Murine K2P5.1 Deficiency Has No Impact on Autoimmune Neuroinflammation due to Compensatory K2P3.1- and KV1.3-Dependent Mechanisms
Abstract
:1. Introduction
2. Results
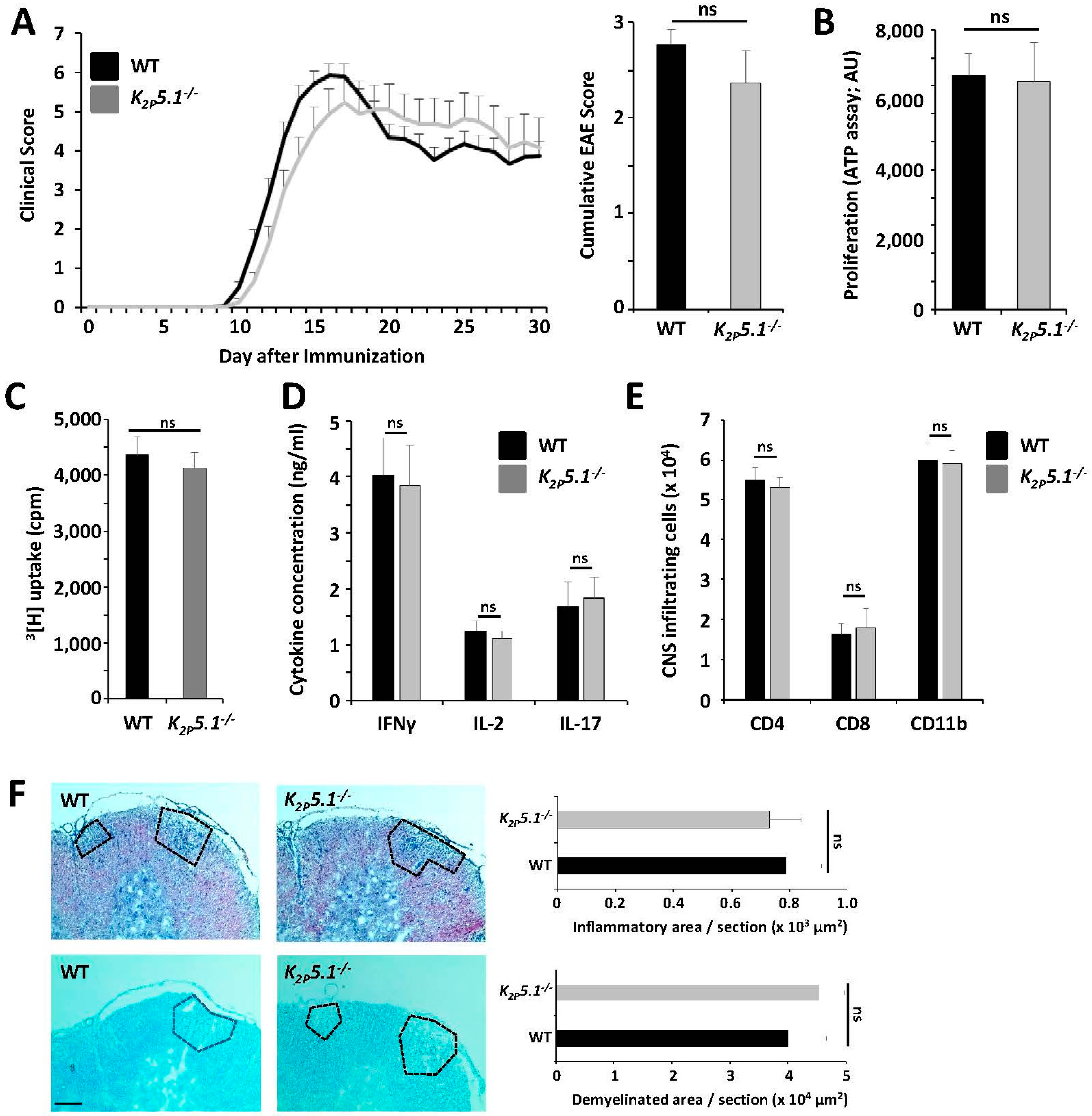
2.1. K2P5.1−/− and Wild-Type Mice Show a Comparable Disease Course in the EAE Model
2.2. K2P5.1−/− Mice Show No Obvious Alterations of the Immune System
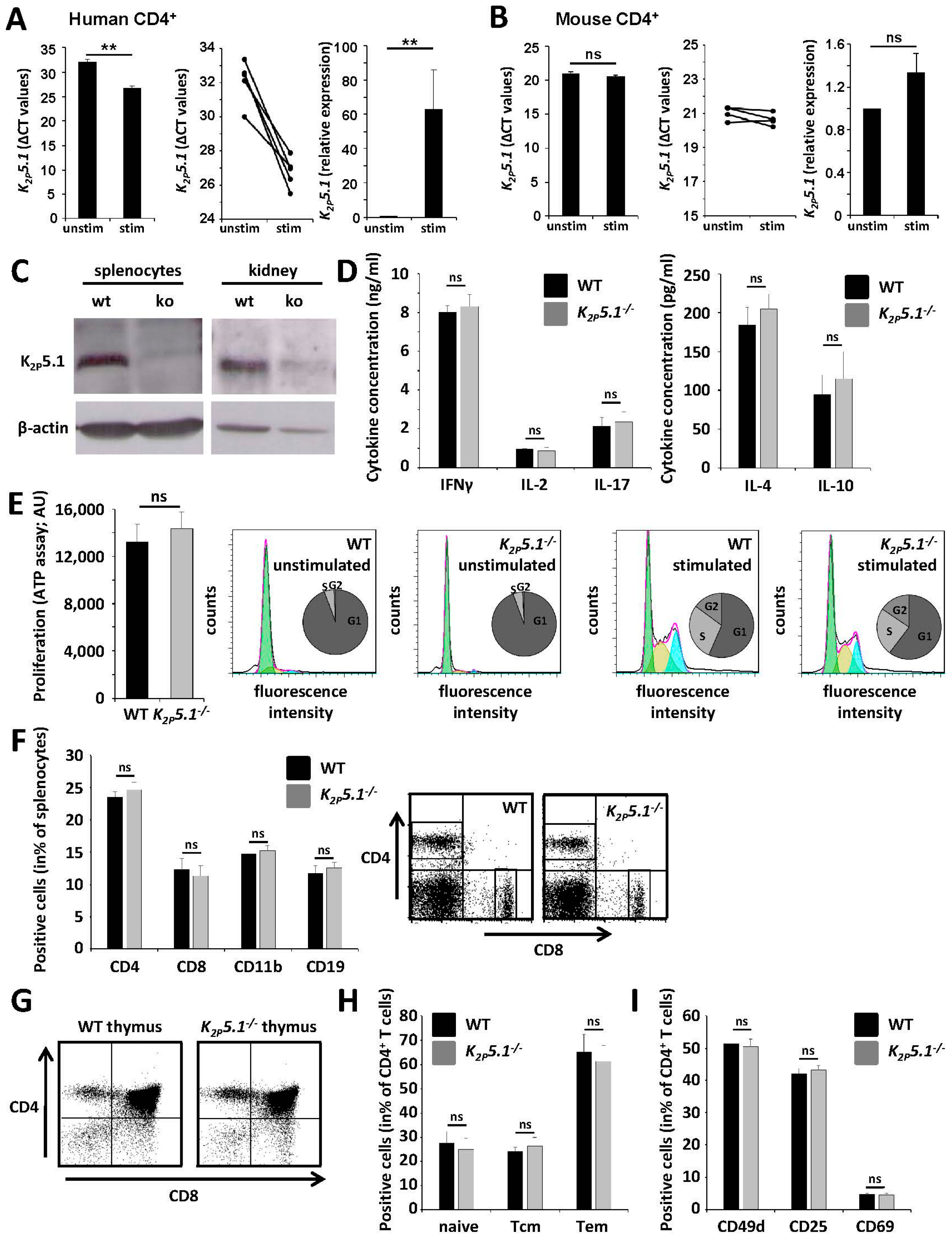
2.3. Compensatory Upregulation of K2P3.1 and KV1.3 in K2P5.1−/− T Lymphocytes


2.4. K2P5.1−/− T Lymphocytes Display No Alterations in TCR-Dependent Signaling Pathways
2.5. Common Marmoset T Lymphocytes Functionally Express K2P5.1
| Commercial Kit | Company | Functional | Not Functional |
|---|---|---|---|
| CD4 non-human primate MACS kit | Miltenyi Biotec | X | |
| CD8 non-human primate MACS kit | Miltenyi Biotec | X | |
| Mouse anti-human CD4 antibody (RPA-T4) | BioLegend | X | |
| Mouse anti-human CD8 antibody (RPA-T8) | BioLegend | X | |
| Human CD3/CD28 microbeads | Life Technologies | X | |
| Mouse CD3/CD28 microbeads | Life Technologies | X | |
| Phytohemagglutinin | Sigma-Aldrich | X | |
| ATP Assay | PerkinElmer | X | |
| Rabbit anti-human/mouse K2P5.1 | Sigma-Aldrich | X | |
| Cytokine flow cytomix | BenderMed Systems | X | |
| Human ELISA IFNγ, IL-2 | eBioscience, RD Systems | X | |
| Mouse ELISA IFNγ, IL-2 | eBioscience, RD Systems | X | |
| Human RT-PCR primers | Applied Biosystems | X | |
| Quinidine | Sigma-Aldrich | X |

3. Discussion
4. Experimental Section
4.1. Experimental Autoimmune Encephalomyelitis Induction and Evaluation
4.2. Murine Cell Isolation and Culture
4.3. Immunological Analysis
4.4. Immunohistochemical Analysis
4.5. Isolation of Human CD4+ T Lymphocytes
4.6. Flow Cytometry
4.7. Real-Time PCR
4.8. Electrophysiology
4.9. Western Blots
4.10. Calcium Imaging
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed]
