Mapping of Powdery Mildew Resistance Gene pmCH89 in a Putative Wheat-Thinopyrum intermedium Introgression Line
Abstract
:1. Introduction
2. Results
2.1. Powdery Mildew Responses
| Line | Bgt Pathotype | ||||||
|---|---|---|---|---|---|---|---|
| E09 | E20 | E21 | E23 | E26 | Bg1 | Bg2 | |
| Th. intermedium Z1141 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TAI7045 | 0 | 0 | 0; | 0 | 0; | 0 | 0 |
| Jinchun 5 a | 4 | 4 | 4 | 4 | 3 | 4 | 4 |
| Jin T2250 a | 4 | 4 | 3 | 4 | 4 | 4 | 4 |
| CH09W89 | 0, 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Jintai 170 b | 4 | 4 | 3 | 4 | 4 | 4 | 4 |
| Jinmai 33 b | 4 | 4 | 4 | 4 | 3 | 4 | 4 |
| SY95-71 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Mianyang 11 | 4 | 4 | 4 | 4 | 4 | 3 | 4 |
| Jingshuang 16 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
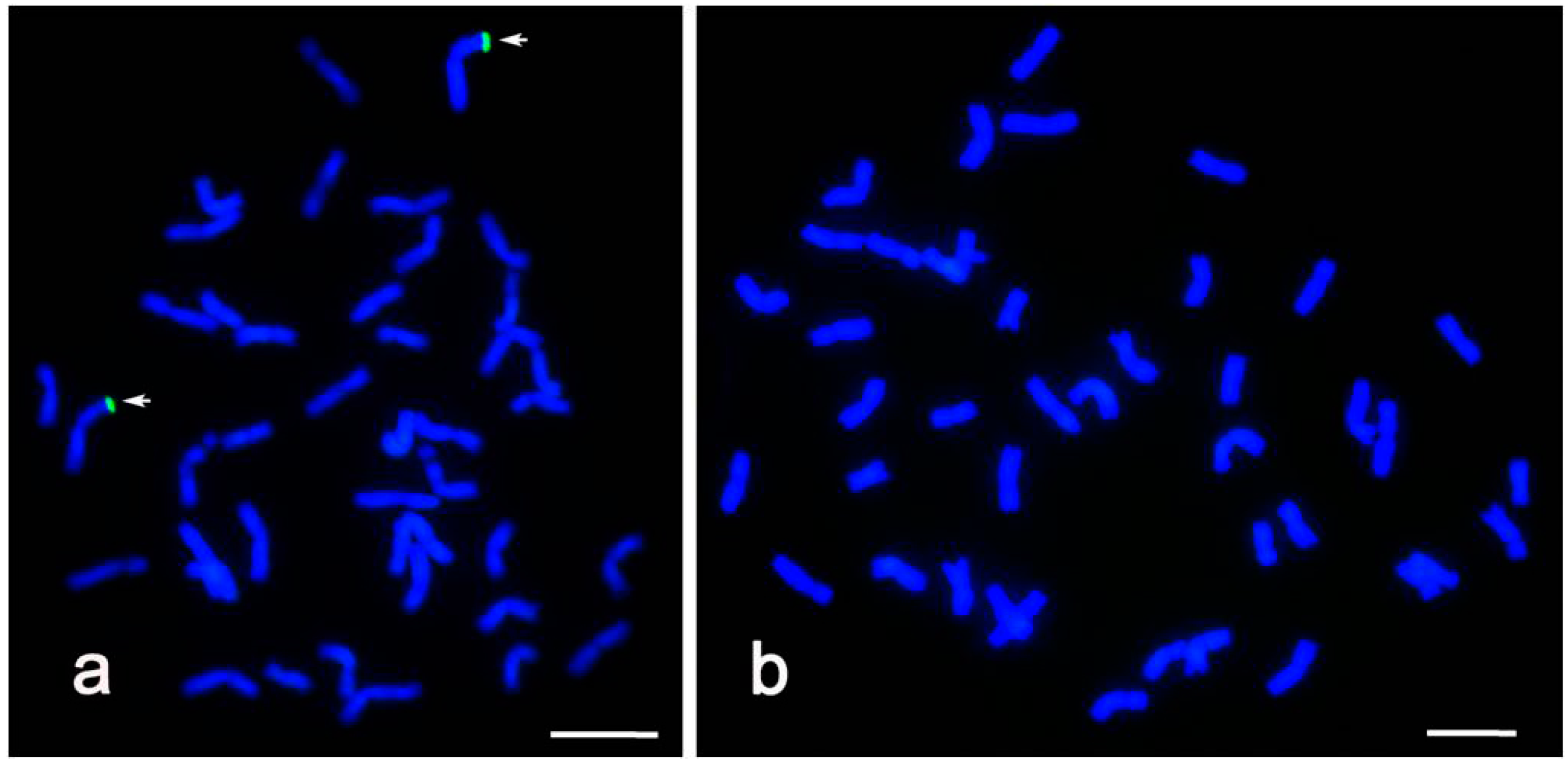
2.2. GISH Identification of Alien Chromatin in CH09W89
2.3. Inheritance of the Resistance to Powdery Mildew in CH09W89
| IT | Parent | P2/P1 a | P1/P3 | P1/P3//P1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Plants | No. of Lines | No. of Plants | No. of Plants | |||||||
| P1 | P2 | P3 | F1 | F2 | F2:3 b | F1 | BC1 | |||
| HR | Seg | HS | ||||||||
| 0 | 15 | 11 | 9 c | 0 | 0 | 7 | ||||
| 0 | 3 | 23 | 23 | 0 | 0 | 25 | ||||
| 1 | 9 | 8 c | 0 | 0 | 4 | |||||
| 2 | 3 | 3 | 0 | 0 | 1 | |||||
| 3 | 3 | 78 | 0 | 62 | 16 | 5 | 24 | |||
| 4 | 16 | 17 | 15 | 51 | 0 | 14 | 31 d | 12 | 17 | |
| Total | 18 | 16 | 17 | 18 | 175 | 43 | 76 | 47 | 17 | 78 |
| χ2 e (3:1) = 0.15 | χ2(1:2:1) = 1.37 | χ2(1:1) = 0.21 | ||||||||
