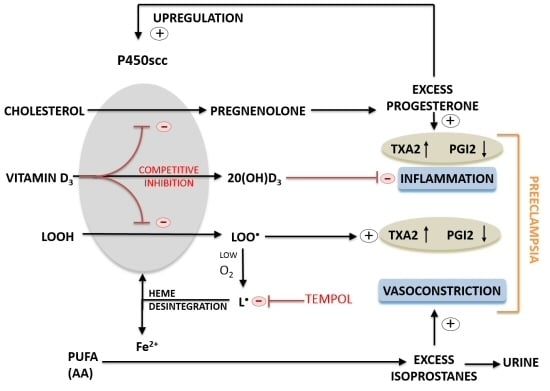
A Proposed Molecular Mechanism of High-Dose Vitamin D3 Supplementation in Prevention and Treatment of Preeclampsia
Abstract
:1. Introduction
2. Results
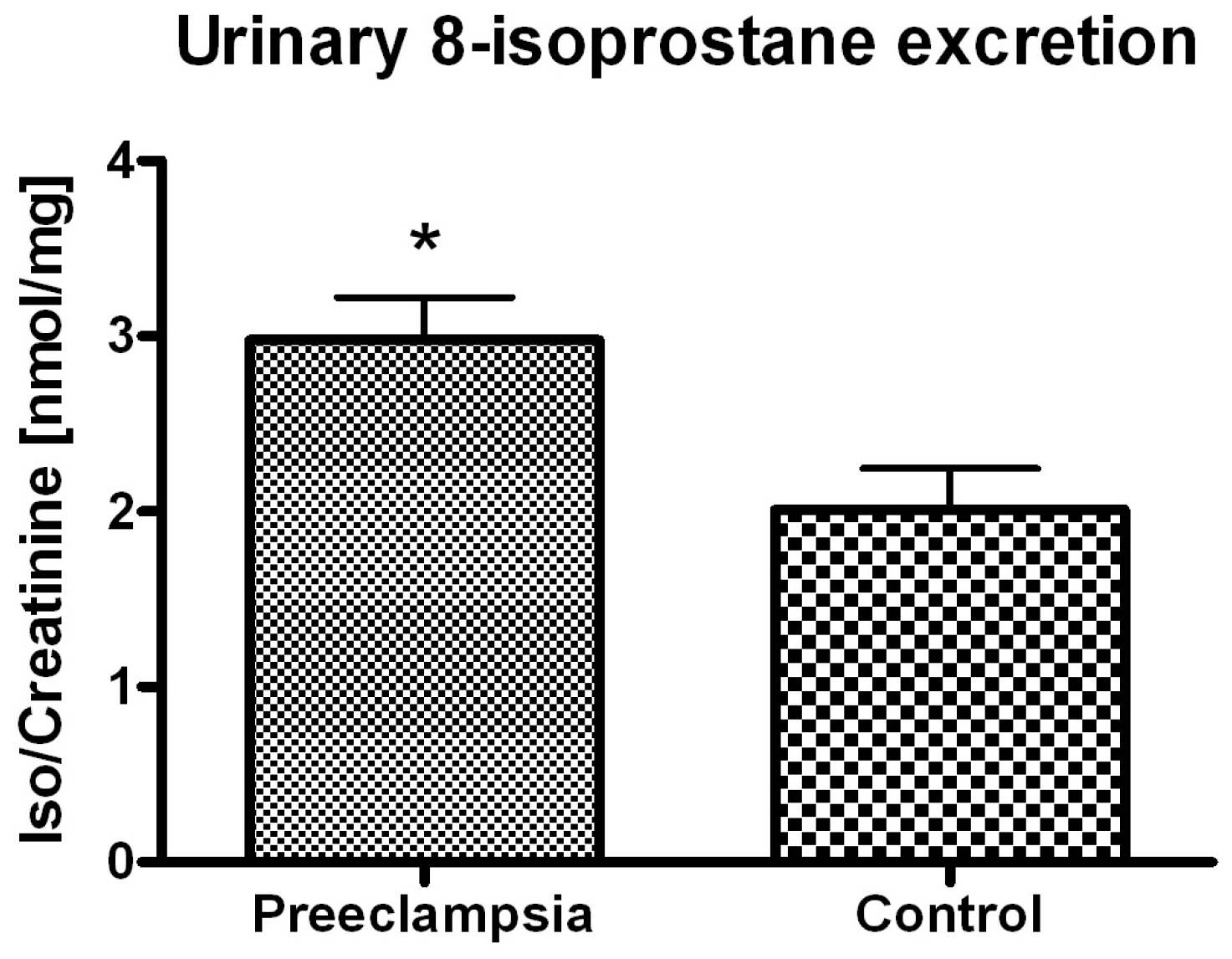


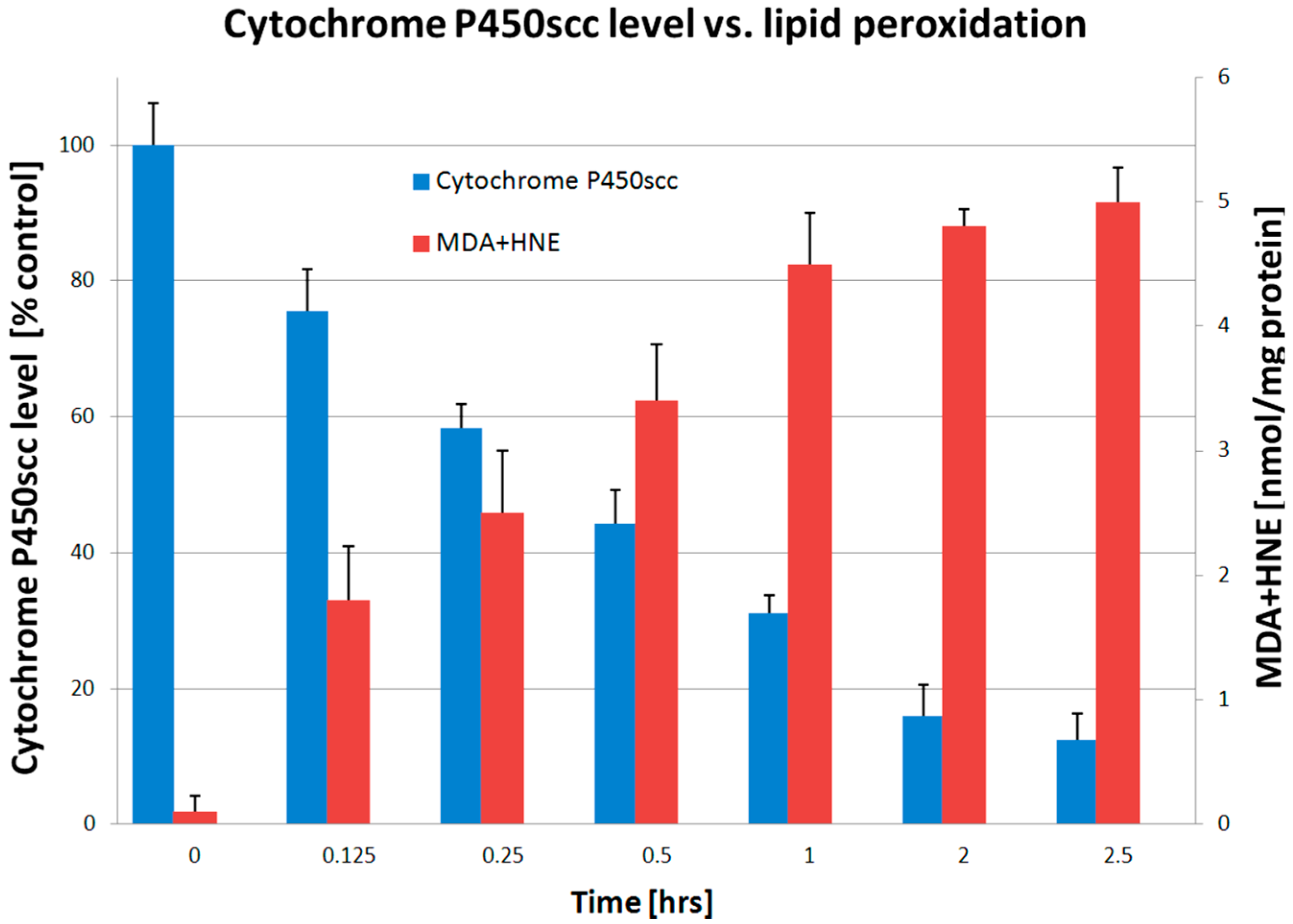
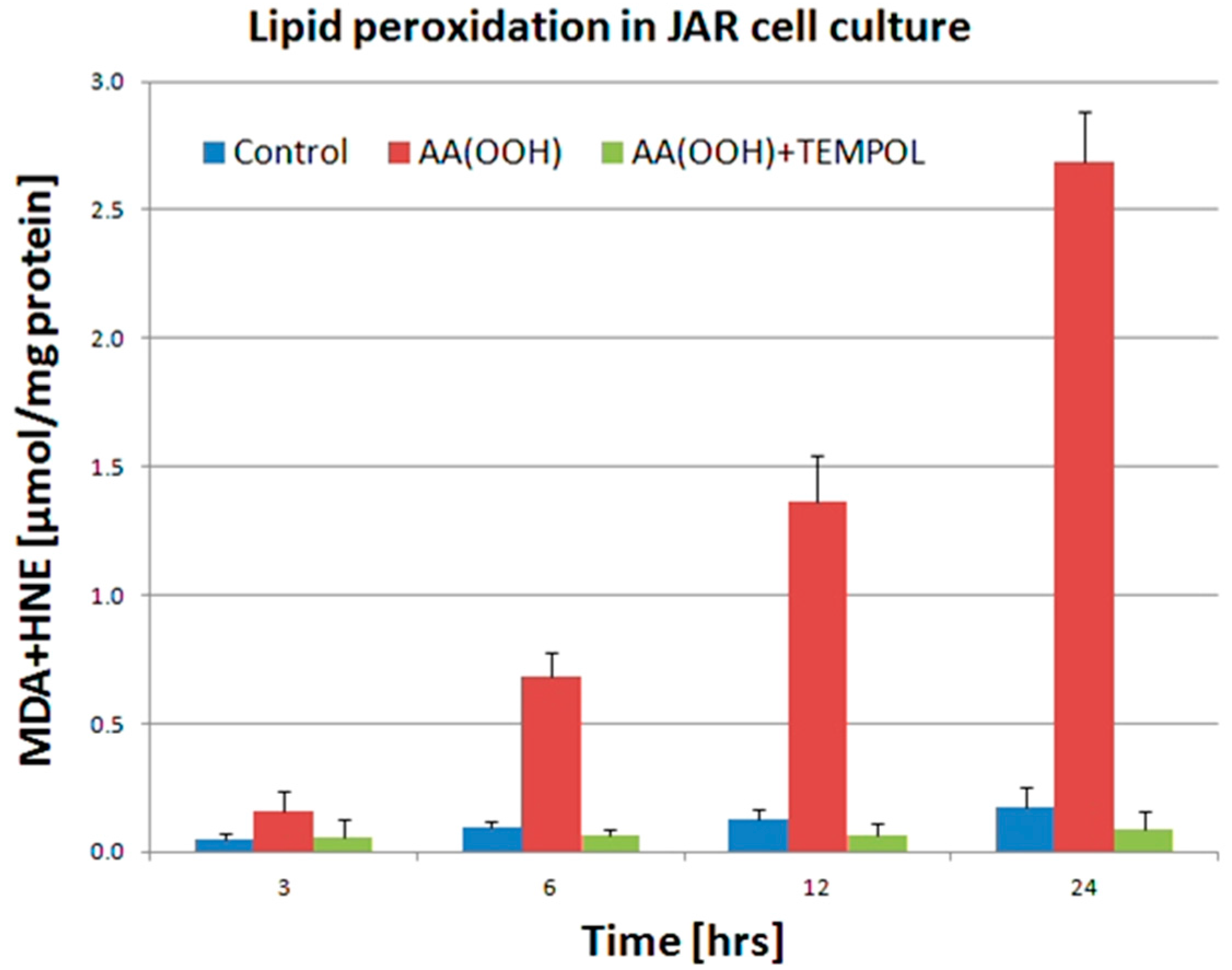
3. Discussion
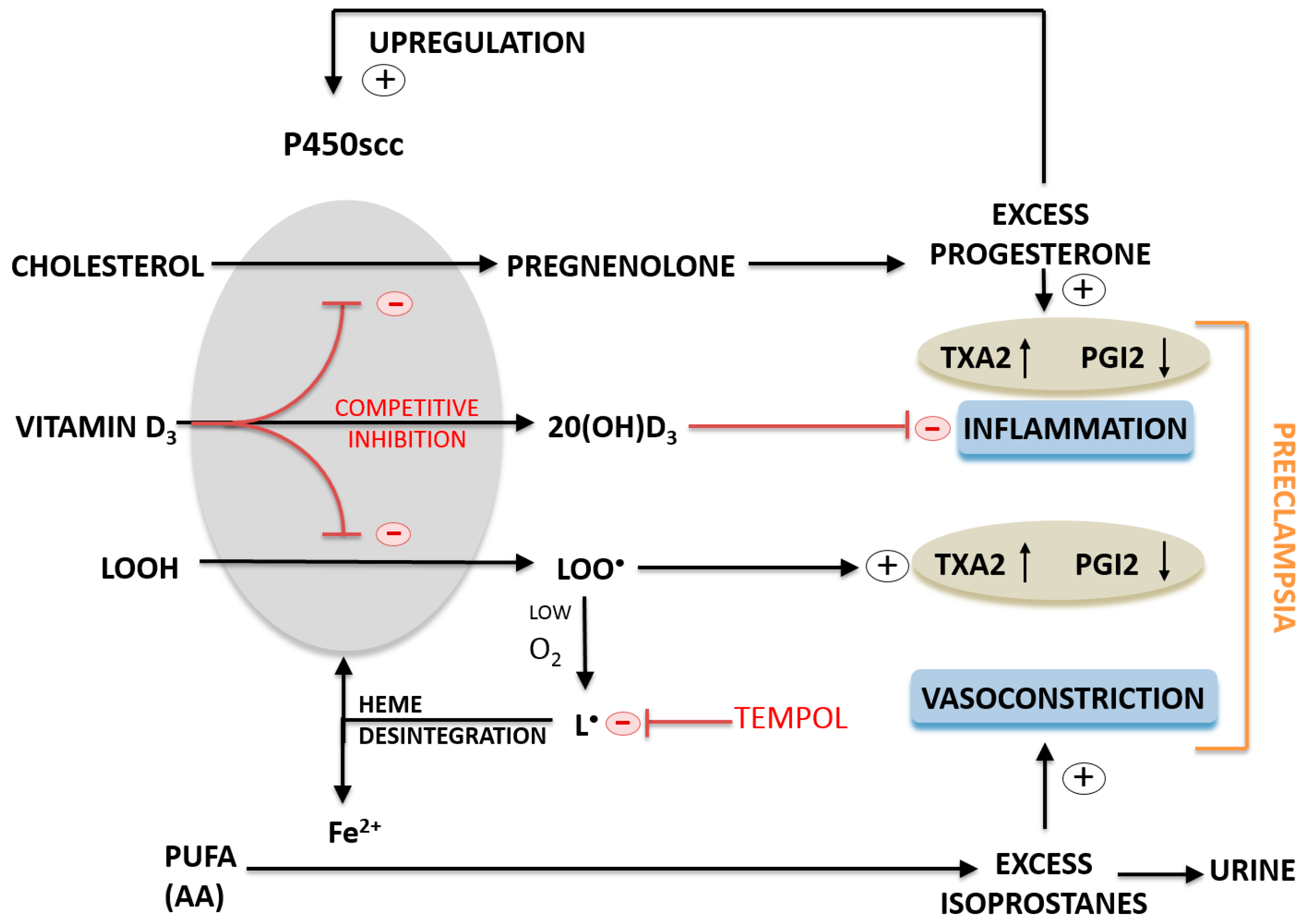
4. Experimental Section
4.1. Clinical Study Design
| Characteristic | Non-Preeclamptic (Mean ± SD) | Preeclamptic (Mean ± SD) | t-Test (p-Value) |
|---|---|---|---|
| Age (years) | 29 ± 6 | 30 ± 7 | p > 0.3 |
| Systolic blood pressure (mm Hg) | 122 ± 8 | 164 ± 7 | p < 0.0001 |
| Diastolic blood pressure (mm Hg) | 74 ± 10 | 99 ± 5 | p < 0.0001 |
| Gravidity | 1.7 ± 1.0 | 1.6 ± 1.1 | p > 0.2 |
| Parity | 0.5 ± 0.7 | 0.4 ± 0.6 | p > 0.1 |
| Gestational age (weeks) | 39 ± 2 | 37 ± 3 | p < 0.001 |
| Newborn’s weight (g) | 3500 ± 500 | 2780 ± 900 | p < 0.0001 |
| Ethnicity | Caucasian | Caucasian | NA |
4.2. Immunochemical Assays
4.3. Preparation of Placental Mitochondria
4.4. Cell Culture
4.5. Cell Treatment with an Oxidative Stress Inducer
4.6. Placental Mitochondria Treatment with Oxidative Stress Inducer
4.7. Lipid Peroxidation Assays
4.8. Progesterone Biosynthesis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, D.K.; Wi, S. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am. J. Epidemiol. 2000, 151, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Reslan, O.M.; Khalil, R.A. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc. Hematol. Agents Med. Chem. 2010, 8, 204–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Holmes, V.A.; McCance, D.R. Could antioxidant supplementation prevent pre-eclampsia? Proc. Nutr. Soc. 2005, 64, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.A.; Strauss, J.F.; Walsh, S.W. Reduced methylation of the thromboxane synthase gene is correlated with its increased vascular expression in preeclampsia. Hypertension 2012, 59, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Vaughan, J.E.; Wang, Y.; Roberts, L.J. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000, 14, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nammour, T.M.; Fukunaga, M.; Ebert, J.; Morrow, J.D.; Roberts, L.J.; Hoover, R.L.; Badr, K.F. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 α, in the rat. Evidence for interaction with thromboxane A2 receptors. J. Clin. Investig. 1992, 90, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Moore, K.P.; Awad, J.A.; Ravenscraft, M.D.; Marini, G.; Badr, K.F.; Williams, R.; Roberts, L.J. Marked overproduction of non-cyclooxygenase derived prostanoids (F2-isoprostanes) in the hepatorenal syndrome. J. Lipid Mediat. 1993, 6, 417–420. [Google Scholar] [PubMed]
- Renke, M.; Tylicki, L.; Knap, N.; Rutkowski, P.; Neuwelt, A.; Larczynski, W.; Wozniak, M.; Rutkowski, B. Spironolactone attenuates oxidative stress in patients with chronic kidney disease. Hypertension 2008, 52, e132–e133. [Google Scholar] [CrossRef] [PubMed]
