G.; Chi, G.C.; Dong, H.; Lee, A.; Carpenter, E.M.; Waschek, J.A. Pituitary adenylyl cyclase-activating peptide counteracts hedgehog-dependent motor neuron production in mouse embryonic stem cell cultures. J. Neurosci. Res. 2011, 89, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, P.; Zhujiang, A.; Youssef, M.; Waschek, J.A. Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA. Cell Signal. 2013, 25, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ali, S.A.; Al-Jazrawe, M.; Kandel, R.; Wunder, J.S.; Alman, B.A. Primary cilia attenuate hedgehog signalling in neoplastic chondrocytes. Oncogene 2013, 32, 5388–5396. [Google Scholar] [CrossRef] [PubMed]
- Joeng, K.S.; Long, F. The Gli2 transcriptional activator is a crucial effector for IHH signaling in osteoblast development and cartilage vascularization. Development 2009, 136, 4177–4185. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Ramaswamy, G.; Serra, R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthr. Cartil. 2012, 20, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Jozsa, R.; Hollosy, T.; Tamas, A.; Toth, G.; Lengvari, I.; Reglodi, D. Pituitary adenylate cyclase activating polypeptide plays a role in olfactory memory formation in chicken. Peptides 2005, 26, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, T.; Szentléleky, E.; Somogyi, C.S.; Takács, R.; Dobrosi, N.; Engler, M.; Tamás, A.; Reglődi, D.; Zákány, R. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Pathway Is Induced by Mechanical Load and Reduces the Activity of Hedgehog Signaling in Chondrogenic Micromass Cell Cultures. Int. J. Mol. Sci. 2015, 16, 17344-17367. https://doi.org/10.3390/ijms160817344
Juhász T, Szentléleky E, Somogyi CS, Takács R, Dobrosi N, Engler M, Tamás A, Reglődi D, Zákány R. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Pathway Is Induced by Mechanical Load and Reduces the Activity of Hedgehog Signaling in Chondrogenic Micromass Cell Cultures. International Journal of Molecular Sciences. 2015; 16(8):17344-17367. https://doi.org/10.3390/ijms160817344
Chicago/Turabian StyleJuhász, Tamás, Eszter Szentléleky, Csilla Szűcs Somogyi, Roland Takács, Nóra Dobrosi, Máté Engler, Andrea Tamás, Dóra Reglődi, and Róza Zákány. 2015. "Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Pathway Is Induced by Mechanical Load and Reduces the Activity of Hedgehog Signaling in Chondrogenic Micromass Cell Cultures" International Journal of Molecular Sciences 16, no. 8: 17344-17367. https://doi.org/10.3390/ijms160817344
APA StyleJuhász, T., Szentléleky, E., Somogyi, C. S., Takács, R., Dobrosi, N., Engler, M., Tamás, A., Reglődi, D., & Zákány, R. (2015). Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Pathway Is Induced by Mechanical Load and Reduces the Activity of Hedgehog Signaling in Chondrogenic Micromass Cell Cultures. International Journal of Molecular Sciences, 16(8), 17344-17367. https://doi.org/10.3390/ijms160817344








