Synthesis, DNA Binding, and Antiproliferative Activity of Novel Acridine-Thiosemicarbazone Derivatives
Abstract
:1. Introduction
2. Results and Discussion
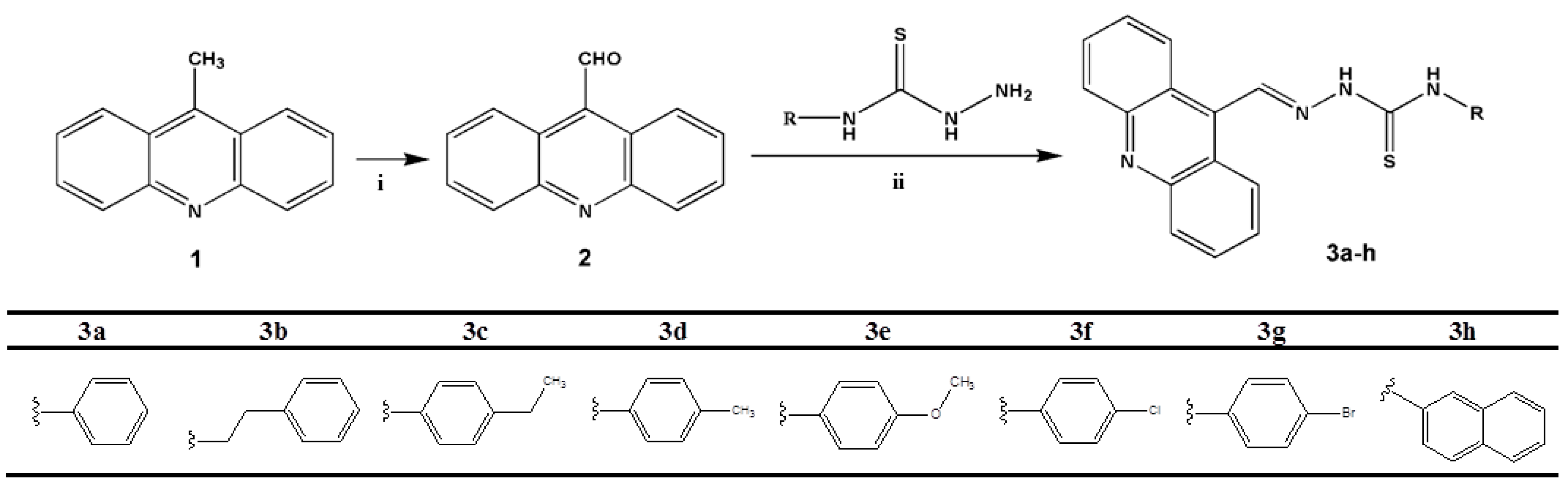
2.1. Chemistry
2.2. DNA Binding Studies

| Compound | λmax Absent (nm) | λmax Present (nm) | Extinction Coefficient (ε, M−1) | Δλ (nm) | Hypochr. (%) a | Hyperchr. (%) b | Kb (M−1) | λ Excitation (nm) | λ Emission (nm) | KSV c (M−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 360 | 366 | 7.300 | 6 | – | 92.58 | 3.77 × 105 | 359 | 439 | 0.92 × 104 |
| 3b | 375 | 372 | 8.600 | 3 | – | 28.13 | 1.84 × 105 | 370 | 441 | −0.2 × 104 |
| 3c | 376 | 375 | 13.780 | 1 | 20.32 | – | 1.2 × 105 | 370 | 440 | 0.18 × 104 |
| 3d | 375 | 371 | 14.040 | 4 | 4.69 | – | 1.74 × 104 | 370 | 441 | 0.87 × 104 |
| 3e | 375 | 371 | 8.760 | 4 | – | 25.22 | 6.5 × 104 | 361 | 441 | −0.27 × 104 |
| 3f | 357 | 358 | 14.860 | 1 | 39.59 | – | 1.0 × 106 | 355 | 440 | 2.18 × 104 |
| 3g | 362 | 362 | 9.200 | 0 | – | 24.4 | 9.91 × 104 | 352 | 439 | 0.5 × 104 |
| 3h | 383 | 371 | 11.546 | 12 | 11.54 | – | 8.47 × 105 | 350 | 439 | 1.75 × 104 |


2.3. Antiproliferative Activity of 3a–h

| Compound | Cancer Cell Lines a | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U251 | MCF-7 | NCI-ADR | 786-O | NCI-H460 | PC-3 | OVCAR-3 | HT-29 | K-562 | HaCaT | |||||||||||
| GI50 | TGI | GI50 | TGI | GI50 | TGI | GI50 | TGI | GI50 | TGI | GI50 | TGI | GI50 | TGI | GI50 | TGI | GI50 | TGI | GI50 | TGI | |
| 3a | 8.74 | 33.75 | 6.85 | 12.92 | 6.35 | 17.24 | 7.99 | 41.66 | 6.97 | 10.58 | 6.30 | 18.88 | 4.41 | 21.13 | 7.54 | 29.28 | 9.26 | >100 | 4.17 | 51.42 |
| 3b | 80.84 | >100 | 66.72 | n.a. | >100 | n.a. | 64.44 | 92.31 | 16.75 | >100 | 73.74 | >100 | 7.26 | 98.48 | >100 | n.a. | n.a. | n.a. | >100 | n.a. |
| 3c | >100 | n.a. | 62.73 | n.a. | 62.94 | n.a. | 28.75 | >100 | 8.63 | n.a. | 22.98 | n.a. | 8.38 | >100 | n.a. | n.a. | n.a. | n.a. | 7.00 | >100 |
| 3d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 3e | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 3f | >100 | n.a. | 27.19 | >100 | 73.15 | >100 | >100 | n.a. | 28.36 | n.a. | 40.33 | n.a. | 62.41 | >100 | >100 | n.a. | 64.64 | >100 | >100 | n.a. |
| 3g | >100 | n.a. | n.a. | n.a. | n.a. | n.a. | >100 | n.a. | >100 | n.a. | 45.89 | >100 | 0.98 | 65.7 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 3h | >100 | n.a. | 69.24 | n.a. | 68.26 | >100 | >100 | n.a. | 70.37 | n.a. | 50.88 | n.a. | 71.39 | >100 | n.a. | n.a. | n.a. | n.a. | 70.06 | n.a. |
| DOX b | 0.046 | 1.20 | 0.036 | 1.48 | 0.24 | 3.80 | 0.051 | 0.63 | 0.04 | 0.54 | 0.075 | 1.20 | 0.17 | 1.52 | 0.43 | 8.37 | 0.18 | 27.64 | 0.053 | 0.42 |
| m-AMSA | 0.26 | 2.81 | 0.23 | 6.96 | 2.01 | 11.94 | 0.52 | 3.14 | 0.47 | 2.58 | 0.39 | 5.37 | 0.87 | 4.26 | 0.64 | 4.66 | 0.18 | 1.82 | 0.46 | 0.86 |
3. Experimental Section
3.1. Materials and Methods
3.2. General Preparation of the Acridine-Thiosemicarbazone Derivatives 3a–h
