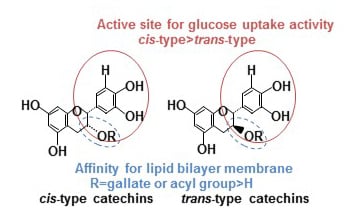
3-O-Acyl-epicatechins Increase Glucose Uptake Activity and GLUT4 Translocation through Activation of PI3K Signaling in Skeletal Muscle Cells
Abstract
:1. Introduction
2. Results
2.1. 3-O-Acyl-(−)-epicatechins Promote Glucose Uptake in L6 Myotubes


2.2. 3-O-Acyl-(−)-epicatechins Promote GLUT4 Translocation in L6 Myotubes
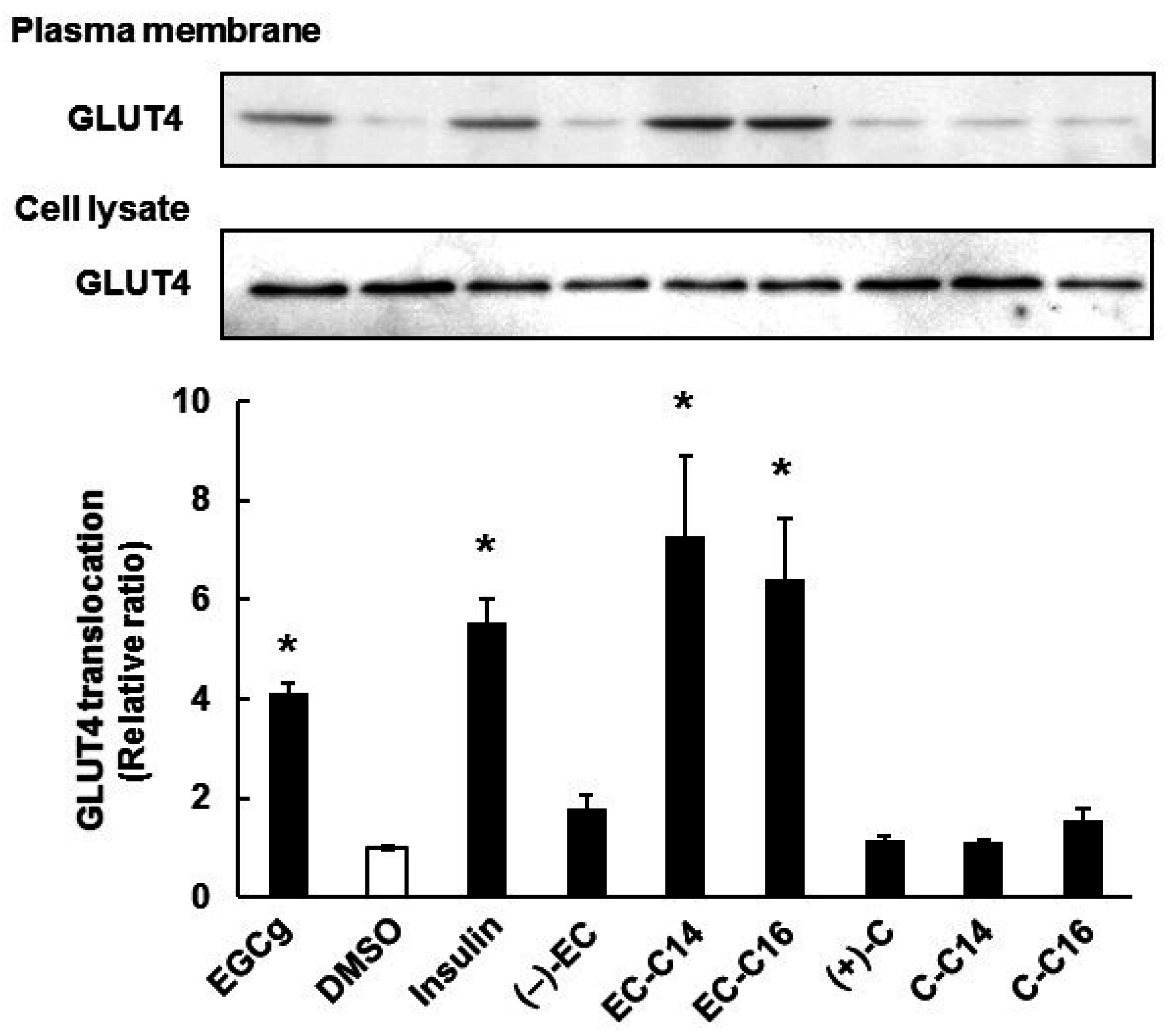


2.3. 3-O-Acyl-(−)-epicatechins Promote GLUT4 Translocation in L6 Myotubes

| Catechin | KA (1/M) | KD (M) |
|---|---|---|
| (+)-C | 1.71 × 103 | 5.84 × 10−4 |
| (−)-EC | 7.19 × 107 | 1.39 × 10−8 |
| EGCg | 1.21 × 109 | 8.26 × 10−1° |
| C-C16 | 6.12 × 106 | 1.63 × 10−7 |
| EC-C16 | 1.27 × 107 | 7.87 × 10−8 |
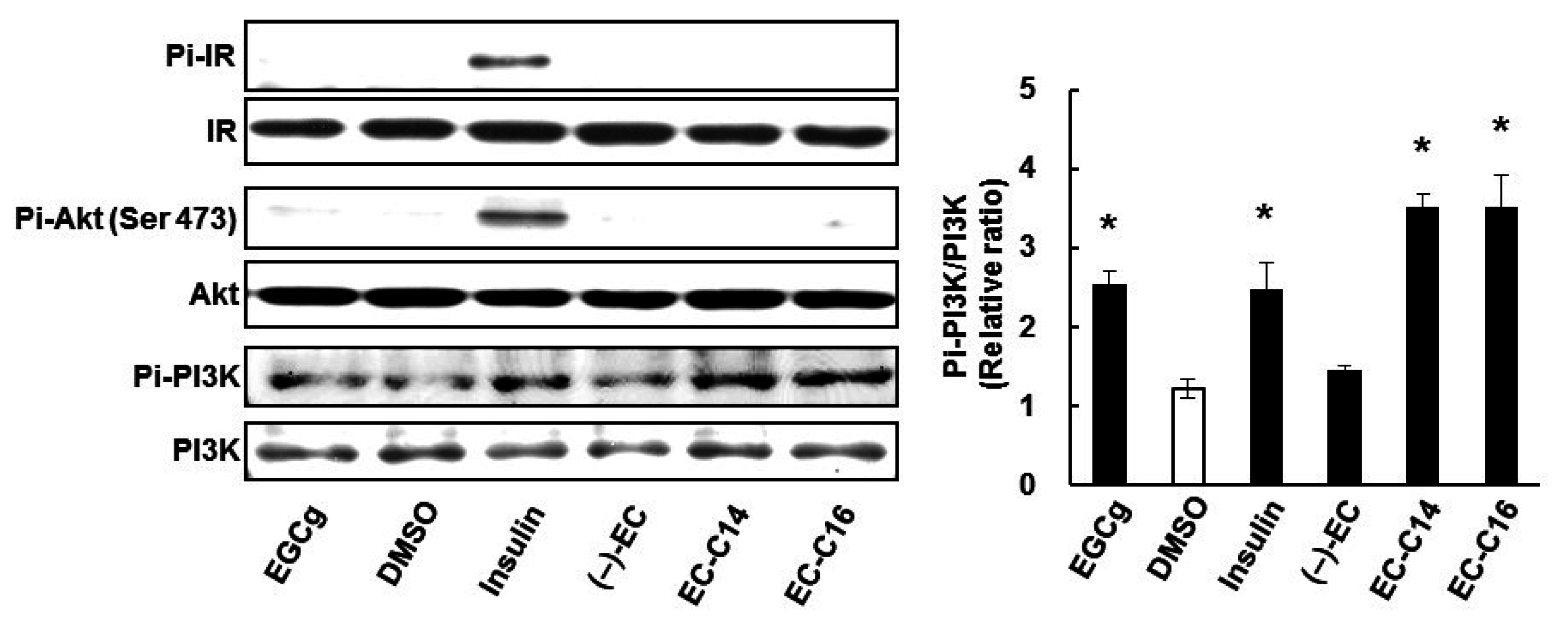
2.4. 3-O-Acyl-(−)-epicatechins Promote GLUT4 Translocation through a PI3K-Dependent and Insulin-Independent Pathway
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Cell Culture and Glucose Uptake Assay
4.3. Western Blot Analysis and Immunoprecipitation
4.4. Surface Plasmon Resonance (SPR) Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shahidi, F. Antioxidants in food and food antioxidants. Nahrung 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Intra, J.; Kuo, S.M. Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal Caco-2 cells. Chem. Biol. Interact. 2007, 169, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.; Harrington, K.; Vollmer, T.; Ward, K.; Zhang, J.Z. Antiinflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Yang, C.; Ju, J.; Lu, G.; Xiao, A. Cancer prevention by tea and tea polyphenols. Asia Pac. J. Clin. Nutr. 2008, 17, 245–248. [Google Scholar] [PubMed]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Nishiumi, S.; Nagayasu, H.; Fukuda, I.; Yoshida, K.; Ashida, H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem. Biophys. Res. Commun. 2008, 377, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Furuyashiki, T.; Yamada, K.; Aoki, Y.; Sakane, I.; Fukuda, I.; Yoshida, K.; Ashida, H. Tea catechins modulate the glucose transport system in 3T3-L1 adipocytes. Food Funct. 2010, 1, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Belman, J.P.; Habtemichael, E.N.; Bogan, J.S. A proteolytic pathway that controls glucose uptake in fat and muscle. Rev. Endocr. Metab. Disord. 