Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits
Abstract
:1. Introduction
2. Results and Discussion
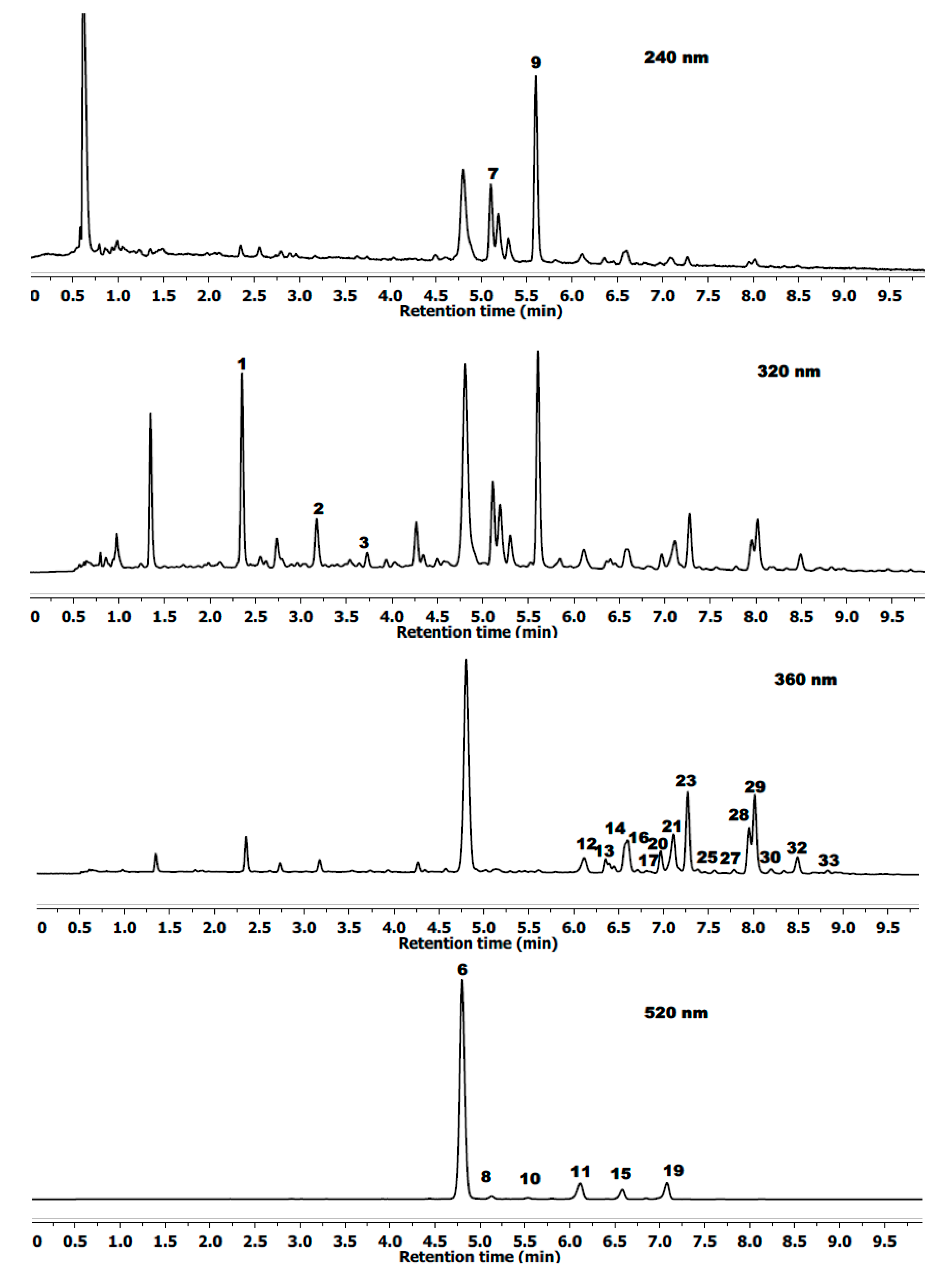
2.1. Peak Identification and Assignment
| Compounds ‡ | Rt | λmax | (MS)− | (MS/MS)− |
|---|---|---|---|---|
| (min) | (nm) | (m/z) | (m/z) | |
| Chlorogenic acid | 2.35 | 323 | 353.0866 | 235.9249/191.0553/146.9378 |
| Caffeoyl hexoside | 3.14 | 320 | 341.0849 | 179.0349/135.0464 |
| p-Coumaric acid | 3.69 | 312 | 163.0380 | |
| Cyanidin-3-O-diglucoside | 4.21 | 514 | 611.1664+ † | 287.0571+ |
| Cyanidin-3-glucosylrutinoside | 4.36 | 517 | 757.2241+ | 611.1513/449.1063/287.0571+ |
| Cyanidin-3-O-glucoside | 4.74 | 514 | 449.1063+ | 287.0571+ |
| Ellagitannins Lambertianin C | 5.00 | 244 | 1401.3730 | 633.075/300.9999 |
| Cyanidin-3-O-rutinoside | 5.08 | 516 | 595.1664+ | 287.0571+ |
| Ellagitannins hex (casuarinin) | 5.51 | 244 | 935.0760 | 633.075/300.9999 |
| Cyanidin-3-(3ʹ-malonyl)glucoside | 5.74 | 515 | 535.1084+ | 287.0571+ |
| Cyanidin-3-O-xyloside | 6.08 | 514 | 419.0987+ | 287.0571+ |
| Ellagic acid pentoside | 6.28 | 360 | 433.0777 | 300.9999 |
| Quercetin-3-methoxyhexoside | 6.38 | 360 | 493.1001 | 463.3010 |
| Ellagic acid | 6.51 | 364 | 300.9999 | |
| Cyanidin-3-(6ʹ-malonyl)glucoside | 6.54 | 517 | 535.1084+ | 287.0571+ |
| Ellagic acid rhamnoside | 6.64 | 360 | 447.0527 | 300.9999 |
| Kaempferol-3-O-glucoside-rhamnoside-7-O-rhamnoside | 6.73 | 346 | 739.1930 | 593.1559/285.0187 |
| Quercetin-3-O-rutinoside | 6.90 | 352 | 609.1080 | 463.0397/301.0277/151.0034 |
| Cyanidin-3-dioxalylglucoside | 7.03 | 517 | 593.1520+ | 287.0571 |