3.2.1. (Z)-2-(Acridin-9-ylmethylene)-N-phenylhydrazinecarbothioamide (3a)
3.2.2. (Z)-2-(Acridin-9-ylmethylene)-N-phenethylhydrazinecarbothioamide (3b)
3.2.3. (Z)-2-(Acridin-9-ylmethylene)-N-(4-ethylphenyl)hydrazinecarbothioamide (3c)
3.2.4. (Z)-2-(Acridin-9-ylmethylene)-N-(p-tolyl)hydrazinecarbothioamide (3d)
3.2.5. (Z)-2-(Acridin-9-ylmethylene)-N-(4-methoxyphenyl)hydrazinecarbothioamide (3e)
3.2.6. (Z)-2-(Acridin-9-ylmethylene)-N-(4-chlorophenyl)hydrazinecarbothioamide (3f)
3.2.7. (Z)-2-(Acridin-9-ylmethylene)-N-(4-bromophenyl)hydrazinecarbothioamide (3g)
3.2.8. (Z)-2-(Acridin-9-ylmethylene)-N-(naphtalen-1-yl)hydrazinecarbothioamide (3h)
3.3. UV–Vis Absorption Measurements
3.4. Fluorescence Measurements
3.5. Determination of Antiproliferative Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maaloum, M.; Muller, P.; Harlepp, S. DNA-intercalator interactions: Structural and physical analysis using atomic force microscopy in solution. Soft Matter 2013, 9, 11233–11240. [Google Scholar] [CrossRef]
- Rescifina, A.; Zagni, C.; Varrica, M.G.; Pistarà, V.; Corsaro, A. Recent advances in small organic molecules as DNA intercalating agents: Synthesis, activity, and modeling. Eur. J. Med. Chem. 2014, 74, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Gan, J.; Huang, Z. Structure-based DNA-targeting strategies with small molecule ligands for drug discovery. Med. Res. Rev. 2013, 33, 1119–1173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaur, M.; Kumari, M. Acridine: A versatile heterocyclic nucleus. Acta Pol. Pharm. 2012, 69, 3–9. [Google Scholar] [PubMed]
- Ketron, A.C.; Denny, W.A.; Graves, D.E.; Osheroff, N. Amsacrine as a topoisomerase II poison: Importance of drug-DNA interactions. Biochemistry 2012, 51, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Jangir, D.K.; Dey, S.K.; Kundu, S.; Mehrotra, R. Assessment of amsacrine binding with DNA using UV–Visible, circular dichroism and Raman spectroscopic techniques. J. Photochem. Photobiol. B 2012, 114, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Belmont, P.; Bosson, J.; Godet, T.; Tiano, M. Acridine and acridone derivatives, anticancer properties and synthetic methods: Where are we now? Anticancer Agents Med. Chem. 2007, 7, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Chilin, A.; Marzaro, G.; Marzano, C.; Via, L.D.; Ferlin, M.G.; Pastorini, G.; Guiotto, A. Synthesis and antitumor activity of novel amsacrine analogs: The critical role of the acridine moiety in determining their biological activity. Bioorg. Med. Chem. 2009, 17, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Lafayette, E.A.; Almeida, S.M.V.; Pitta, M.G.R.; Beltrão, E.I.C.; Silva, T.G.; Moura, R.O.; Pitta, I.R.; Carvalho Júnior, L.B.; Lima, M.C.A. Synthesis, DNA binding and topoisomerase I inhibition activity of thiazacridine and imidazacridine derivatives. Molecules 2013, 18, 15035–15050. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.W.A.; Silva, T.G.; Pitta, M.G.R.; Bezerra, D.P.; Costa-Lotufo, L.V.; Moraes, M.O.; Pessoa, C.; Moura, M.A.F.B.; Abreu, F.C.; Lima, M.C.A.; et al. Synthesis and cytotoxic activity of new acridine-thiazolidine derivatives. Bioorg. Med. Chem. 2012, 20, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gutierrez, E.; Kovacevic, Z.; Saletta, F.; Obeidy, P.; Rahmanto, Y.S.; Richardson, D.R. Iron chelators for the treatment of cancer. Curr. Med. Chem. 2012, 19, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Viñuelas-Zahínos, E.; Luna-Giles, F.; Torres-García, P.; Fernández-Calderón, M.C. Co(III), Ni(II), Zn(II) and Cd(II) complexes with 2-acetyl-2-thiazoline thiosemicarbazone: Synthesis, characterization, X-ray structures and antibacterial activity. Eur. J. Med. Chem. 2011, 46, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Chellan, P.; Nasser, S.; Vivas, L.; Chibale, K.; Smith, G.S. Cyclopalladated complexes containing tridentate thiosemicarbazone ligands of biological significance: Synthesis, structure and antimalarial activity. J. Organomet. Chem. 2010, 695, 2225–2232. [Google Scholar] [CrossRef]
- Ferraz, K.O.S.; Cardoso, G.M.M.; Bertollo, C.M.; Souza-Fagundes, E.M.; Speziali, N.; Zani, C.L.; Mendes, I.C.; Gomes, M.A.; Beraldo, H. N(4)-tolyl-2-benzoylpyridine-derived thiosemicarbazones and their palladium(II) and platinum(II) complexes: Cytotoxicity against human solid tumor cells. Polyhedron 2011, 30, 315–321. [Google Scholar] [CrossRef]
- Ali, A.Q.; Teoh, S.G.; Eltayeb, N.E.; Ahamed, M.B.K.; Majid, A.M.S.A. Synthesis of copper (II) complexes of isatin thiosemicarbazone derivatives: In vitro anti-cancer, DNA binding, and cleavage activities. Polyhedron 2014, 74, 6–15. [Google Scholar] [CrossRef]
- Chandra, S.; Vandana. Synthesis, spectroscopic, anticancer and antibacterial studies of Ni(II) and Cu(II) complexes with 2-carboxybenzaldehyde thiosemicarbazone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Ma, Z-Y.; Li, A.; Liu, Y.-H.; Xie, C.-Z.; Qiang, Z-Y.; Xu, J-Y. Thiosemicarbazone Cu(II) and Zn(II) complexes as potential anticancer agents: Syntheses, crystal structure, DNA cleavage, cytotoxicity and apoptosis induction activity. J. Inorg. Biochem. 2014, 136, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; Guan, T.S.; Haque, R.A.; Ahamed, M.B.K.; Abdul Majid, A.M.S. Synthesis and characterization of thiosemicarbazonato molybdenum(VI) complexes: In vitro DNA binding, cleavage, and antitumor activities. Polyhedron 2015, 85, 93–103. [Google Scholar] [CrossRef]
- Kumar, S.M.; Rajesh, J.; Anitha, K.; Dhahagani, K.; Marappan, M.; Gandhi, N.I.; Rajagopal, G. Synthesis, characterization, crystal structure and cytotoxic properties of thiosemicarbazide Ni(II) and Zn(II) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Thota, S.; Vallala, S.; Yerra, R.; Barreiro, E.J. Design, synthesis, characterization, cytotoxic and structure activity relationships of novel Ru(II) complexes. Chin. Chem. Lett. 2015, in press. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Kalinowski, D.S.; Lovejoy, D.B.; Yu, Y.; Rahmanto, Y.S.