- Renke, M.; Tylicki, L.; Knap, N.; Rutkowski, P.; Neuwelt, A.; Petranyuk, A.; Larczynski, W.; Wozniak, M.; Rutkowski, B. High-dose angiotensin-converting enzyme inhibitor attenuates oxidative stress in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Mulla, Z.D. Seasonal trend in the occurrence of preeclampsia and eclampsia in Texas. Am. J. Hypertens. 2012, 25, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [PubMed]
- Bikle, D.D. Vitamin D: Newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol. Metab. 2010, 21, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2 vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Seuter, S.; Heikkinen, S. The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res. 2012, 32, 271–282. [Google Scholar] [PubMed]
- Woodham, P.C.; Brittain, J.E.; Baker, A.M.; Long, D.L.; Haeri, S.; Camargo, C.A., Jr.; Boggess, K.A.; Stuebe, A.M. Midgestation maternal serum 25-hydroxyvitamin D level and soluble fms-like tyrosine kinase 1/placental growth factor ratio as predictors of severe preeclampsia. Hypertension 2011, 58, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993, 326, 285–288. [Google Scholar] [CrossRef]
- Phillips, J.K.; Bernstein, I.M.; Mongeon, J.A.; Badger, G.J. Seasonal variation in preeclampsia based on timing of conception. Obstet. Gynecol. 2004, 104, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Abdel-Aleem, H.; Merialdi, M.; Mathai, M.; Ali, M.M.; Zavaleta, N.; Purwar, M.; Hofmeyr, J.; Nguyen, T.N.; Campodonico, L.; et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am. J. Obstet. Gynecol. 2006, 194, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.L.; Felton, S.K.; Riek, A.E.; Bernal-Mizrachi, C. Implications of vitamin D deficiency in pregnancy and lactation. Am. J. Obstet. Gynecol. 2010, 202, 429.e1–429.e9. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Tabacova, S.; Little, R.E.; Balabaeva, L.; Pavlova, S.; Petrov, I. Complications of pregnancy in relation to maternal lipid peroxides, glutathione, and exposure to metals. Reprod. Toxicol. 1994, 8, 217–224. [Google Scholar] [CrossRef]
- Walsh, S.W. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin. Reprod. Endocrinol. 1998, 16, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, S.J.; Wilson, R.; McKillop, J.H.; Walker, J.J. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am. J. Obstet. Gynecol. 1991, 165, 1701–1704. [Google Scholar] [CrossRef]
- Davidge, S.T.; Hubel, C.A.; Brayden, R.D.; Capeless, E.C.; McLaughlin, M.K. Sera antioxidant activity in uncomplicated and preeclamptic pregnancies. Obstet. Gynecol. 1992, 79, 897–901. [Google Scholar] [CrossRef]
- Morris, J.M.; Gopaul, N.K.; Endresen, M.J.; Knight, M.; Linton, E.A.; Dhir, S.; Anggard, E.E.; Redman, C.W. Circulating markers of oxidative stress are raised in normal pregnancy and pre-eclampsia. Br. J. Obstet. Gynaecol. 1998, 105, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.F., III; Martinez, F.; Kiriakidou, M. Placental steroid hormone synthesis: Unique features and unanswered questions. Biol. Reprod. 1996, 54, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C. Progesterone synthesis by the human placenta. Placenta 2005, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Klimek, J.; Wozniak, M.; Szymanska, G.; Zelewski, L. Inhibitory effect of free radicals derived from organic hydroperoxide on progesterone synthesis in human term placental mitochondria. Free Radic. Biol. Med. 1998, 24, 1168–1175. [Google Scholar] [CrossRef]