| Quercetin-3-O-galactoside | 7.04 | 353 | 463.0843 | 301.0277/151.0034 |
| Quercetin-3-O-glucuronide | 7.14 | 351 | 477.0670 | 301.0277/151.0034 |
| Quercetin-3-O-glucoside | 7.20 | 352 | 463.0843 | 301.0277/151.0034 |
| Kaempferol derivative | 7.27 | 345 | 475.0125 | 447.0968/285.0187 |
| Quercetin-3-O-hexoside | 7.36 | 352 | 463.0843 | 301.0277/151.0034 |
| Kaempferol-3-O-rutinoside | 7.48 | 350 | 593.1559 | 447.0968/285.0187 |
| Luteolin-3-O-glucuronide | 7.59 | 340 | 461.0710 | 285.0187 |
| Quercetin-3-O-pentoside | 7.88 | 352 | 433.0777 | 301.0277/151.0034 |
| Quercetin-3-(6ʹ-(3-hydroxy-3-methylglutaroyl)-galactoside | 7.94 | 345 | 607.1293 | 463.0843/301.0277/151.0034 |
| Quercetin-3-O-pentoside | 8.12 | 352 | 433.0777 | 301.0277/151.0034 |
| Quercetin-3-O-rhamnoside | 8.28 | 350 | 447.0968 | 301.0277/151.0034 |
| Kaempferol-3-O-glucuronide | 8.43 | 346 | 461.0710 | 285.0187 |
| Methyl ellagic acid pentose | 8.60 | 360 | 477.1082 | 314.0421/300.9996 |
| Kaempferol-3-O-pentoside | 8.76 | 350 | 417.0397 | 285.0187 |
| Apigenin-3-O-glucuronide | 8.90 | 338 | 445.0710 | 269.0450 |
| Number of Sample | Blackberry Species | Geographical Location | |
|---|---|---|---|
| 1 | Rubus radula | Albigowa Honie | N 50°0ʹ19.28ʹʹ E 22°10ʹ22.06ʹʹ |
| 2 | Rubus montanus | Berendowice | N 49°40ʹ14.85ʹʹ E 22°43ʹ39.58ʹʹ |
| 3 | Rubus gracilis | Las Niechciałka | N 50°5ʹ45.38ʹʹ E 22°35ʹ45.06ʹʹ |
| 4 | Rubus macrophyllus | Las Niechciałka | N 50°5ʹ45.38ʹʹ E 22°35ʹ45.06ʹʹ |
| 5 | Rubus pericrispatus | Kopystno | N 49°41ʹ8.38ʹʹ E 22°38ʹ32.49ʹʹ |
| 6 | Rubus austoslovacus | Długie k/Przemyśla | N 49°45ʹ49.61ʹʹ E 22°42ʹ4.59ʹʹ |
| 7 | Rubus subcatus | Łazy k/Birczy | N 49°42ʹ49.56ʹʹ E 22°32ʹ3.14ʹʹ |