- Ehling, P.; Bittner, S.; Budde, T.; Wiendl, H.; Meuth, S.G. Ion channels in autoimmune neurodegeneration. FEBS Lett. 2011, 585, 3836–3842. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, M.D.; Chandy, K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009, 231, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Meuth, S.G. Targeting ion channels for the treatment of autoimmune neuroinflammation. Ther. Adv. Neurol. Disord. 2013, 6, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Chandy, K.G.; Wulff, H.; Beeton, C.; Pennington, M.; Gutman, G.A.; Cahalan, M.D. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004, 25, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, P.; Czirjak, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Meuth, S.G.; Bittner, S.; Meuth, P.; Simon, O.J.; Budde, T.; Wiendl, H. TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 critically influence T lymphocyte effector functions. J. Biol. Chem. 2008, 283, 14559–14570. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Bauer, M.A.; Ehling, P.; Bobak, N.; Breuer, J.; Herrmann, A.M.; Golfels, M.; Wiendl, H.; Budde, T.; Meuth, S.G. The TASK1 channel inhibitor A293 shows efficacy in a mouse model of multiple sclerosis. Exp. Neurol. 2012, 238, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Meuth, S.G.; Gobel, K.; Melzer, N.; Herrmann, A.M.; Simon, O.J.; Weishaupt, A.; Budde, T.; Bayliss, D.A.; Bendszus, M.; et al. TASK1 modulates inflammation and neurodegeneration in autoimmune inflammation of the central nervous system. Brain 2009, 132, 2501–2516. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Bobak, N.; Herrmann, A.M.; Gobel, K.; Meuth, P.; Hohn, K.G.; Stenner, M.P.; Budde, T.; Wiendl, H.; Meuth, S.G. Upregulation of K2P5.1 potassium channels in multiple sclerosis. Ann. Neurol. 2010, 68, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Bobak, N.; Feuchtenberger, M.; Herrmann, A.M.; Gobel, K.; Kinne, R.W.; Hansen, A.J.; Budde, T.; Kleinschnitz, C.; Frey, O.; et al. Expression of K2P5.1 potassium channels on CD4+ T lymphocytes correlates with disease activity in rheumatoid arthritis patients. Arthritis Res. Ther. 2011, 13, R21. [Google Scholar] [CrossRef] [PubMed]
