A New Ligustrazine Derivative-Selective Cytotoxicity by Suppression of NF-κB/p65 and COX-2 Expression on Human Hepatoma Cells. Part 3
Abstract
:1. Introduction
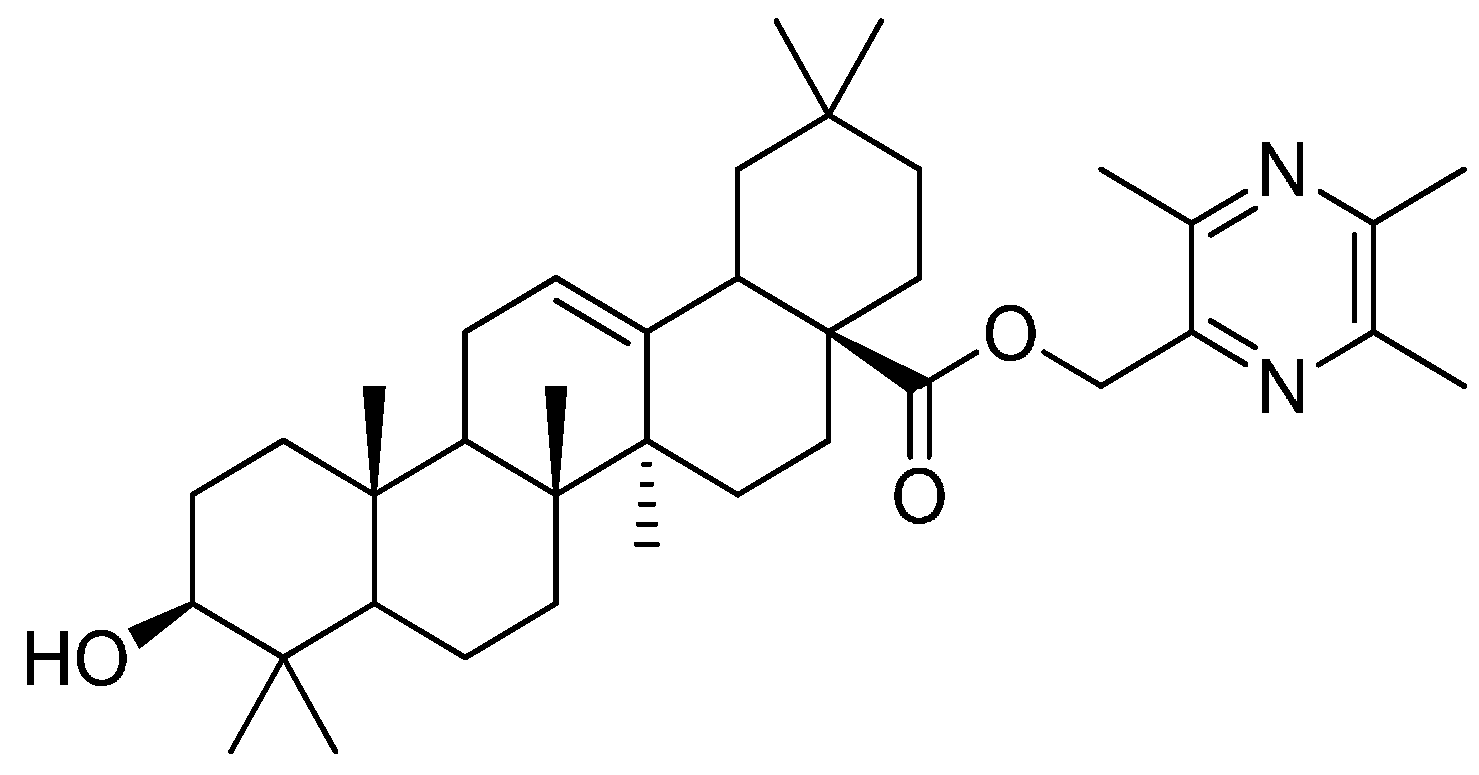
2. Results and Discussion
2.1. In Vitro Cytotoxicity
| Compound | IC50 Values (µM) | ||||
|---|---|---|---|---|---|
| Bel-7402 | HeLa | HT-29 | BGC-823 | MDCK | |
| T-OA | 6.36 ± 1.56 | 28.73 ± 5.89 | 29.84 ± 6.73 | >30 | >150 |
| Cisplatin | 5.94 ± 1.48 | 6.22 ± 2.06 | 6.11 ± 2.31 | 6.82 ± 1.79 | 3.70 ± 2.89 |

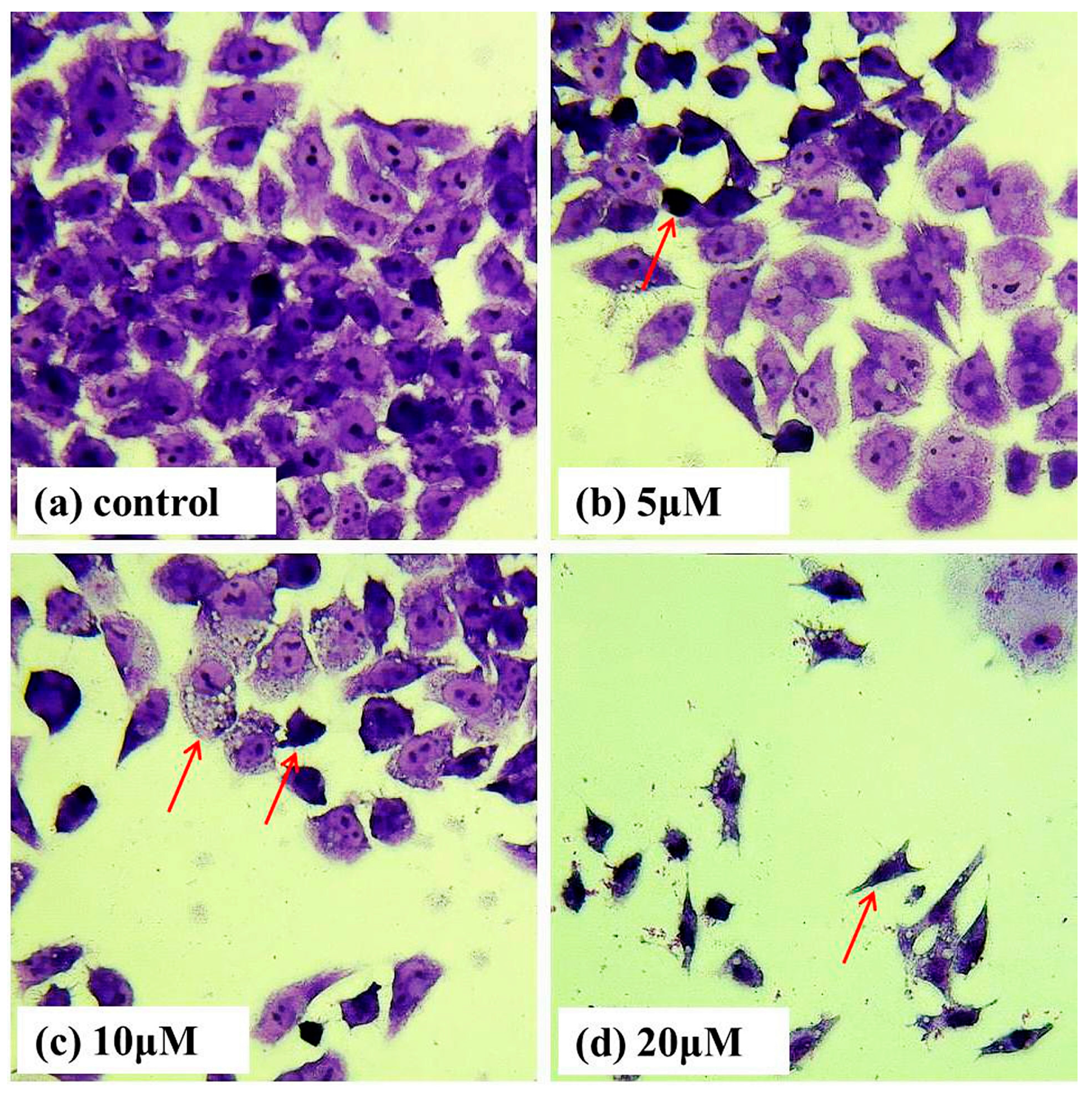
2.2. Giemsa Staining
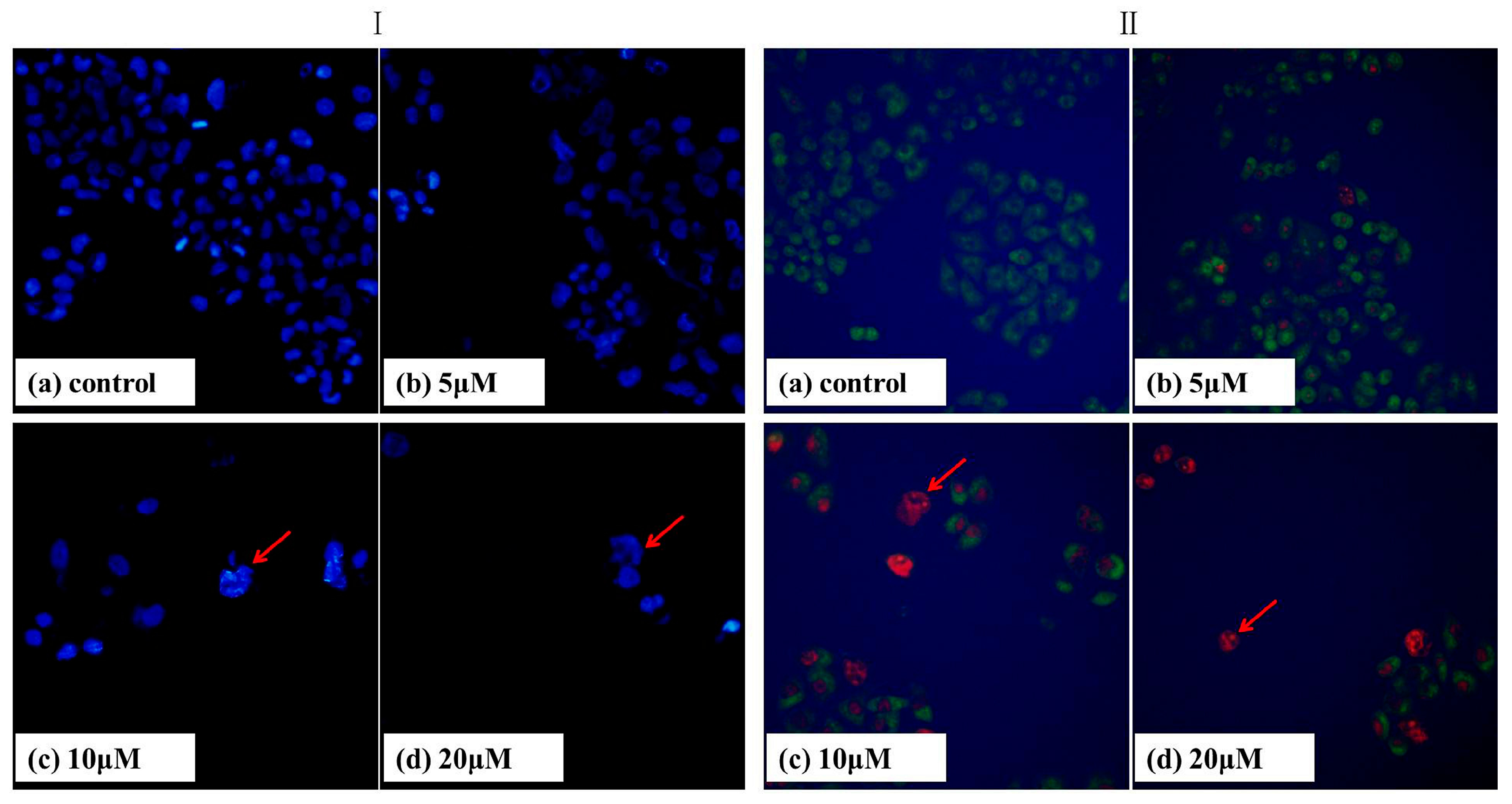
2.3. DAPI Staining
2.4. AO/EB Staining
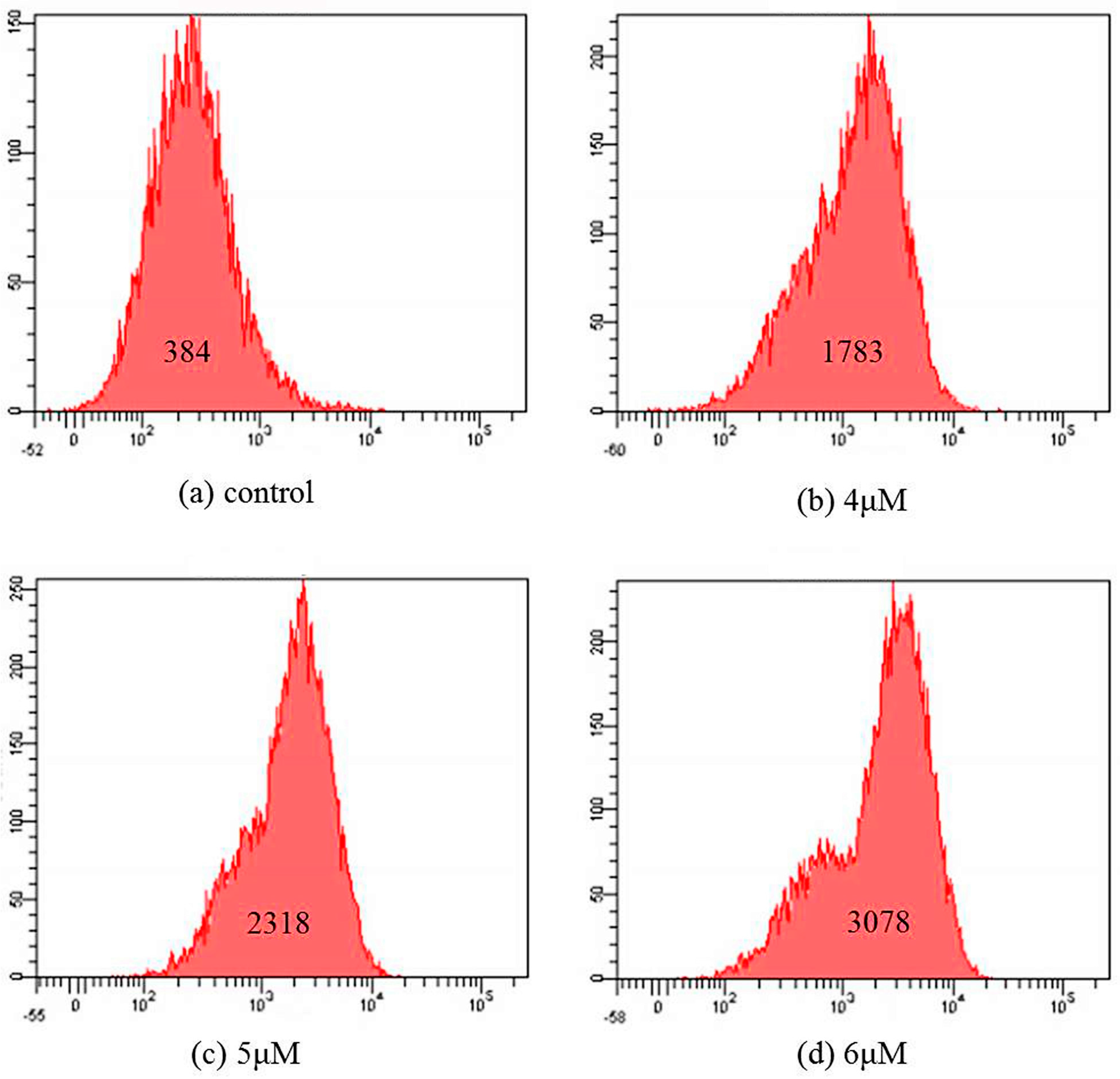
2.5. Apoptosis Analysis

2.6. Mitochondrial Membrane Potential Assay
2.7. Immumohistochemical Staining
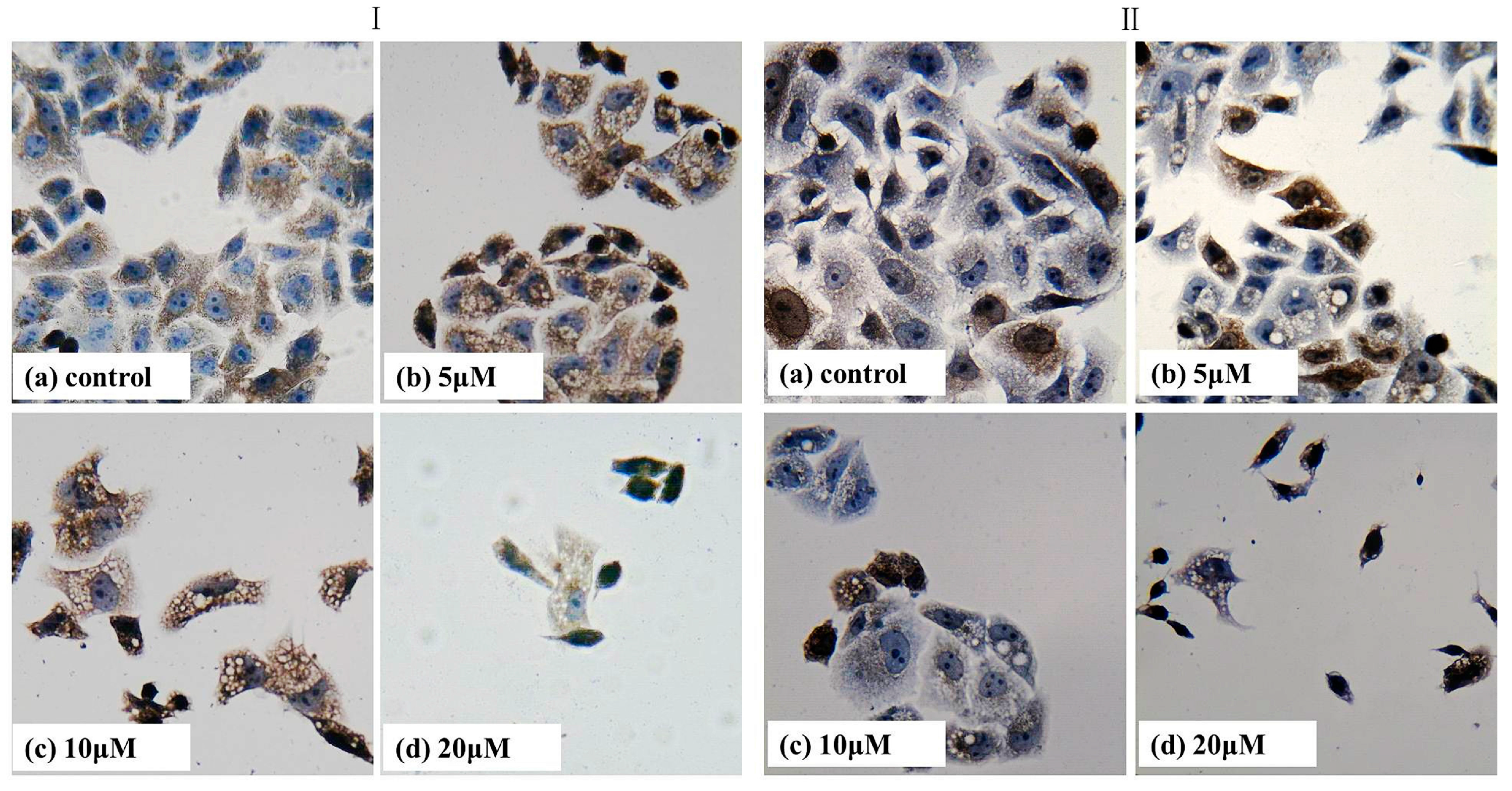
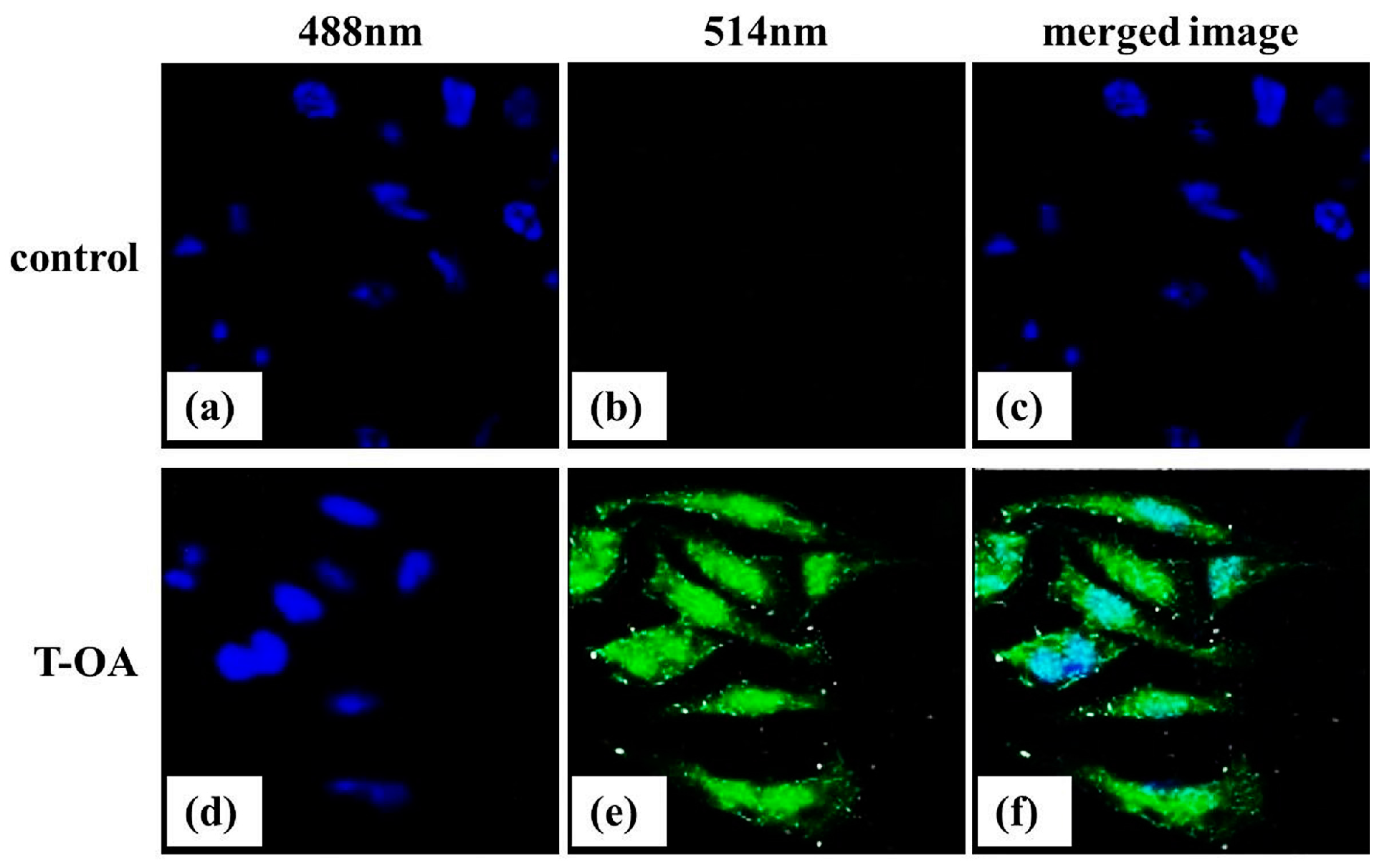
2.8. Subcellular Localization
3. Experimental Section
3.1. General
3.2. Cytotoxicity Evaluation
3.3. Morphological Examination by Giemsa Staining
3.4. DAPI Staining
3.5. AO/EB Staining
3.6. Apoptosis Analysis
3.7. Mitochondrial Membrane Potential Assay
3.8. Immumohistochemical Staining