| 8 | Rubus ambrosius | Zmysłówka | N 50°9ʹ58.91ʹʹ E 22°22ʹ43.39ʹʹ |
| 9 | Rubus fasciculatus | Łazy k/Birczy | N 49°42ʹ49.56ʹʹ E 22°32ʹ3.14ʹʹ |
| 10 | Rubus nessersis | Las Niechciałka | N 50°5ʹ45.38ʹʹ E 22°35ʹ45.06ʹʹ |
| 11 | Rubus glivicensis | Zmysłówka | N 50°9ʹ58.91ʹʹ E 22°22ʹ43.39ʹʹ |
| 12 | Rubus caesius | Długie k/Przemyśla | N 49°45ʹ49.61ʹʹ E 22°42ʹ4.59ʹʹ |
| 13 | Rubus bifronus | Berendowice | N 49°40ʹ26.44ʹʹ E 22°43ʹ6.76ʹʹ |
| 14 | Rubus praecox | Ławy k/Birczy | N 49°42ʹ49.56ʹʹ E 22°32ʹ3.14ʹʹ |
| 15 | Rubus bifronus | Honie | N 50°0ʹ19.28ʹʹ E 22°10ʹ22.06ʹʹ |
| 16 | Rubus perrobustus | Łazy k/Birczy | N 49°42ʹ49.56ʹʹ E 22°32ʹ3.14ʹʹ |
| 17 | Rubus parthenocisus | Berendowice | N 49°40ʹ26.44ʹʹ E 22°43ʹ6.76ʹʹ |
| 18 | Rubus pseudidaeus | Białobrzeszki | N 50°7ʹ18.26ʹʹ E 22°31ʹ29.98ʹʹ |
| 19 | Rubus constrictus | Berendowice | N 49°40ʹ14.85ʹʹ E 22°43ʹ39.58ʹʹ |
| 20 | Rubus chaerophylloides | Gruszowa | N 49°40ʹ27.7ʹʹ E 22°41ʹ36.99ʹʹ |
| 21 | Rubus wimmerianus | Zmysłówka | N 50°9ʹ58.91ʹʹ E 22°22ʹ43.39ʹʹ |
| 22 | Rubus crispomarginatus | Łazy k/Birczy | N 49°42ʹ49.56ʹʹ E 22°32ʹ3.14ʹʹ |
| 23 | Rubus orthostachys | Berendowice | N 49°40ʹ14.85ʹʹ E 22°43ʹ39.58ʹʹ |
2.2. Analysis of the Extracted Amounts of Phenolic Compounds Using Pressure Liquid Extraction and Ultrasonic-Assisted Extraction Methods
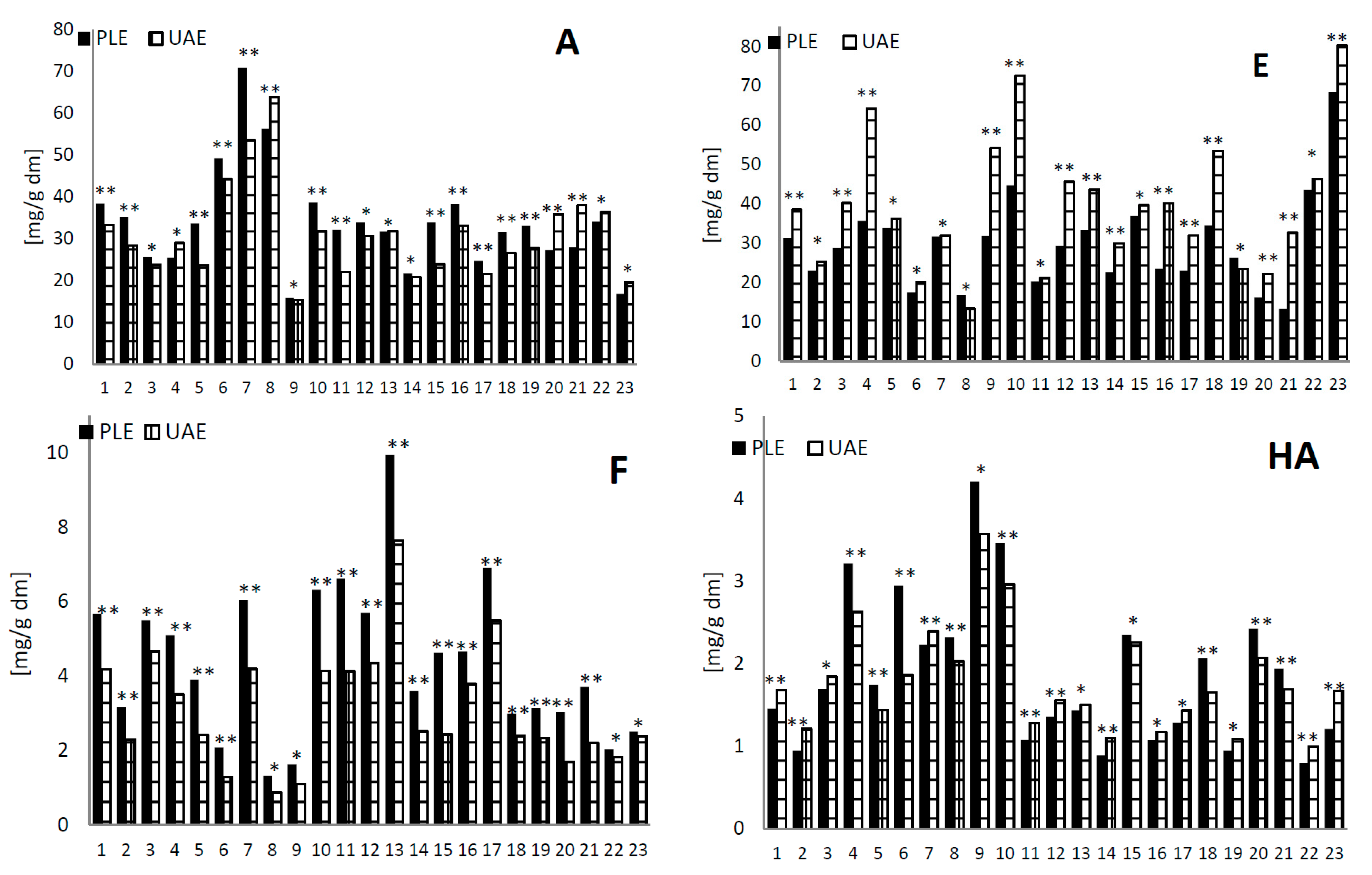
2.3. Analysis of the Antioxidant Activity Using Pressure Liquid Extraction and Ultrasonic-Assisted Extraction Methods

2.4. Principal Component Analysis
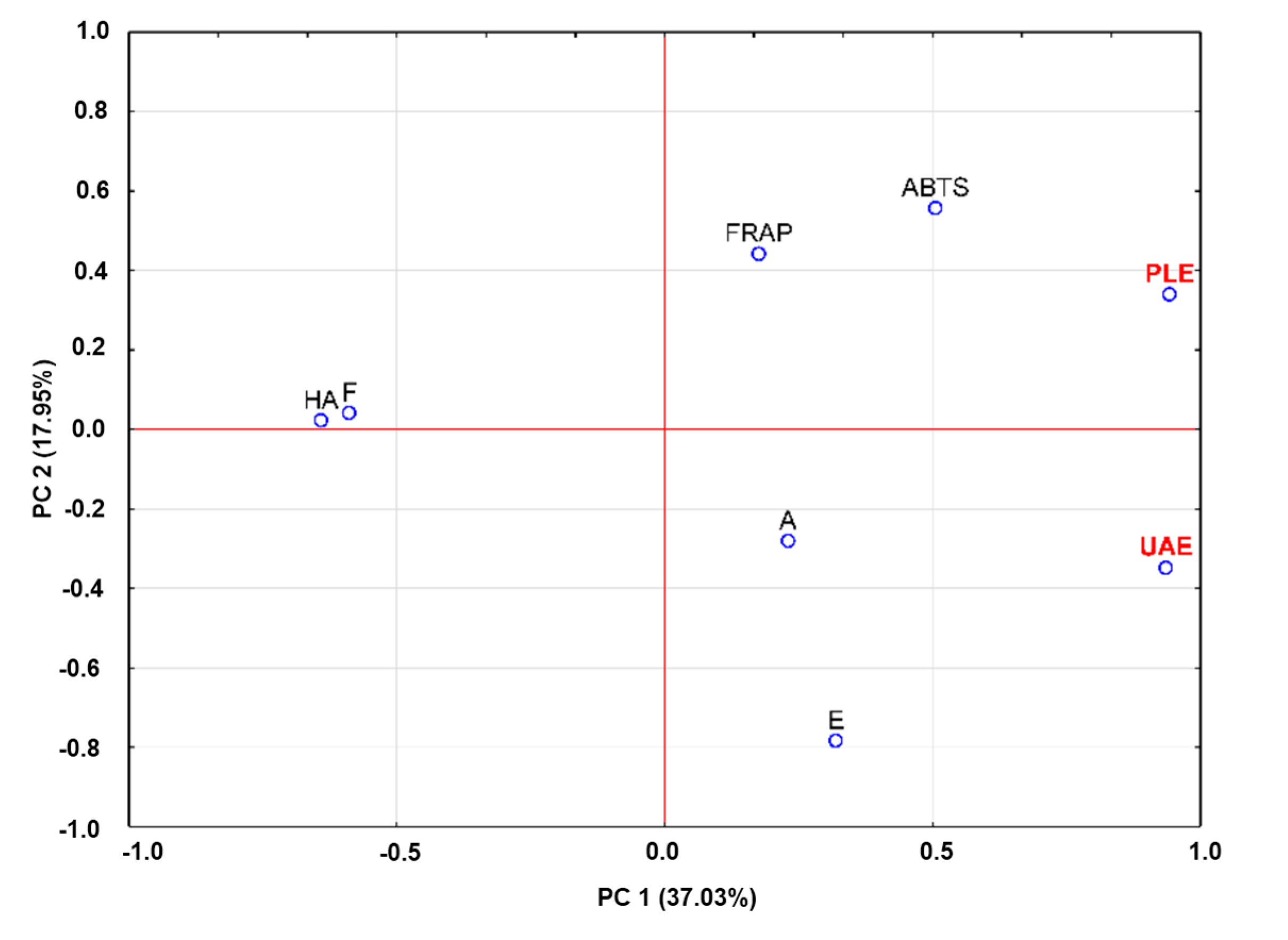
3. Experimental Section
3.1. Reagent and Standard