| p = 0.69 | p = 0.50 | p = 0.65 | ||||||||

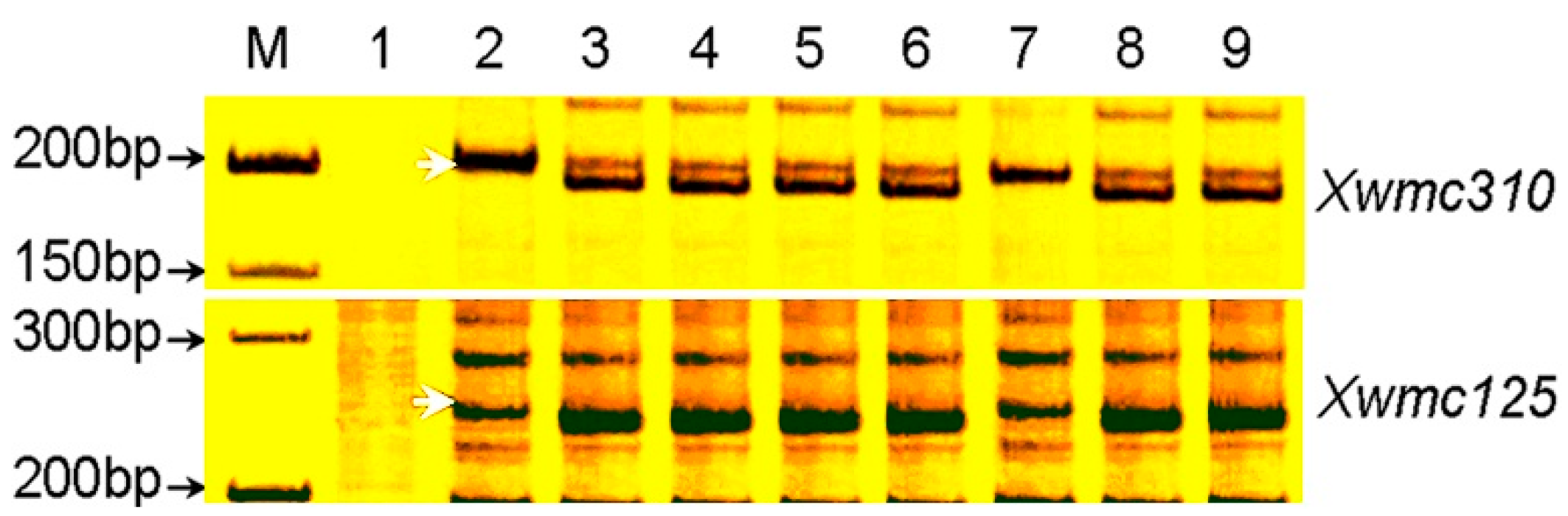
2.4. Identification of Microsatellite Markers Linked to pmCH89
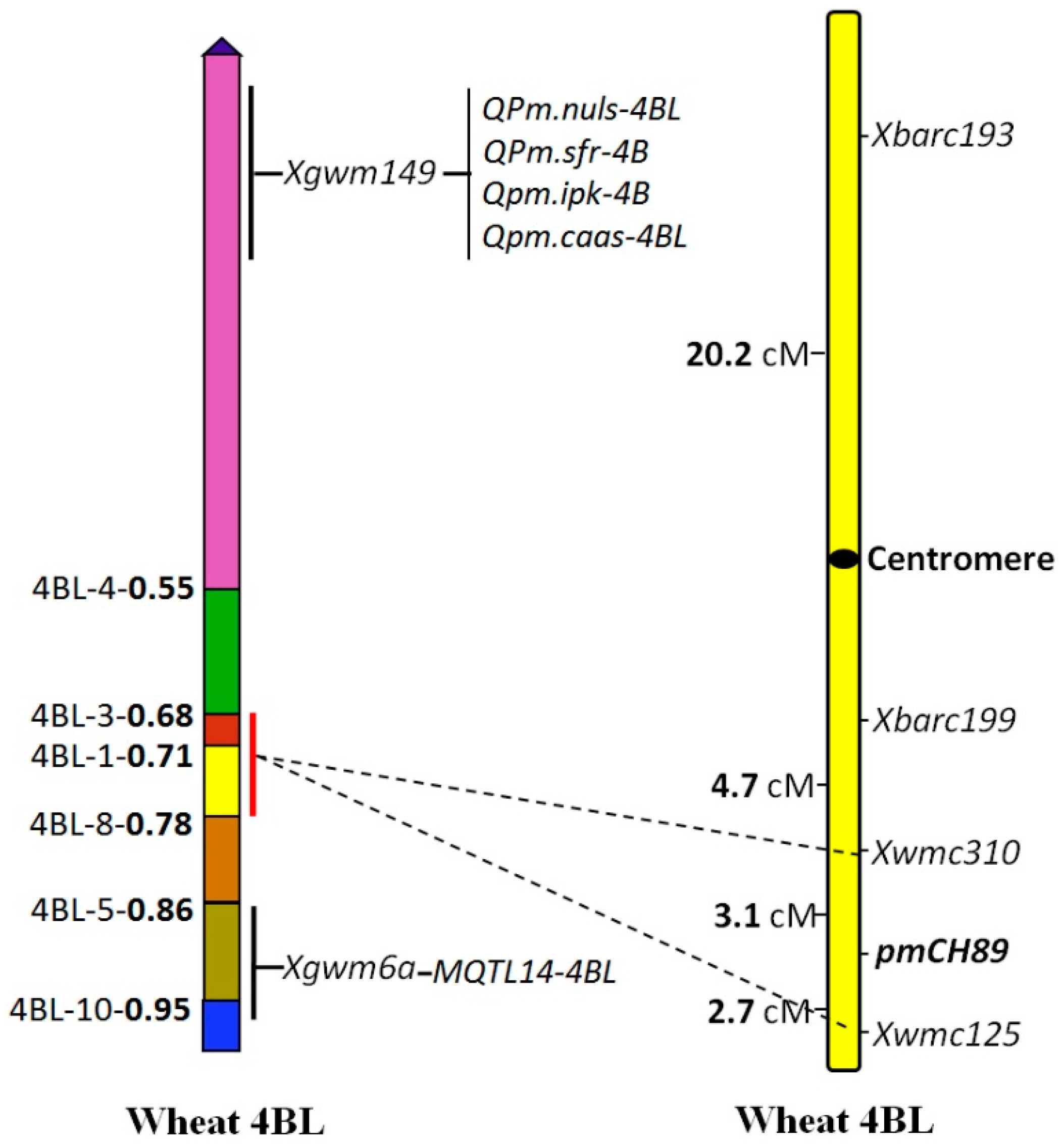
2.5. Chromosome Arm Assignment and Deletion Bin Mapping
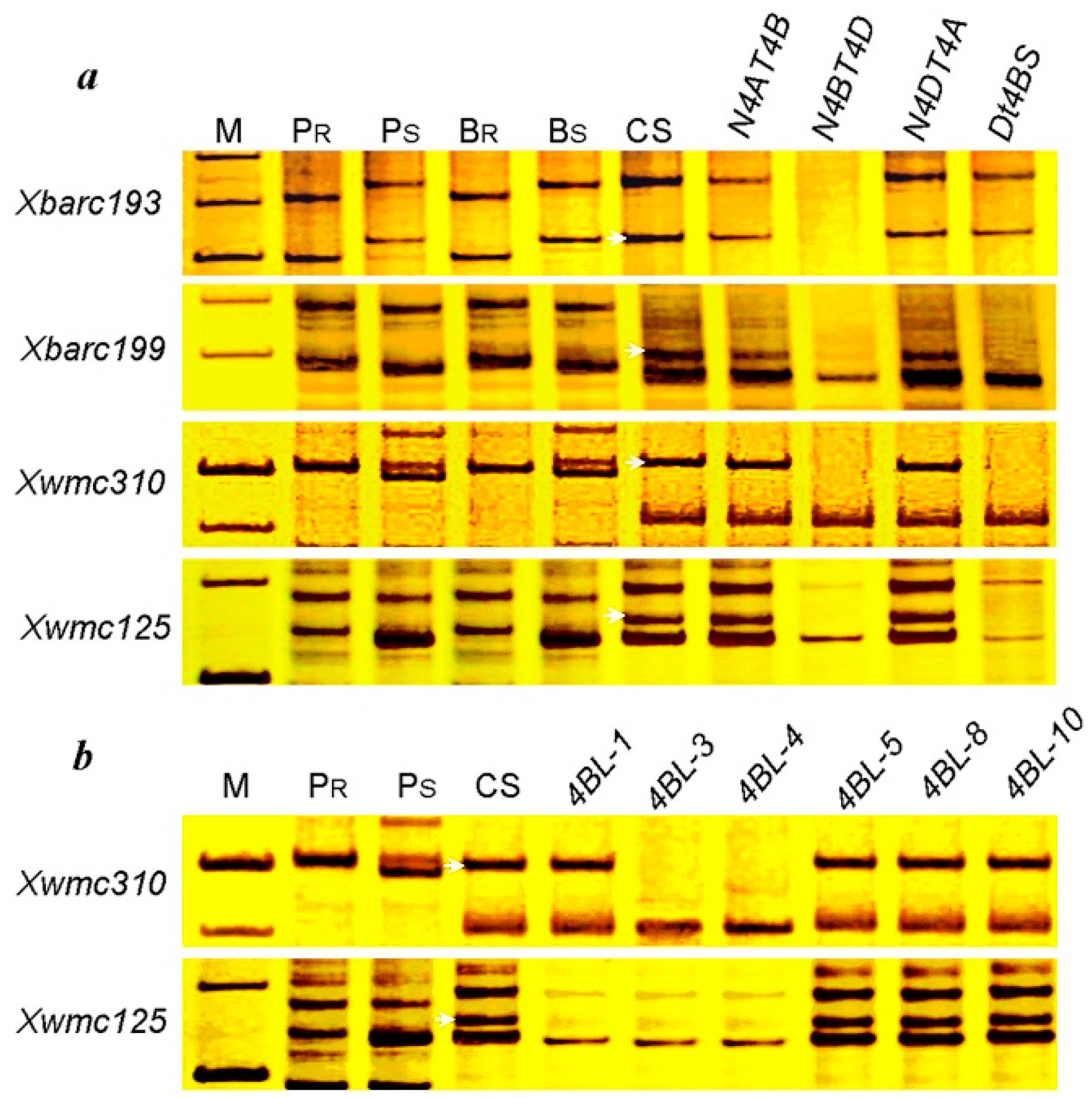
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Cytogenetic Analysis
4.3. Evaluation of Powdery Mildew Responses
4.4. Molecular Marker Analysis
4.5. Data Analysis and Chromosomal Assignment
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhuang, Q.S.; Li, Z.S. Present status of wheat breeding and related genetic study in China. Wheat Inf. Serv. 1993, 76, 1–5. [Google Scholar]
- Morgounov, A.; Tufan, H.A.; Sharma, R.; Akin, B.; Bagei, A.; Braun, H.J.; Kaya, Y.; Keser, M.; Payne, T.S.; Sonder, K.; et al. Global incidence of