2014, 15, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sheena, A.; Mohan, S.S.; Haridas, N.P.; Anilkumar, G. Molecular dynamics simulation studies of GLUT4: Substrate-free and substrate-induced dynamics and ATP-mediated glucose transport inhibition. PLoS ONE 2011, 6, e25747. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Yamamoto, M.; Ema, K.; Monobe, M.; Tokuda, Y.; Tachibana, H. Epicatechin-3-O-(3″-O-methyl)-gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release. J. Agric. Food Chem. 2012, 60, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Lee, S.G.; Kim, S.U.; Kim, S.H.; Sun, W.S.; Cho, S.J.; Jeong, D.H. Anticancer activity of 3-O-acyl and alkyl-(−)-epicatechin derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 5189–5192. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Cho, S.J. Synthesis and antimicrobial activities of 3-O-alkyl analogues of (+)-catechin: Improvement of stability and proposed action mechanism. Eur. J. Med. Chem. 2010, 45, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Saito, A.; Tanaka, A.; Nakajima, N.; Akagi, R.; Mori, M.; Mizushina, Y. Epicatechin conjugated with fatty acid is a potent inhibitor of DNA polymerase and angiogenesis. Life Sci. 2007, 80, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Caturla, N.; Vera-Samper, E.; Villalaín, J.; Mateo, C.R.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef]
- Kajiya, K.; Hojo, H.; Suzuki, M.; Nanjo, F.; Kumazawa, S.; Nakayama, T. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J. Agric. Food Chem. 2004, 52, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Hamilton-Miller, J.M.; Hara, Y.; Nagaoka, Y.; Kumagai, A.; Uesato, S.; Taylor, P.W. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents 2004, 24, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Uekusa, Y.; Kamihira, M.; Nakayama, T. Dynamic behavior of tea catechins interacting with lipid membranes as determined by NMR spectroscopy. J. Agric. Food Chem. 2007, 55, 9986–9992. [Google Scholar] [CrossRef] [PubMed]