3.9. Subcellular Localization of T-OA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, J.L.; Wang, H.; Chen, C.; Pi, H.F.; Ruan, H.L.; Zhang, P.; Wu, J.Z. Addictive evaluation of cholic acid-verticinone ester, a potential cough therapeutic agent with agonist action of opioid receptor. Acta Pharmacol. Sin. 2009, 30, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Wang, H.; Pi, H.F.; Ruan, H.L.; Zhang, P.; Wu, J.Z. Structural analysis and antitussive evaluation of five novel esters of verticinone and bile acids. Steroids 2009, 74, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.Z.; Wang, P.L.; Hong, Y.; Lei, H.M. Synthesis and structure identification of tetramethylpyrazine-protocatechuic acid and effects on hypoxic-ischemic brain damage. Pharmacol. Clin. Chin. Mater. Med. 2010, 26, 41–45. [Google Scholar]
- Wang, P.L.; Cheng, Y.T.; Xu, K.; An, Y.W.; Wang, W.; Li, Q.S.; Han, Q.J.; Li, Q.; Zhang, H.G.; Lei, H.M. Synthesis and anti-tumor evaluation of one novel tetramethylpyrazine-rhein derivative. Asian J. Chem. 2013, 25, 4885–4888. [Google Scholar]
- Chu, F.H.; Xu, X.; Li, G.; Gu, S.; Xu, K.; Gong, Y.; Xu, B.; Wang, M.N.; Zhang, W.Z.; Zhang, Y.Z.; et al. Amino acid derivatives of ligustrazine-oleanolic acid as new cytotoxic agents. Molecules 2014, 19, 18215–18231. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, L.; Zhong, J.; Wu, N.; Liu, G.; Lin, X. Oleanolic acid induces protective autophagy in cancer cells through the JNK and mTOR pathways. Oncol. Rep. 2014, 32, 567–572. [Google Scholar] [PubMed]
- Zheng, C.Y.; Xiao, W.; Zhu, M.X.; Pan, X.J.; Yang, Z.H.; Zhou, S.Y. Inhibition of cyclooxygenase-2 by tetramethylpyrazine and its effects on A549 cell invasion and metastasis. Int. J. Radiat. Oncol. 2012, 40, 2029–2037. [Google Scholar]
- Wang, P.L.; She, G.M.; Yang, Y.N.; Li, Q.; Zhang, H.G.; Liu, J.; Cao, Y.Q.; Xu, X.; Lei, H.M. Synthesis and biological evaluation of new ligustrazine derivatives as anti-tumor agents. Molecules 2012, 17, 4972–4985. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Zhang, Y.Z.; Xu, K.; Li, Q.; Zhang, H.G.; Guo, J.; Pang, D.D.; Cheng, Y.T.; Lei, H.M. A new ligustrazine derivative—Pharmacokinetic evaluation and anticancer activity by suppression of NF-κB/p65 and COX-2 expression in S180 mice. Pharmazie 2013, 68, 782–789. [Google Scholar] [PubMed]
- Xu, K.; Gong, Y.; Xu, B.; Tian, Y.F.; Wang, M.X.; Zhang, H.Z.; Zhang, Y.Z.; Lei, H.M. Identification of metabolites of anticancer lead compound T-OA in rat urine by HPLC-HRMS. China J. Chin. Mater. Med. 2014, 39, 911–915. [Google Scholar]