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. The medicinal chemistry of novel iron chelators for the treatment of cancer. Curr. Top. Med. Chem. 2011, 11, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Prathiba, K.; Jayaprakash, V.; Basu, A.; Mishra, N.; Zhou, B.; Hu, S.; Yen, Y. Synthesis and ribonucleotide reductase inhibitory activity of thiosemicarbazones. Bioorg. Med. Chem. Lett. 2008, 18, 6248–6250. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A. Iron chelators with topoisomerase-inhibitory activity and their anticancer applications. Antioxid. Redox Signal. 2013, 18, 930–955. [Google Scholar] [CrossRef] [PubMed]
- Aye, Y.; Long, M.J.C.; Stubbe, J. Mechanistic studies of semicarbazone triapine targeting Human ribonucleotide reductase in vitro and in mammalian cells tyrosyl radical quenching not involving reactive oxygen species. J. Biol. Chem. 2012, 28, 35768–35778. [Google Scholar] [CrossRef] [PubMed]
- Kunos, C.A.; Radivoyevitch, T.; Waggoner, S.; Debernardo, R.; Zanotti, K.; Resnick, K.; Fusco, N.; Adams, R.; Redline, R.; Faulhaber, P.; et al. Radiochemotherapy plus 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in advanced-stage cervical and vaginal cancers. Gynecol. Oncol. 2013, 130, 75–80. [Google Scholar] [PubMed]
- Vandresen, F.; Falzirolli, H.; Batista, S.A.A.; Silva-Giardini, A.P.B.; Oliveira, D.N.; Catharino, R.R.; Ruiz, A.L.T.G.; Carvalho, J.E.; Foglio, M.A.; Silva, C.C. Novel R-(+)-limonene-based thiosemicarbazones and their antitumor activity against human tumor cell lines. Eur. J. Med. Chem. 2014, 79, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xie, S.; Zhou, Y.; Tang, X.; Liu, J.; Yang, W.; Qiu, M. Design and synthesis of novel 5,6-disubstituted pyridine-2,3-dione-3-thiosemicarbazone derivatives as potential anticancer agents. Eur. J. Med. Chem. 2014, 81, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Cui, J.; Su, S.; Lin, Q.; Jia, L.; Fan, L.; Huang, Y. Synthesis and antiproliferative activity of some steroidal thiosemicarbazones, semicarbazones and hydrozones. Steroids 2014, 87, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.Q.; Teoh, S.G.; Salhin, A.; Eltayeb, N.E.; Ahamed, M.B.K.; Abdul Majid, A.M.S. Synthesis of platinum(II) complexes of isatin thiosemicarbazone derivatives: In vitro anti-cancer and deoxyribose nucleic acid binding activities. Inorg. Chim. Acta 2014, 416, 235–244. [Google Scholar] [CrossRef]
- Ali, A.Q.; Teoh, S.G.; Salhin, A.; Eltayeb, N.E.; Ahamed, M.B.K.; Abdul Majid, A.M.S. Synthesis of isatin thiosemicarbazones derivatives: In vitro anti-cancer, DNA binding and cleavage activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 125, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Wu, Q.; Shi, L.; Cui, F. Spectroscopia study one thiosemicarbazone derivative with ctDNA using ethidium bromide as a fluorescence probe. Int. J. Biol. Macromol. 2013, 60, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, O.; Nishinohara, M.; Tashiro, M.B. Compounds related to acridine. I. Condensation of acridine derivatives having active methyl group and aromatic nitroso compounds. Bull. Chem. Soc. 1963, 36, 1477–1485. [Google Scholar] [CrossRef]