- Uekusa, Y.; Kamihira-Ishijima, M.; Sugimoto, O.; Ishii, T.; Kumazawa, S.; Nakamura, K.; Tanji, K.; Naito, A.; Nakayama, T. Interaction of epicatechin gallate with phospholipid membranes as revealed by solid-state NMR spectroscopy. Biochim. Biophys. Acta 2010, 1808, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Suárez, E.; Lizcano, J.M.; Griñó, E.; Shephed, P.R.; Fryer, L.G.D.; Carling, D.; Bertran, J.; Palacín, M.; Zorzano, A.; et al. Neuregulin signaling on glucose transport in muscle cells. J. Biol. Chem. 2004, 279, 12260–12268. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Saito, A.; Tanaka, A.; Nakajima, N.; Akagi, R.; Mori, M.; Mizushina, Y. Catechin conjugated with fatty acid inhibits DNA polymerase and angiogenesis. DNA Cell Biol. 2006, 25, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Ashida, H. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: Application for detection of translocated glucose transporter 4 on the plasma membrane. Biosci. Biotechnol. Biochem. 2007, 71, 2343–2346. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda-Wakagi, M.; Mukai, R.; Fuse, N.; Mizushina, Y.; Ashida, H. 3-O-Acyl-epicatechins Increase Glucose Uptake Activity and GLUT4 Translocation through Activation of PI3K Signaling in Skeletal Muscle Cells. Int. J. Mol. Sci. 2015, 16, 16288-16299. https://doi.org/10.3390/ijms160716288
Ueda-Wakagi M, Mukai R, Fuse N, Mizushina Y, Ashida H. 3-O-Acyl-epicatechins Increase Glucose Uptake Activity and GLUT4 Translocation through Activation of PI3K Signaling in Skeletal Muscle Cells. International Journal of Molecular Sciences. 2015; 16(7):16288-16299. https://doi.org/10.3390/ijms160716288
Chicago/Turabian StyleUeda-Wakagi, Manabu, Rie Mukai, Naoya Fuse, Yoshiyuki Mizushina, and Hitoshi Ashida. 2015. "3-O-Acyl-epicatechins Increase Glucose Uptake Activity and GLUT4 Translocation through Activation of PI3K Signaling in Skeletal Muscle Cells" International Journal of Molecular Sciences 16, no. 7: 16288-16299. https://doi.org/10.3390/ijms160716288
APA StyleUeda-Wakagi, M., Mukai, R., Fuse, N., Mizushina, Y., & Ashida, H. (2015). 3-O-Acyl-epicatechins Increase Glucose Uptake Activity and GLUT4 Translocation through Activation of PI3K Signaling in Skeletal Muscle Cells. International Journal of Molecular Sciences, 16(7), 16288-16299. https://doi.org/10.3390/ijms160716288