- Xu, K.; Wang, P.L.; Xu, X.; Xu, S.X.; Wang, Y.H.; Zhang, S.; Zhang, Y.Z.; Lei, H.M. Equilibrium solubility and apparent oil/water partition coefficient of anticancer primer T-OA in various media. J. Beijing Univ. Tradit. Chin. Med. 2013, 36, 554–557. [Google Scholar]
- Hou, P.; Ni, J.; Cao, S.L.; Lei, H.M.; Cai, Z.J.; Zhang, T.; Yu, F.; Tan, Q.Z. Preparation and evaluation of solid dispersions of a new anticancer compound based on early-stage preparation discovery concept. AAPS PharmSciTech 2013, 14, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Cao, S.L.; Ni, J.; Zhang, T.; Cai, Z.J.; Liu, J.J.; Wang, Y.; Wang, P.L.; Lei, H.M.; Liu, Y. In vitro and in vivo comparison of T-OA microemulsions and solid dispersions based on EPDC. Drug Dev. Ind. Pharm. 2015, 41, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Thanos, D.; Maniatis, T. NF-κB: A lesson in family values. Cell 1995, 80, 529–532. [Google Scholar] [CrossRef]
- Lee, J.I.; Burckart, G.J. Nuclear factor κB: Important transcription factor and therapeutic target. J. Clin. Pharmacol. 1998, 38, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclearfactor-κB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Baldwin, A.S. NF-κB as a therapeutic target in cancer. Trends Mol. Med. 2002, 8, 385–389. [Google Scholar] [CrossRef]