- Andronic, J.; Bobak, N.; Bittner, S.; Ehling, P.; Kleinschnitz, C.; Herrmann, A.M.; Zimmermann, H.; Sauer, M.; Wiendl, H.; Budde, T.; et al. Identification of two-pore domain potassium channels as potent modulators of osmotic volume regulation in human T lymphocytes. Biochim. Biophys. Acta 2013, 1828, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Bobak, N.; Bittner, S.; Andronic, J.; Hartmann, S.; Muhlpfordt, F.; Schneider-Hohendorf, T.; Wolf, K.; Schmelter, C.; Gobel, K.; Meuth, P.; et al. Volume regulation of murine T lymphocytes relies on voltage-dependent and two-pore domain potassium channels. Biochim. Biophys. Acta 2011, 1808, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lin, H.; Zheng, H.; Kim, K.S.; Kim, J.Y.; Chun, Y.S.; Park, J.W.; Nam, J.H.; Kim, W.K.; Zhang, Y.H.; et al. HIF-1α-mediated upregulation of TASK-2 K+ channels augments Ca2+ signaling in mouse B cells under hypoxia. J. Immunol. 2014, 193, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Shin, D.H.; Zheng, H.; Lee, D.S.; Park, S.J.; Park, K.S.; Kim, S.J. Expression of TASK-2 and its upregulation by B cell receptor stimulation in WEHI-231 mouse immature B cells. Am. J. Physiol. Cell. Physiol. 2011, 300, C1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Huse, M. The T-cell-receptor signaling network. J. Cell Sci. 2009, 122, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Bart, A.; Gran, B.; Weissert, R. EAE: Imperfect but useful models of multiple sclerosis. Trends Mol. Med. 2011, 17, 119–125. [Google Scholar]
- A’t Hart, B.; van Meurs, M.; Brok, H.P.; Massacesi, L.; Bauer, J.; Boon, L.; Bontrop, R.E.; Laman, J.D. A new primate model for multiple sclerosis in the common marmoset. Immunol. Today 2000, 21, 290–297. [Google Scholar] [CrossRef]
- Reyes, R.; Duprat, F.; Lesage, F.; Fink, M.; Salinas, M.; Farman, N.; Lazdunski, M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 1998, 273, 30863–30869. [Google Scholar] [CrossRef] [PubMed]
- Duprat, F.; Lesage, F.; Fink, M.; Reyes, R.; Heurteaux, C.; Lazdunski, M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997, 16, 5464–5471. [Google Scholar] [CrossRef] [PubMed]