- Mosher, M.D.; Natale, N.R. The preparation of intercalating isoxazoles via a nitrile oxide cycloaddition. J. Heterocycl. Chem. 1995, 32, 779–781. [Google Scholar] [CrossRef]
- Gürsoy, A.; Terzioglu, N.; Ötuk, G. Synthesis of some new hydrazide-hydrazones, thiosemicarbazides and thiazolidinones as possible antimicrobials. Eur. J. Med. Chem. 1997, 32, 753–757. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, T.L.; Ding, L.; Wang, W.H. Synthesis, characterization, fluorescence and DNA-binding studies of europium(III) pirates complexes with amide-based 2,3-dihydroxynaphthalene derivatives. J. Rare Earths 2012, 30, 297–303. [Google Scholar] [CrossRef]
- Baranovsky, S.F.; Bolotin, P.A.; Evstigneev, M.P.; Chenyshev, D.N. Interaction of ethidium bromide and caffeine with DNA in aqueous solution. J. Appl. Spectrosc. 2009, 76, 132–139. [Google Scholar] [CrossRef]
- Raja, D.S.; Bhuvanesh, N.S.P.; Natarajan, K. DNA binding, protein interaction, radical scavenging and cytotoxic activity of 2-oxo-1,2-dihydroquinoline-3-carbaldehyde(2'-hydroxybenzoyl)hydrazone and its Cu(II) complexes: A structure activity relationship study. Inorg. Chim. Acta 2012, 385, 81–93. [Google Scholar] [CrossRef]
- Faulhaber, K.; Granzhan, A.; Ihmels, H.; Otto, D.; Thomas, L.; Wells, S. Studies of the fluorescence light-up effect of amino-substituted benzo[b]quinolizinium derivatives in the presence of biomacromolecules. Photochem. Photobiol. Sci. 2011, 10, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Xi, X.L.; Yang, P. Studies on DNA binding to metal complexes of sal2trien. Biochemistry 2007, 72, 37–43. [Google Scholar] [CrossRef]
- McGhee, J.D.; von Hippel, P.H. Theoretical aspects of DNA-Protein interactions: Co-operative and non-cooperative binding of large ligands to a one-dimensional homogeneous lattice. J. Mol. Biol. 1974, 86, 469–489. [Google Scholar] [CrossRef]
- Ihmels, H.; Otto, D. Intercalation of organic dye molecules into double-stranded DNA general principles and recent developments. Top. Curr. Chem. 2005, 258, 161–204. [Google Scholar]
- Plsikova, J.; Janovec, L.; Koval, J.; Ungvarsky, J.; Mikes, J.; Jendzelovsky, R.; Fedorocko, P.; Imrich, J.; Kristian, P.; Kasparkova, J.; et al. 3,6-Bis(3-alkyl-guanidino)acridines as DNA-intercalating antitumor agents. Eur. J. Med. Chem. 2012, 57, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Sabolova, D.; Kozurkova, M.; Kristian, P.; Danihel, I.; Podhradsk, D.; Imrich, J. Determination of the binding affinities of plasmid DNA using fluorescent intercalators possessing an acridine skeleton. Int. J. Biol. Macromol. 