- Chwalisz, K.; Garfield, R.E. Role of progesterone during pregnancy: Models of parturition and preeclampsia. Z. Geburtshilfe Perinatol. 1994, 198, 170–180. [Google Scholar] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Zbytek, B.; Pisarchik, A.; Li, W.; Zjawiony, J.; Tuckey, R.C. Cytochromes P450 and skin cancer: Role of local endocrine pathways. Anticancer Agents Med. Chem. 2014, 14, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Li, W.; Zjawiony, J.K.; Zmijewski, M.A.; Nguyen, M.N.; Sweatman, T.; Miller, D.; Slominski, A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008, 275, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Sanchez, I.; Avila, E.; Halhali, A.; Vilchis, F.; Larrea, F. Identification of a 25-hydroxyvitamin D3 1α-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J. Clin. Endocrinol. Metab. 2000, 85, 2543–2549. [Google Scholar] [PubMed]
- Pospechova, K.; Rozehnal, V.; Stejskalova, L.; Vrzal, R.; Pospisilova, N.; Jamborova, G.; May, K.; Siegmund, W.; Dvorak, Z.; Nachtigal, P.; et al. Expression and activity of vitamin D receptor in the human placenta and in choriocarcinoma BeWo and JEG-3 cell lines. Mol. Cell. Endocrinol. 2009, 299, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Anari, M.R.; Khan, S.; O’Brien, P.J. The involvement of cytochrome P450 peroxidase in the metabolic bioactivation of cumene hydroperoxide by isolated rat hepatocytes. Chem. Res. Toxicol. 1996, 9, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.H.; Estabrook, R.W. The mechanism of cumene hydroperoxide-dependent lipid peroxidation: The function of cytochrome P-450. Arch. Biochem. Biophys. 1986, 251, 348–360. [Google Scholar] [CrossRef]
- Levin, W.; Lu, A.Y.; Jacobson, M.; Kuntzman, R.; Poyer, J.L.; McCay, P.B. Lipid peroxidation and the degradation of cytochrome P-450 heme. Arch. Biochem. Biophys. 1973, 158, 842–852. [Google Scholar] [CrossRef]
- Yao, K.; Falick, A.M.; Patel, N.; Correia, M.A. Cumene hydroperoxide-mediated inactivation of cytochrome P450 2B1. Identification of an active site heme-modified peptide. J. Biol. Chem. 1993, 268, 59–65. [Google Scholar] [PubMed]
- Barr, D.P.; Martin, M.V.; Guengerich, F.P.; Mason, R.P. Reaction of cytochrome P450 with cumene hydroperoxide: ESR spin-trapping evidence for the homolytic scission of the peroxide O–O bond by ferric cytochrome P450 1A2. Chem. Res. Toxicol. 1996, 9, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Barr, D.P.; Martin, M.V.; Guengerich, F.P.; Tomasi, A.; Mason, R.P. Detection of free radicals produced from the reaction of cytochrome P-450 with linoleic acid hydroperoxide. Biochem. J. 1997, 328, 565–571. [Google Scholar] [PubMed]
- Szymczyk, G.; Beltowski, J.; Marciniak, A.; Kotarski, J. Serum isoprostanes levels in patients after abdominal hysterectomy. Rocz. Akad. Med. Bialymst. 2005, 50, 322–324. [Google Scholar] [PubMed]
- Scholl, T.O.; Leskiw, M.; Chen, X.; Sims, M.; Stein, T.P. Oxidative stress, diet, and the etiology of preeclampsia. Am. J. Clin. Nutr. 2005, 81, 1390–1396. [Google Scholar] [PubMed]