- Cid, L.P.; Roa-Rojas, H.A.; Niemeyer, M.I.; Gonzalez, W.; Araki, M.; Araki, K.; Sepulveda, F.V. TASK-2: A K2P K+ channel with complex regulation and diverse physiological functions. Front. Physiol. 2013, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cayuqueo, K.I.; Pena-Munzenmayer, G.; Niemeyer, M.I.; Sepulveda, F.V.; Cid, L.P. TASK-2 K2P K+ channel: Thoughts about gating and its fitness to physiological function. Pflugers Arch. 2015, 467, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.; Barhanin, J. Molecular physiology of pH-sensitive background K(2P) channels. Physiology 2011, 26, 424–37. [Google Scholar] [CrossRef] [PubMed]
- Barriere, H.; Belfodil, R.; Rubera, I.; Tauc, M.; Lesage, F.; Poujeol, C.; Guy, N.; Barhanin, J.; Poujeol, P. Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J. Gen. Physiol. 2003, 122, 177–190. [Google Scholar] [CrossRef] [PubMed]
- L’Hoste, S.; Poet, M.; Duranton, C.; Belfodil, R.; e Barriere, H.; Rubera, I.; Tauc, M.; Poujeol, C.; Barhanin, J.; Poujeol, P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J. Biol. Chem. 2007, 282, 36692–36703. [Google Scholar] [CrossRef] [PubMed]
- Skatchkov, S.N.; Eaton, M.J.; Shuba, Y.M.; Kucheryavykh, Y.V.; Derst, C.; Veh, R.W.; Wurm, A.; Iandiev, I.; Pannicke, T.; Bringmann, A.; Reichenbach, A. Tandem-pore domain potassium channels are functionally expressed in retinal (Muller) glial cells. Glia 2006, 53, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Warth, R.; Barriere, H.; Meneton, P.; Bloch, M.; Thomas, J.; Tauc, M.; Heitzmann, D.; Romeo, E.; Verrey, F.; Mengual, R.; et al. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc. Natl. Acad. Sci. USA 2004, 101, 8215–8220. [Google Scholar] [CrossRef] [PubMed]