3.2. Plant Material
3.3. Extraction Procedure
3.3.1. Pressurized Liquid Extraction
3.3.2. Ultrasound-Assisted Extraction
3.4. Identification and Quantification of Polyphenols by the Ultra-Performance Liquid Chromatography–Mass Spectrometry Method
3.5. Analysis of Antioxidant Activity
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Talcott, S.T. Chemical components of berry fruits. In Berry Fruit Value Added Products for Health Promotion, 1st ed.; Zhao, Y., Ed.; CRC: Boca Raton, FL, USA, 2007; pp. 51–72. [Google Scholar]
- Cho, M.J.; Howard, L.R.; Prior, R.L; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food 2007, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Siriwoharn, T.; Wrolstad, R.E. Characterisation of phenolics in Marion and Evergreen blackberries. J. Food Sci. 2004, 69, 233–240. [Google Scholar]
- Lei, Z.; Jervis, J.; Helm, R.F. Use of methanolysis for the determination of total ellagic and gallic acid contents of wood and food products. J. Agric. Food Chem. 2001, 49, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Plaza, M.; Bahrim, G.; Ibanez, E.; Herrero, M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. J. Chromatogr. 2010, 1218, 4918–4927. [Google Scholar] [CrossRef] [PubMed]
- Dunford, N.; Irmak, S.; Jonnala, R. Pressurised solvent extraction of policosanol from wheat straw, germ and bran. Food Chem. 2010, 119, 1246–1249. [Google Scholar] [CrossRef]