- Tetteh, P.W.; Antwi-Boasiako, C.; Gyan, B.; Antwi, D.; Adzaku, F.; Obed, S. Impaired renal function and increased urinary isoprostane excretion in Ghanaian women with pre-eclampsia. Res. Rep. Trop. Med. 2013, 4, 7–13. [Google Scholar]
- Barden, A.; Ritchie, J.; Walters, B.; Michael, C.; Rivera, J.; Mori, T.; Croft, K.; Beilin, L. Study of plasma factors associated with neutrophil activation and lipid peroxidation in preeclampsia. Hypertension 2001, 38, 803–808. [Google Scholar] [CrossRef] [PubMed]
- McKinney, E.T.; Shouri, R.; Hunt, R.S.; Ahokas, R.A.; Sibai, B.M. Plasma, urinary, and salivary 8-epi-prostaglandin f2α levels in normotensive and preeclamptic pregnancies. Am. J. Obstet. Gynecol. 2000, 183, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, O.; Hayashi, M.; Osawa, H.; Kobayashi, K.; Takeda, S.; Vessby, B.; Basu, S. Isoprostanes, prostaglandins and tocopherols in pre-eclampsia, normal pregnancy and non-pregnancy. Free Radic. Res. 2004, 38, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Isoprostanes: Novel bioactive products of lipid peroxidation. Free Radic. Res. 2004, 38, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Fatty acid oxidation and isoprostanes: Oxidative strain and oxidative stress. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Fam, S.S.; Morrow, J.D. The isoprostanes: Unique products of arachidonic acid oxidation—A review. Curr. Med. Chem. 2003, 10, 1723–1740. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Beilin, L.J.; Ritchie, J.; Croft, K.D.; Walters, B.N.; Michael, C.A. Plasma and urinary 8-iso-prostane as an indicator of lipid peroxidation in pre-eclampsia and normal pregnancy. Clin. Sci. 1996, 91, 711–718. [Google Scholar] [PubMed]
- Regan, C.L.; Levine, R.J.; Baird, D.D.; Ewell, M.G.; Martz, K.L.; Sibai, B.M.; Rokach, J.; Lawson, J.A.; Fitzgerald, G.A. No evidence for lipid peroxidation in severe preeclampsia. Am. J. Obstet. Gynecol. 2001, 185, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Kalpdev, A.; Saha, S.C.; Dhawan, V. Vitamin C and E supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertens. Pregnancy 2011, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Choi, M.Y.; Longtine, M.S.; Nelson, D.M. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Chen, J.; Nguyen, M.N.; Li, W.; Yates, C.R.; Sweatman, T.; Janjetovic, Z.; Tuckey, R.C. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int. J. Biochem. Cell Biol. 2012, 44, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, R.P.; Thorp, J.M. Vitamin D in pregnancy: Current concepts. Curr. Opin. Obstet. Gynecol. 2012, 24, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements and supplementation during pregnancy. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, F.; Griz, L.; Dreyer, P.; Eufrazino, C.; Bandeira, C.; Freese, E. Vitamin D deficiency: A global perspective. Arq Bras. Endocrinol. Metabol. 2006, 50, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Simhan, H.N.; Powers, R.W.; Frank, M.P.; Cooperstein, E.; Roberts, J.M. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J. Nutr. 2007, 137, 447–452. [Google Scholar] [PubMed]
