Effect of Oral Taurine on Morbidity and Mortality in Elderly Hip Fracture Patients: A Randomized Trial
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Patients
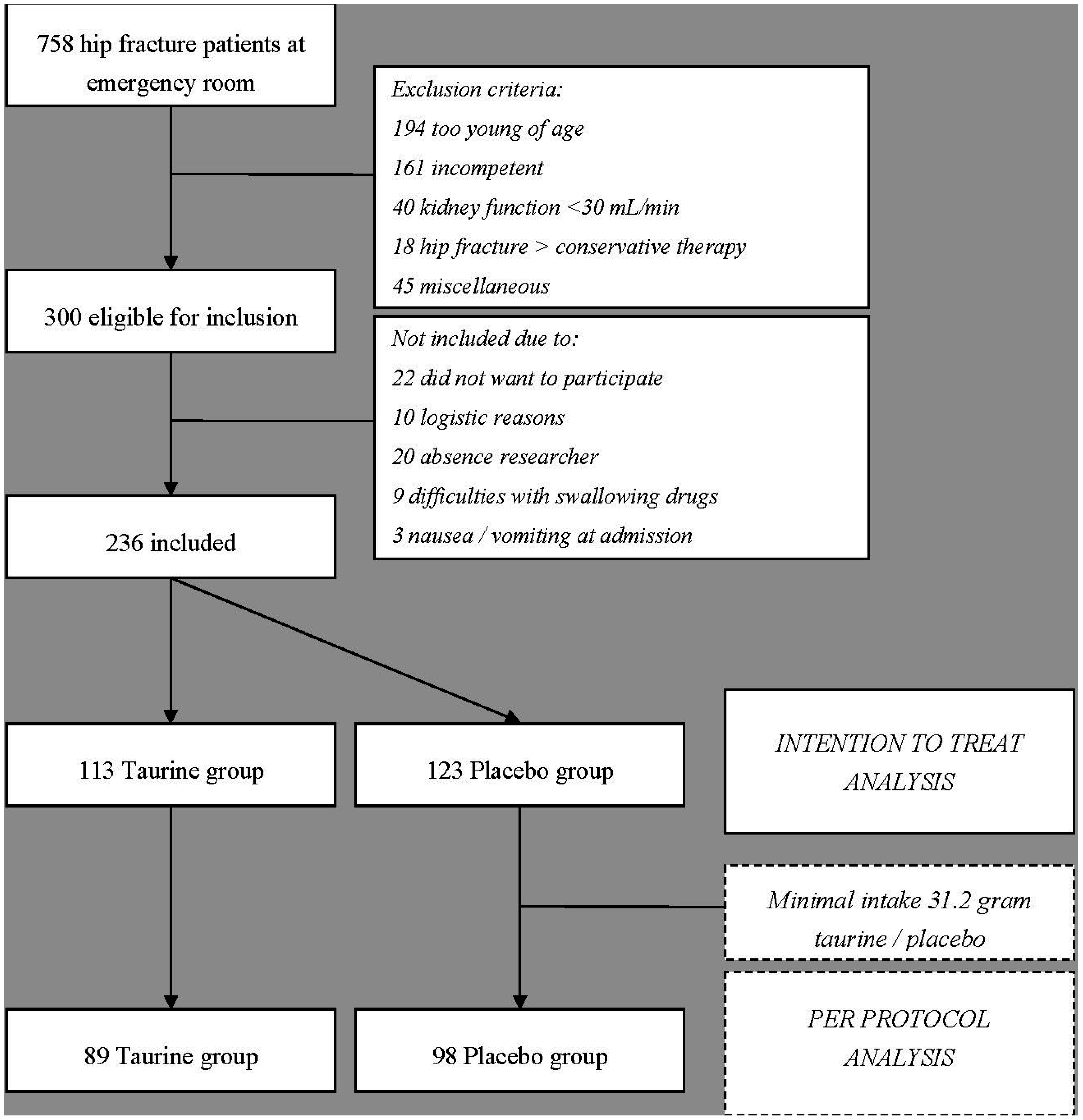
| Parameter | Taurine | Placebo | p-Value |
|---|---|---|---|
| Male/Female | 33/80 | 30/93 | 0.41 |
| Age (years) | 84.4 ± 5.4 | 84.4 ± 4.9 | 0.95 |
| BMI (kg/m2) | 24.1 ± 4.0 | 24.5 ± 3.8 | 0.35 |
| Barthel ADL index (max. 20 points) | 17 ± 3 | 18 ± 3 | 0.20 |
| Hemoglobin (mmol/L) | 7.7 ± 0.9 | 7.8 ± 0.9 | 0.43 |
| Creatinine clearance * (mL/min) | 56 ± 19 | 55 ± 23 | 0.90 |
| Vitamin B6 (nmol/L) | 83.7 ± 73.4 | 80.1 ± 51.6 | 0.67 |
| Albumin (g/L) | 34.5 ± 4.7 | 35.1 ± 3.6 | 0.35 |
| Mini Nutritional Assessment | |||
| Screening score (max. 14 points) | 11.6 ± 2.7 | 11.9 ± 2.8 | 0.39 |
| Assessment (max. 16 points) | 12.5 ± 1.7 | 12.7 ± 1.9 | 0.52 |
| Total Assessment (max. 30 points) | 24.2 ± 3.8 | 24.6 ± 4.3 | 0.39 |
| Nutritional Status ** | 0.40 | ||
| Malnourished, n/N (%) | 6/109 (5) | 7/122 (6) | |
| At risk of malnutrition, n/N (%) | 29/109 (27) | 24/122 (20) | |
| Well nourished, n/N (%) | 74/109 (68) | 91/122 (74) | |
| Medical comorbidities (quantity) | 2.1 ± 1.6 | 2.1 ± 1.3 | 0.93 |
| 0, n/N (%) | 14/113 (12) | 12/123 (10) | |
| 1n/N (%) | 33/113 (29) | 33/123 (27) | |
| 2, n/N (%) | 29/113 (26) | 32/123 (26) | |
| ≥3, n/N (%) | 37/113 (33) | 46/123 (37) | |
| Cardiovascular disease, n/N (%) | 108/113 (95) | 113/123 (92) | 0.76 |
| Cerebral vascular disease, n/N (%) | 20/113 (18) | 17/123 (14) | 0.41 |