- Breinig, M.; Schirmacher, P.; Kern, M.A. Cyclooxygenase-2 (COX-2)—A therapeutic target in liver cancer? Curr. Pharm. Des. 2007, 13, 3305–3315. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.C.; Poon, R.T.; Lau, C.P.; Xie, D.; Fan, S.T. Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J. Gastroenterol. 2005, 11, 1896–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazhar, D.; Gillmore, R.; Waxman, J. COX and cancer. QJM 2005, 98, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Adsule, S.; Li, Y.; Padhye, S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therpy. Mini Rev. Med. Chem. 2007, 7, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Cai, X.; Chennamaneni, S.; Yi, X.; Liu, L.; Pink, J.J.; Dowlati, A.; Xu, Y.; Zhou, A.; Su, B. From COX-2 inhibitor nimesulide to potent anticancer agent: Synthesis, in vitro, in vivo and pharmacokinetic evaluation. Eur. J. Med. Chem. 2012, 47, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.C.; O’Byrne, K.J.; Reynolds, J.V.; O’Sullivan, J.; Pidgeon, G.P. COX-derived prostanoid pathways in gastrointestinal cancer development and progression: Novel targets for prevention and intervention. Biochim. Biophys. Acta Rev. Cancer 2012, 1825, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Chu, W.; Hu, Y.; Delhase, M.; Deerinek, T.; Ellisman, M.; Johnson, R.; Karin, M. The IKKβ subunit of I–B kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 1999, 189, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Yim, T.K.; Wu, W.K.; Pak, W.F.; Ko, K.M. Hepatoprotective action of an oleanolic acid-enriched extract of Ligustrum lucidum fruits is mediated through an enhancement on hepatic glutathione regeneration capacity in mice. Phytother. Res. 2001, 15, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, B.Z. Research process on oleanolic acid. Chin. Pharm. J. 2008, 19, 711–712. [Google Scholar]
- Li, X.Y.; He, J.L.; Liu, H.T.; Li, W.M.; Yu, C. Tetramethylpyrazine suppresses interleukin-8 expression in LPS-stimulated human umbilical vein endothelial cell by blocking ERK, p38 and nulear factor-κB signaling pathways. J. Ethnopharmacol. 2009, 125, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.J.; Jin, U.H.; Kim, K.W.; Son, J.K.; Lee, S.H.; Son, K.H.; Chang, H.W.; Lee, Y.C.; Kim, C.H. Triterpenoid saponin, oleanolic acid 3-O-β-d-glucopyranosyl(1→3)-α-l-rhamnopyranosyl (1→2)-α-l-arabinopyranoside (OA) from Aralia elata inhibits LPS-induced nitric oxide production by down-regulated NF-κB in raw 264.7 cells. Arch. Biochem. Biophys. 