- Holmes, V.A.; Barnes, M.S.; Alexander, H.D.; McFaul, P.; Wallace, J.M. Vitamin D deficiency and insufficiency in pregnant women: A longitudinal study. Br. J. Nutr. 2009, 102, 876–881. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, M.N.; Kiely, M.; Higgins, J.R.; Cashman, K.D. Prevalence of suboptimal vitamin D status during pregnancy. Ir. Med. J. 2008, 101, 240, 242–240, 243. [Google Scholar] [PubMed]
- Kmiec, P.; Zmijewski, M.; Waszak, P.; Sworczak, K.; Lizakowska-Kmiec, M. Vitamin D deficiency during winter months among an adult, predominantly urban, population in Northern Poland. Endokrynol. Pol. 2014, 65, 105–113. [Google Scholar] [PubMed]
- Kmiec, P.; Zmijewski, M.; Lizakowska-Kmiec, M.; Sworczak, K. Widespread vitamin D deficiency among adults from northern Poland (54 degrees N) after months of low and high natural UVB radiation. Endokrynol. Pol. 2015, 66, 30–38. [Google Scholar] [PubMed]
- Pludowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokol, D.; Czech-Kowalska, J.; Debski, R.; Decsi, T.; Dobrzanska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe-recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Simhan, H.N.; Catov, J.M.; Roberts, J.M.; Platt, R.W.; Diesel, J.C.; Klebanoff, M.A. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef]
- Walsh, S.W.; Wang, Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J. Clin. Endocrinol. Metab. 1995, 80, 1888–1893. [Google Scholar] [PubMed]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Kurauchi, O.; Tanaka, M.; Yoneda, M.; Uchida, K.; Itakura, A.; Furugori, K.; Mizutani, S.; Tomoda, Y. Increased mitochondrial damage by lipid peroxidation in trophoblast cells of preeclamptic placentas. Biochem. Mol. Biol. Int. 1997, 41, 767–775. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Xu, W.; Chen, Y.; Liu, X.; Xi, M. Abnormal apoptosis of trophoblastic cells is related to the up-regulation of CYP11A gene in placenta of preeclampsia patients. PLoS ONE 2013, 8, e59609. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Moon, M.H.; Kim, K.T.; Jeong, D.H.; Kim, Y.N.; Chung, B.C.; Choi, M.H. Cytochrome P450-mediated metabolic alterations in preeclampsia evaluated by quantitative steroid signatures. J. Steroid Biochem. Mol. Biol. 2014, 139, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, A.; Klimek, J.; Masaoka, M.; Kaminski, M.; Kedzior, J.; Majczak, A.; Niemczyk, E.; Wozniak, M.; Trzonkowski, P.; Wakabayashi, T. Partial characterization of human choriocarcinoma cell line JAR cells in regard to oxidative stress. Acta Biochim. Pol. 2004, 51, 1023–1038. [Google Scholar] [PubMed]
- Hallmann, A.; Milczarek, R.; Lipinski, M.; Kossowska, E.; Spodnik, J.H.; Wozniak, M.; Wakabayashi, T.; Klimek, J. Fast perinuclear clustering of mitochondria in oxidatively stressed human choriocarcinoma cells. Folia Morphol. 2004, 63, 407–412. [Google Scholar]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E928–E935. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Arranz, C.; Avila, E.; Halhali, A.; Vilchis, F.; Larrea, F. Expression and activity of 25-hydroxyvitamin D-1α-hydroxylase are restricted in cultures of human syncytiotrophoblast cells from preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2002, 87, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Halhali, A.; Tovar, A.R.; Torres, N.; Bourges, H.; Garabedian, M.; Larrea, F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J. Clin. Endocrinol. Metab. 2000, 85, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Haeri, S.; Camargo, C.A., Jr.; Espinola, J.A.; Stuebe, A.M. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J. Clin. Endocrinol. Metab. 2010, 95, 5105–5109. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986, 201, 291–295. [Google Scholar] [CrossRef]