| Respiratory disease, n/N (%) | 11/113 (10) | 11/123 (9) | 0.83 |
| Diabetes, n/N (%) | 24/113 (21) | 24/123 (19) | 0.74 |
| Musculoskeletal, n/N (%) | 34/113 (30) | 27/123 (22) | 0.24 |
| Other, n/N (%) | 40/113 (35) | 64/123 (52) | 0.04 |
| Concomitant Medication (quantity) | 5 ± 4 | 5 ± 4 | 0.61 |
| 0–1, n/N (%) | 23/113 (20) | 24/123 (20) | |
| 2–5, n/N (%) | 45/113 (40) | 60/123 (49) | |
| 6–10, n/N (%) | 37/113 (33) | 30/123 (24) | |
| >10, n/N (%) | 8/113 (7) | 9/123 (7) | |
| ASA Score | |||
| 1, n/N (%) | 19/113 (17) | 22/123 (18) | 0.17 |
| 2, n/N (%) | 50/113 (44) | 67/123 (54) | |
| 3, n/N (%) | 44/113 (39) | 34/123 (28) | |
| Type of Surgery | |||
| Hemiarthroplasty, n/N (%) | 48/110 (44) | 65/122 (53) | 0.08 |
| Cannulated screws, n/N (%) | 4/110 (4) | 7/122 (6) | |
| Sliding hip screw, n/N (%) | 7/110 (6) | 7/122 (6) | |
| Gamma nail, n/N (%) | 51/110 (46) | 43/122 (35) | |
| Type of Anesthesia | |||
| Spinal, n/N (%) | 89/110 (81) | 93/122 (76) | 0.33 |
| Spinal + general, n/N (%) | 2/110 (2) | 1/122 (1) | |
| General, n/N (%) | 19/110 (17) | 28/122 (23) | |
| Time of Admission to Surgery (h) | 20.2 ± 15.0 | 21.4 ± 20.6 | 0.61 |
| Duration of surgery (min) | 49 ± 25 | 48 ± 18 | 0.69 |
| Group | Preoperative | Postoperative | Beta (95% CI) p * | ||
|---|---|---|---|---|---|
| Day 1 | Day 5 | ||||
| WBC (109/L) | T | 8.0 (3.4–16.9) | 9.8 (4.5–16.1) | 8.1 (2.7–11.4) | −0.13 (−1.07–0.81) 0.78 |
| P | 9.2 (4.4–18.2) | 9.9 (5.2–15.9) | 7.4 (3.5–13.7) | ||