2006, 38, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Benner, K.; Ihmels, H.; Kölsch, S.; Pithan, P.M. Targeting abasic site-containing DNA with annelated quinolizinium derivatives: the influence of size, shape and substituents. Org. Biomol. Chem. 2014, 12, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Janovec, L.; Kozurkova, M.; Sabolova, D.; Ungvarsky, J.; Paulikova, H.; Plsikova, J.; Vantosa, Z.; Imrich, J. Cytotoxic 3,6-bis((imidazolidionone)imino)acridines: Synthesis, DNA binding and molecular modeling. Bioorg. Med. Chem. 2011, 19, 1790–1801. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Gao, C.; Liu, F.; Luan, X.; Tan, C.; Liu, H.; Xie, Y.; Jin, Y.; Jiang, Y. Novel synthetic 2-amino-10-(3,5-dimethoxy)benzyl-9(10H)-acridinone derivatives as potent DNA-binding antiproliferative agents. Bioorg. Med. Chem. 2010, 18, 7507–7514. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Liu, Q.; Luo, H.; Zhang, G. Spectroscopic, viscositc and molecular modeling studies on the interaction of 3ʹ-azido-daunorubicin thiosemicarbazone with DNA. J. Fluoresc. 2014, 24, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; Gray-Goodrich, M.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Nat. Cancer. Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Stefanska, B.; Bontemps-Gracz, M.M.; Antonini, I.; Martelli, S.; Arciemiuk, M.; Piwkowska, A.; Rogacka, D.; Borowski, E. 2,7-Dihydro-3H-pyridazino[5,4,3-kl]acridin-3-one derivatives, novel type of cytotoxic agents active on multidrug-resistant cell lines. Synthesis and biological evaluation. Bioorg. Med. Chem. 2005, 13, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Liesen, A.P.; Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; Araújo, J.M.; Lima, J.G.; Faria, A.R.; Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.; Shimer, G.H.; Meehan, T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Almeida, S.M.V.; Lafayette, E.A.; Da Silva, L.P.B.G.; Amorim, C.A.d.C.; De Oliveira, T.B.; Ruiz, A.L.T.G.; De Carvalho, J.E.; De Moura, R.O.; Beltrão, E.I.C.; De Lima, M.D.C.A.; et al. Synthesis, DNA Binding, and Antiproliferative Activity of Novel Acridine-Thiosemicarbazone Derivatives. Int. J. Mol. Sci. 2015, 16, 13023-13042. https://doi.org/10.3390/ijms160613023
De Almeida SMV, Lafayette EA, Da Silva LPBG, Amorim CAdC, De Oliveira TB, Ruiz ALTG, De Carvalho JE, De Moura RO, Beltrão EIC, De Lima MDCA, et al. Synthesis, DNA Binding, and Antiproliferative Activity of Novel Acridine-Thiosemicarbazone Derivatives. International Journal of Molecular Sciences. 2015; 16(6):13023-13042. https://doi.org/10.3390/ijms160613023
Chicago/Turabian StyleDe Almeida, Sinara Mônica Vitalino, Elizabeth Almeida Lafayette, Lúcia Patrícia Bezerra Gomes Da Silva, Cézar Augusto da Cruz Amorim, Tiago Bento De Oliveira, Ana Lucia Tasca Gois Ruiz, João Ernesto De Carvalho, Ricardo Olímpio De Moura, Eduardo Isidoro Carneiro Beltrão, Maria Do Carmo Alves De Lima, and et al. 2015. "Synthesis, DNA Binding, and Antiproliferative Activity of Novel Acridine-Thiosemicarbazone Derivatives" International Journal of Molecular Sciences 16, no. 6: 13023-13042. https://doi.org/10.3390/ijms160613023