- Braughler, J.M.; Duncan, L.A.; Chase, R.L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 1986, 261, 10282–10289. [Google Scholar] [PubMed]
- Walsh, S.W.; Parisi, V.M. The role of arachidonic acid metabolites in preeclampsia. Semin. Perinatol. 1986, 10, 334–355. [Google Scholar] [PubMed]
- Wang, Y.P.; Walsh, S.W.; Guo, J.D.; Zhang, J.Y. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am. J. Obstet. Gynecol. 1991, 165, 1695–1700. [Google Scholar] [CrossRef]
- Warso, M.A.; Lands, W.E. Lipid peroxidation in relation to prostacyclin and thromboxane physiology and pathophysiology. Br. Med. Bull. 1983, 39, 277–280. [Google Scholar] [PubMed]
- Huang, C.H.; Ren, F.R.; Shan, G.Q.; Qin, H.; Mao, L.; Zhu, B.Z. Molecular mechanism of metal-independent decomposition of organic hydroperoxides by the halogenated quinoid carcinogens and the potential biological implications. Chem. Res. Toxicol. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Vargas, F.R.; Zoltan, T.; Proverbio, T.; Pinero, S.; Proverbio, F.; Marin, R. Magnesium sulfate affords protection against oxidative damage during severe preeclampsia. Placenta 2015, 36, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, S.; Bayar, U.O.; Can, M.; Guven, B.; Mungan, G.; Dogan, S.; Sumbuloglu, V. Levels of oxidized LDL, estrogens, and progesterone in placenta tissues and serum paraoxonase activity in preeclampsia. Mediators Inflamm. 2013, 2013, 862982. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W. Progesterone and estradiol production by normal and preeclamptic placentas. Obstet. Gynecol. 1988, 71, 222–226. [Google Scholar] [CrossRef]
- Walsh, S.W. Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am. J. Obstet. Gynecol. 1985, 152, 335–340. [Google Scholar] [CrossRef]
- Walsh, S.W.; Coulter, S. Increased placental progesterone may cause decreased placental prostacyclin production in preeclampsia. Am. J. Obstet. Gynecol. 1989, 161, 1586–1592. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Rocki, W.; Murray, R.; Mayo, G.; Fitzgerald, G.A. Thromboxane A2 synthesis in pregnancy-induced hypertension. Lancet 1990, 335, 751–754. [Google Scholar] [CrossRef]
- Hogg, K.; Blair, J.D.; McFadden, D.E.; von, D.P.; Robinson, W.P. Early onset pre-eclampsia is associated with altered DNA methylation of cortisol-signalling and steroidogenic genes in the placenta. PLoS ONE 2013, 8, e62969. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, R.; Lagiou, P.; Vatten, L.J.; Mucci, L.; Trichopoulos, D.; Hellerstein, S.; Ekbom, A.; Adami, H.O.; Hsieh, C.C. Pregnancy hormones, pre-eclampsia, and implications for breast cancer risk in the offspring. Cancer Epidemiol. Biomark. Prev. 2003, 12, 647–650. [Google Scholar]