- Da Fonseca Machado, A.P.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Engl. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valero, J.R. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Wibisono, R.; Zhang, J.; Saleh, Z.; Stevenson, D.E.; Joyce, N.I. Optimization of accelerated solvent extraction for screening of the health benefits of plant food materials. Health 2009, 1, 220–230. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Ann. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Herrero, M.; Cifuentes, A.; Ibanez, E. Use of compressed fluids for sample preparation: Food applications. J .Chromatogr. A 2007, 1152, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Maksinovic, Z.; Malencic, D.; Kovacevic, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Li, S.A.; Zhu, R.H.; Zhong, M.; Zhang, Y.P.; Huang, K.L.; Zhi, X.; Fu, S.T. Effects of ultrasonic-assistant extraction parameters on total flavones yield of Selaginella doederleinii and its antioxidant activity. J. Med. Plants Res. 2010, 4, 1743–1750. [Google Scholar]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Biesaga, M. Influence of extraction methods on stability of flavonoids. J. Chromatogr. A 2011, 1218, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC: Boca Raton, FL, USA, 2004; Volume 1, pp. 558–595. [Google Scholar]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Wrolstad, R.E. A novel Zwitterionic anthocyanin from Evergreen blackberry (Rubus laciniatus Wild). J. Agric. Food Chem. 2002, 50, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.W.S.; Woods, F.M.; Dozier, W.A., Jr.; Ebel, R.C.; Nesbitt, M.; Jee, J.; Fields, D. Phenolic content and antioxidant capacities of Alabama-grown thornless blackberries. Int. J. Fruit Sci. 2008, 7, 33–46. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC–ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.P.; Ossipov, V.; Loponen, J.; Haukioja, E.; Pihlaja, K. Characterization of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography–mass spectrometry. J. Chromatogr. A 1999, 865, 283–291. [Google Scholar] [CrossRef]
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Torronen, R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (Family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Zafrilla, P.; Ferreres, F.; Tomas-Barberan, F.A. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001, 49, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Pineiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oszmiański, J.; Nowicka, P.; Teleszko, M.; Wojdyło, A.; Cebulak, T.; Oklejewicz, K. Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits. Int. J. Mol. Sci. 2015, 16, 14540-14553. https://doi.org/10.3390/ijms160714540
Oszmiański J, Nowicka P, Teleszko M, Wojdyło A, Cebulak T, Oklejewicz K. Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits. International Journal of Molecular Sciences. 2015; 16(7):14540-14553. https://doi.org/10.3390/ijms160714540
Chicago/Turabian StyleOszmiański, Jan, Paulina Nowicka, Mirosława Teleszko, Aneta Wojdyło, Tomasz Cebulak, and Krzysztof Oklejewicz. 2015. "Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits" International Journal of Molecular Sciences 16, no. 7: 14540-14553. https://doi.org/10.3390/ijms160714540
APA StyleOszmiański, J., Nowicka, P., Teleszko, M., Wojdyło, A., Cebulak, T., & Oklejewicz, K. (2015). Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits. International Journal of Molecular Sciences, 16(7), 14540-14553. https://doi.org/10.3390/ijms160714540