wheat rusts and powdery mildew during 1969–2010 and durability of resistance of winter wheat variety Bezostaya 1. Eur. J. Plant Pathol. 2012, 132, 323–340. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Sun, H.G.; Song, W.; Lu, M.; Huang, J.; Wu, L.F.; Wang, X.M.; Li, H.J. Genetic analysis and detection of the gene MlLX99 on chromosome 2BL conferring resistance to powdery mildew in the wheat cultivar Liangxing 99. Theor. Appl. Genet. 2013, 126, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Line, R.F.; Chen, X.M. Success in breeding for and managing durable resistance to wheat rusts. Plant Dis. 1995, 79, 1254–1255. [Google Scholar]
- Zhan, H.X.; Li, G.R.; Zhang, X.J.; LI, X.; Guo, H.J.; Gong, W.P.; Jia, J.Q.; Qiao, L.Y. Chromosomal location and comparative genomics analysis of powdery mildew resistance gene Pm51 in a putative wheat-Thinopyrum ponticum introgression line. PLoS ONE 2014, 9, e113455. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Lyerly, J.H.; Worthington, M.L.; Parks, W.R.; Cowger, C.; Marshall, D.S.; Brown-Guedira, G.; Murphy, J.P. Mapping of powdery mildew resistance gene Pm53 introgressed from Aegilops speltoides into soft red winter wheat. Theor. Appl. Genet. 2015, 128, 303–312. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Yamazaki, Y.Y.; Dubcovsky, J.; Rogers, J.; Morris, C.; Appels, R.; Xia, X.C. Gene Catalogue 2013: Catalogue of gene symbols for wheat. 12th International Wheat Genetics Symposium: Yokohama, Japan, 2013. Available online: http://www.shigen.nig.ac.jp/wheat/komugi/genes/macgene/2013/GeneSymbol.pdf (accessed on 8 June 2015).