- Gestreau, C.; Heitzmann, D.; Thomas, J.; Dubreuil, V.; Bandulik, S.; Reichold, M.; Bendahhou, S.; Pierson, P.; Sterner, C.; Peyronnet-Roux, J.; et al. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Benamer, N.; Zanella, S.; Kumar, N.N.; Shi, Y.; Bevengut, M.; Penton, D.; Guyenet, P.G.; Lesage, F.; Gestreau, C.; et al. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J. Neurosci. 2013, 33, 16033–16044. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, D.A.; Barhanin, J.; Gestreau, C.; Guyenet, P.G. The role of pH-sensitive TASK channels in central respiratory chemoreception. Pflugers Arch. 2015, 467, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Gob, E.; Bittner, S.; Bobak, N.; Kraft, P.; Gobel, K.; Langhauser, F.; Homola, G.A.; Brede, M.; Budde, T.; Meuth, S.G.; et al. The two-pore domain potassium channel KCNK5 deteriorates outcome in ischemic neurodegeneration. Pflugers Arch. 2015, 467, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Ruck, T.; Schuhmann, M.K.; Herrmann, A.M.; Moha ou Maati, H.; Bobak, N.; Gobel, K.; Langhauser, F.; Stegner, D.; Ehling, P.; et al. Endothelial TWIK-related potassium channel-1 (TREK1) regulates immune-cell trafficking into the CNS. Nat. Med. 2013, 19, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bittner, S.; Bobak, N.; Hofmann, M.-S.; Schuhmann, M.K.; Ruck, T.; Göbel, K.; Brück, W.; Wiendl, H.; Meuth, S.G. Murine K2P5.1 Deficiency Has No Impact on Autoimmune Neuroinflammation due to Compensatory K2P3.1- and KV1.3-Dependent Mechanisms. Int. J. Mol. Sci. 2015, 16, 16880-16896. https://doi.org/10.3390/ijms160816880
Bittner S, Bobak N, Hofmann M-S, Schuhmann MK, Ruck T, Göbel K, Brück W, Wiendl H, Meuth SG. Murine K2P5.1 Deficiency Has No Impact on Autoimmune Neuroinflammation due to Compensatory K2P3.1- and KV1.3-Dependent Mechanisms. International Journal of Molecular Sciences. 2015; 16(8):16880-16896. https://doi.org/10.3390/ijms160816880
Chicago/Turabian StyleBittner, Stefan, Nicole Bobak, Majella-Sophie Hofmann, Michael K. Schuhmann, Tobias Ruck, Kerstin Göbel, Wolfgang Brück, Heinz Wiendl, and Sven G. Meuth. 2015. "Murine K2P5.1 Deficiency Has No Impact on Autoimmune Neuroinflammation due to Compensatory K2P3.1- and KV1.3-Dependent Mechanisms" International Journal of Molecular Sciences 16, no. 8: 16880-16896. https://doi.org/10.3390/ijms160816880