- Beaudoin, C.; Blomquist, C.H.; Bonenfant, M.; Tremblay, Y. Expression of the genes for 3 β-hydroxysteroid dehydrogenase type 1 and cytochrome P450scc during syncytium formation by human placental cytotrophoblast cells in culture and the regulation by progesterone and estradiol. J. Endocrinol. 1997, 154, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Risberg, A.; Olsson, K.; Lyrenas, S.; Sjoquist, M. Plasma vasopressin, oxytocin, estradiol, and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet. Gynecol. Scand. 2009, 88, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.; Duley, L. Progesterone for preventing pre-eclampsia and its complications. Cochrane. Database. Syst. Rev. 2006, 4. [Google Scholar] [CrossRef]
- Al, E.S.; Hammoudeh, M. Vitamin D study in pregnant women and their babies. Qatar Med. J. 2013, 2013, 32–37. [Google Scholar]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2014, 151, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Li, W.; Szczesniewski, A.; Tuckey, R.C. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005, 272, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Nguyen, M.N.; Slominski, A. Kinetics of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) in phospholipid vesicles and cyclodextrin. Int. J. Biochem. Cell Biol. 2008, 40, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.; Brantsaeter, A.L.; Trogstad, L.; Alexander, J.; Roth, C.; Magnus, P.; Meltzer, H.M. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 2009, 20, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [PubMed]
- Heaney, R.P. Vitamin D: Criteria for safety and efficacy. Nutr. Rev. 2008, 66, S178–S181. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.F.; Patel, M.; Dimaano, R.; Li-Ng, M.; Talwar, S.A.; Mikhail, M.; Pollack, S.; Yeh, J.K. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am. J. Clin. Nutr. 2008, 87, 1952–1958. [Google Scholar] [PubMed]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Al, M.A.; Raqib, R.; Akhtar, E.; Perumal, N.; Pezzack, B.; Baqui, A.H. Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: The AViDD trial. Nutr. J. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Sablok, A.; Batra, A.; Thariani, K.; Batra, A.; Bharti, R.; Aggarwal, A.R.; Kabi, B.C.; Chellani, H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. 2015; in press. [Google Scholar]
- Wang, J.; Slominski, A.; Tuckey, R.C.; Janjetovic, Z.; Kulkarni, A.; Chen, J.; Postlethwaite, A.E.; Miller, D.; Li, W. 20-hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012, 32, 739–746. [Google Scholar] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE 2010, 5, e9907. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Bhattacharya, K.G.; Wang, J.; Li, W.; Jiao, Y.; Gu, W.; Brown, M.; et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J. Clin. Endocrinol. Metab. 2013, 98, E298–E303. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Zmijewski, M.A.; Tuckey, R.C.; DeLeon, D.A.; Nguyen, M.N.; Pfeffer, L.M.; Slominski, A.T. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-κB activity by increasing IkappaB α levels in human keratinocytes. PLoS ONE 2009, 4, e5988. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Thorpe, E.M., Jr.; Slominski, A.T. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J. Cell Physiol. 2010, 223, 36–48. [Google Scholar] [PubMed]