- Li, Z.F.; Lan, C.X.; He, Z.H.; Singh, R.P.; Rosewarne, G.M.; Chen, X.M.; Xia, X.C. Overview and application of QTL for adult plant resistance to leaf rust and powdery mildew in wheat. Crop Sci. 2014, 54, 1907–1925. [Google Scholar] [CrossRef]
- Marone, D.; Russo, M.A.; Laidò, G.; Vita, P.D.; Papq, R.; Blanco, A.; Gadaleta, A.; Rubiales, D.; Mastrangelo, A.M. Genetic basis of qualitative and quantitative resistance to powdery mildew in wheat: From consensus regions to candidate genes. BMC Genomics 2013, 14, 562. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.G.; Luo, H.Y.; Chang, Z.J.; Zhang, H.Y.; Zhang, M.; Ren, Z.L. Characterization and chromosomal location of Pm40 in common wheat: A new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 2009, 118, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- He, R.L.; Chang, Z.J.; Yang, Z.J.; Zhan, H.X.; Zhang, X.J.; Liu, J.X. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor. Appl. Genet. 2009, 118, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Liu, J.; Chang, Z.J.; Zhang, X.J.; Yang, Z.J.; Li, X.; Jia, J.Q.; Zhan, H.X.; Guo, H.J.; Wang, J.M. Putative Thinopyrum intermedium-derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor. Appl. Genet. 2013, 126, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.J.; Isaac, P.; Edwards, K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.J.; Zhang, X.J.; Yang, Z.J.; Zhan, H.X.; Li, X.; Liu, C.; Zhang, C.Z. Characterization of a partial wheat-Thinopyrum intermedium amphiploid and its reaction to fungal diseases of wheat. Hereditas 2010, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Wang, H.G. Characterization of a wheat-Thinopyron intermedium substitution line with resistance to powdery mildew. Euphytica 2005, 143, 229–233. [Google Scholar]
- Ayala-Navarrete, L.; Thompson, N.; Ohm, H.; Anderson, J. Molecular markers show a complex mosaic pattern of wheat-Thinopyrum intermedium translocations carrying resistance to BYDV. Theor. Appl. Genet. 2010, 121, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Zhang, Y.; Lin, Z.S.; Ye, X.G.; Yuan, Y.P.; Ma, W.; Xin, Z.Y. Development of EST-PCR markers for Thinopyrum intermedium chromosome 2Ai#2 and their application in characterization of novel wheat-grass recombinants. Theor. Appl. Genet. 2010, 121, 1369–1380. [Google Scholar] [PubMed]
- Tang, X.Q.; Shi, D.; Xu, J.; Li, Y.L.; Li, W.J.; Ren, Z.L.; Fu, T.H. Molecular cytogenetic characteristics of a translocation line between common wheat and Thinopyrum intermedium with resistance to powdery mildew. Euphytica 2014, 197, 201–210. [Google Scholar] [CrossRef]
- Dong, Y.; Bu, X.; Luan, Y.; He, M.; Liu, B. Molecular characterization of a cryptic wheat-Thinopyrum intermedium translocation line: Evidence for genomic instability in nascent allopolyploid and aneuploid lines. Genet. Mol. Biol. 2004, 27, 237–241. [Google Scholar]
- Huang, Q.; Li, X.; Chen, W.Q.; Xiang, Z.P.; Zhong, S.F.; Chang, Z.J.; Zhang, M.; Zhang, H.Y.; Tan, F.Q.; Ren, Z.L.; et al. Genetic mapping of a putative Thinopyrum intermedium-derived stripe rust resistance gene on wheat chromosome 1B. Theor. Appl. Genet. 2014, 127, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M.E.; Pupilli, F.; Ceccarelli, M.; Vaccino, P.; Sarri, V.; Pace, C.D. Cryptic introgression of Dasypyrum villosum parental DNA in wheat lines derived from intergeneric hybridization. Cytogenet. Genome Res. 2012, 136, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.G.; Song, F.J.; Jiao, J.F.; Wang, X.M.; Xu, H.X.; Li, H.J. Identification of the gene Pm47 on chromosome 7BS conferring resistance to powdery mildew in the Chinese wheat landrace Hongyanglazi. Theor. Appl. Genet. 2013, 126, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Liu, Z.J.; Zhu, J.; Xie, C.J.; Yang, T.; Zhou, Y.L.; Duan, X.Y.; Sun, Q.X.; Liu, Z.Y. Identification and genetic mapping of Pm42, a new recessive wheat powdery mildew resistance gene derived from wild emmer (Triticum turgidum var. dicoccoides). Theor. Appl. Genet. 2009, 119, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Liu, D.; Zhang, H.; Huo, N.; Zhou, R.; Jia, J. Identification and mapping of two new genes conferring resistance to powdery mildew from Aegilops tauschii (Coss.) Schmal. J. Integr. Plant Biol. 2006, 48, 1204–1209. [Google Scholar] [CrossRef]