2007, 467, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Tang, H.M.; Mak, K.H.; Hu, S.; Wang, S.S.; Wong, K.M.; Wong, C.S.; Wu, H.Y.; Law, H.T.; Liu, K. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell. 2012, 23, 2240–2252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Qin, Q.; Jiang, Y.; Yang, G.; Rao, K.; Wang, Q.; Xiong, W.; Yuan, J. Mono-2-ethylhexyl phthalate induced loss of mitochondrial membrane potential and activation of Caspase3 in HepG2 cells. Environ. Toxicol. Pharmacol. 2012, 33, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Butachlor induced dissipation of mitochondrial membrane potential, oxidative DNA damage and necrosis in human peripheral blood mononuclear cells. Toxicology 2012, 302, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kucharczak, J.; Simmons, M.J.; Fan, Y.; Gelinas, C. To be, or not to be: NF-κB is the answer-role of Rel/NF-κB in the regulation of apoptosis. Oncogene 2003, 22, 8961–8982. [Google Scholar] [CrossRef] [PubMed]
- Von Rahden, B.H.A.; Stein, H.J.; Pühringer, F. Co-expression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005, 65, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Soares, R.; Reis-Filho, J.S. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J. Clin. Pathol. 2002, 55, 429–434. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yan, W.; Li, B.; Xu, B.; Gong, Y.; Chu, F.; Zhang, Y.; Yao, Q.; Wang, P.; Lei, H. A New Ligustrazine Derivative-Selective Cytotoxicity by Suppression of NF-κB/p65 and COX-2 Expression on Human Hepatoma Cells. Part 3. Int. J. Mol. Sci. 2015, 16, 16401-16413. https://doi.org/10.3390/ijms160716401
Zhang C, Yan W, Li B, Xu B, Gong Y, Chu F, Zhang Y, Yao Q, Wang P, Lei H. A New Ligustrazine Derivative-Selective Cytotoxicity by Suppression of NF-κB/p65 and COX-2 Expression on Human Hepatoma Cells. Part 3. International Journal of Molecular Sciences. 2015; 16(7):16401-16413. https://doi.org/10.3390/ijms160716401
Chicago/Turabian StyleZhang, Chenze, Wenqiang Yan, Bi Li, Bing Xu, Yan Gong, Fuhao Chu, Yuzhong Zhang, Qiuli Yao, Penglong Wang, and Haimin Lei. 2015. "A New Ligustrazine Derivative-Selective Cytotoxicity by Suppression of NF-κB/p65 and COX-2 Expression on Human Hepatoma Cells. Part 3" International Journal of Molecular Sciences 16, no. 7: 16401-16413. https://doi.org/10.3390/ijms160716401