- Chen, J.; Wang, J.; Kim, T.K.; Tieu, E.W.; Tang, E.K.; Lin, Z.; Kovacic, D.; Miller, D.D.; Postlethwaite, A.; Tuckey, R.C.; et al. Novel vitamin D analogs as potential therapeutics: Metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014, 34, 2153–2163. [Google Scholar] [PubMed]
- Castejon, O.C.S. Mitochondrial dysfunction and apoptosis in trophoblast cells during preeclampsia: And ultrastural study. Electron. J. Biomed. 2011, 2, 30–38. [Google Scholar]
- Sobek, J.; Martschke, R.; Fischer, H. Entropy control of the cross-reaction between carbon-centered and nitroxide radicals. J. Am. Chem. Soc. 2001, 123, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Kalinska, B.; Canapa, A.; Canestrari, S.; Wozniak, M.; Olmo, E.; Greci, L. The effects of nitroxide radicals on oxidative DNA damage. Free Radic. Biol. Med. 2000, 28, 1257–1265. [Google Scholar] [CrossRef]
- Chateauneuf, J.; Lusztyk, J.; Ingold, K.U. Absolute rate constants for the reactions of some carbon-centered radicals with 2,2,6,6-tetramethyl-1-piperidinoxyl. J. Org. Chem. 1988, 53, 1629–1632. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Samuni, A.; Krishna, M.C.; DeGraff, W.G.; Ahn, M.S.; Samuni, U.; Russo, A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry 1990, 29, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R.; Holmes, V.A.; Maresh, M.J.; Patterson, C.C.; Walker, J.D.; Pearson, D.W.; Young, I.S. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): A randomised placebo-controlled trial. Lancet 2010, 376, 259–266. [Google Scholar] [CrossRef]
- Klimek, J. The involvement of superoxide and iron ions in the NADPH-dependent lipid peroxidation in human placental mitochondria. Biochim. Biophys. Acta 1988, 958, 31–39. [Google Scholar] [CrossRef]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [PubMed]
- Thompson, E.A., Jr.; Siiteri, P.K. The involvement of human placental microsomal cytochrome P-450 in aromatization. J. Biol. Chem. 1974, 249, 5373–5378. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabul, P.; Wozniak, M.; Slominski, A.T.; Preis, K.; Gorska, M.; Korozan, M.; Wieruszewski, J.; Zmijewski, M.A.; Zabul, E.; Tuckey, R.; et al. A Proposed Molecular Mechanism of High-Dose Vitamin D3 Supplementation in Prevention and Treatment of Preeclampsia. Int. J. Mol. Sci. 2015, 16, 13043-13064. https://doi.org/10.3390/ijms160613043
Zabul P, Wozniak M, Slominski AT, Preis K, Gorska M, Korozan M, Wieruszewski J, Zmijewski MA, Zabul E, Tuckey R, et al. A Proposed Molecular Mechanism of High-Dose Vitamin D3 Supplementation in Prevention and Treatment of Preeclampsia. International Journal of Molecular Sciences. 2015; 16(6):13043-13064. https://doi.org/10.3390/ijms160613043
Chicago/Turabian StyleZabul, Piotr, Michal Wozniak, Andrzej T. Slominski, Krzysztof Preis, Magdalena Gorska, Marek Korozan, Jan Wieruszewski, Michal A. Zmijewski, Ewa Zabul, Robert Tuckey, and et al. 2015. "A Proposed Molecular Mechanism of High-Dose Vitamin D3 Supplementation in Prevention and Treatment of Preeclampsia" International Journal of Molecular Sciences 16, no. 6: 13043-13064. https://doi.org/10.3390/ijms160613043