- Peng, F.X.; Song, N.; Shen, H.X.; Wu, H.B.; Dong, H.T.; Zhang, J.; Li, Y.H.; Peng, H.R.; Ni, Z.F.; Liu, Z.Y.; et al. Molecular mapping of a recessive powdery mildew resistance gene in spelt wheat cultivar Hubel. Mol. Breed. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Fu, B.S.; Chen, Y.; Li, N.; Ma, H.Q.; Kong, Z.X.; Zhang, L.X.; Jia, H.Y.; Ma, Z.Q. pmX: A recessive powdery mildew resistance gene at the Pm4 locus identified in wheat landrace Xiaohongpi. Theor. Appl. Genet. 2013, 1, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Lillemo, M.; Asalf, B.; Singh, R.P.; Huerta-Espino, J.; Chen, X.M.; He, Z.H.; Bjørnstad, A. The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor. Appl. Genet. 2008, 116, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Marone, D.; Laidò, G.; Gadaleta, A.; Colasuonno, P.; Ficco, D.B.; Giancaspro, A.; Giove, S.; Panio, G.; Russo, M.A.; de Vita, P.; et al. A high-density consensus map of A and B wheat genomes. Theor. Appl. Genet. 2012, 125, 1619–1638. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Devos, K.M.; Chao, S.; Miller, T.E.; Reader, S.M.; Gale, M.D. RFLP-based maps of the homoelogous group-6 chromosomes of wheat and their application in the tagging of Pm12, a powdery mildew resistance gene transferred from Aegilops speltoides to wheat. Theor. Appl. Genet. 1996, 92, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Jarve, K.; Peusha, H.O.; Tsymbalova, J.; Tamm, S.; Devos, K.M.; Enno, T.M. Chromosomal location of a Triticum timopheevii-derived powdery mildew resistance gene transferred to common wheat. Genome 2000, 43, 377–381. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.A.; Linde, C. The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica 2002, 124, 163–180. [Google Scholar] [CrossRef]
- Han, F.; Lamb, J.C.; Bircher, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.J.; Sheng, B.Q.; Zhou, Y.L.; Duan, X.Y.; Zhang, K.C. Analyses of resistance genes of three differential varieties to the isolates of Blumeria graminis f. sp. tritici in wheat. Acta Agric. Boreali-Sin. 1994, 9, 94–97. [Google Scholar]
- Xie, C.J.; Sun, Q.X.; Ni, Z.F.; Yang, T.; Nevo, E.; Fahima, T. Chromosomal location of a Triticum dicoccoides-derived powdery mildew resistance gene in common wheat by using microsatellite markers. Theor. Appl. Genet. 2003, 106, 341–345. [Google Scholar] [PubMed]
- Michelmore, R.M.; Paran, I.; Kesseli, R.V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. USA 1991, 88, 9828–9832. [Google Scholar] [CrossRef] [PubMed]
- Ooijen, J.W.; Voorips, R.E. JoinMap™ Version 3.0: Software for the Calculation of Genetic Linkage Maps; University and Research Center: Wageningen, The Netherlands, 2001. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Zhang, X.; Li, X.; Jia, J.; Yang, H.; Zhan, H.; Qiao, L.; Guo, H.; Chang, Z. Mapping of Powdery Mildew Resistance Gene pmCH89 in a Putative Wheat-Thinopyrum intermedium Introgression Line. Int. J. Mol. Sci. 2015, 16, 17231-17244. https://doi.org/10.3390/ijms160817231
Hou L, Zhang X, Li X, Jia J, Yang H, Zhan H, Qiao L, Guo H, Chang Z. Mapping of Powdery Mildew Resistance Gene pmCH89 in a Putative Wheat-Thinopyrum intermedium Introgression Line. International Journal of Molecular Sciences. 2015; 16(8):17231-17244. https://doi.org/10.3390/ijms160817231
Chicago/Turabian StyleHou, Liyuan, Xiaojun Zhang, Xin Li, Juqing Jia, Huizhen Yang, Haixian Zhan, Linyi Qiao, Huijuan Guo, and Zhijian Chang. 2015. "Mapping of Powdery Mildew Resistance Gene pmCH89 in a Putative Wheat-Thinopyrum intermedium Introgression Line" International Journal of Molecular Sciences 16, no. 8: 17231-17244. https://doi.org/10.3390/ijms160817231