| CRP (mg/L) | T | 4 (1–25) | 85 (19–229) | 66 (22–218) | −1.2 (−27.0–24.5) 0.93 |
| P | 4 (1–117) | 103 (8–209) | 83 (5–207) | ||
| IL-6 (pg/mL) | T | 29.1 (5.5–415.0) | 104.5 (11.9–335.3) | 21.7 (6.7–360.0) | 6.8 (−17.8–31.4) 0.59 |
| P | 39.6 (4.5–116.7) | 91.4 (31.5–622.7) | 18.1 (3.8–57.7) | ||
| Taurine (µmol/L) | T | 102 (49–474) | 689 (169–1629) | 685 (97–1561) | 701 (578–824) 0.00 |
| P | 96 (64–142) | 86 (57–152) | 74 (53–137) | ||
| Vitamin C (µmol/L) | T | 56 (27–135) | 53 (15–104) | 56 (18–92) | 2.2 (−5.5–10.1) 0.57 |
| P | 54 (26–107) | 52 (26–79) | 55 (26–97) | ||
| Vitamin E (µmol/L) | T | 35 (15–52) | 26 (12–40) | 28 (22–40) | −1.3 (−3.9–1.2) 0.31 |
| P | 34 (18–56) | 27 (16–48) | 30 (17–45) | ||
| β-carotene (µmol/L) | T | 0.29 (0.08–1.07) | 0.24 (0.07–0.70) | 0.22 (0.08–0.81) | −0.02 (−0.06–0.02) 0.27 |
| P | 0.28 (0.12–0.89) | 0.23 (0.07–0.71) | 0.27 (0.11–1.04) | ||
| Glutathione (µmol/L) | T | 767 (256–1350) | 683 (223–1195) | 651 (161–1035) | 2.5 (−59.8–64.9) 0.94 |
| P | 745 (505–1228) | 696 (455–1006) | 637 (336–998) | ||
| Ox. LDL/apoB100 ratio (U/g) | T | 94 (76–130) | 100 (81–145) | 117 (96–146) | 2.39 (−2.39–7.17) 0.33 |
| P | 100 (69–126) | 106 (80–139) | 117 (84–150) | ||
| Malondialdehyde (µmol/L) ‡ | T | 7.0 (4.3–10.8) | 9.3 (6.1–19.1) | 7.6 (5.4–12.7) | −0.45 (−1.64–0.74) 0.46 |
| P | 7.3 (4.6–24.0) | 10.8 (6.4–23.3) | 7.5 (4.8–11.6) | ||
| F2-Isoprostane (pmol/mmol creatinine) | T | 106.2 (23.8–359.3) | 76.7 (11.5–184.6) | 115.9 (47.2–184.2) | −1.62 (−18.5–15.2) 0.85 |
| P | 113.7 (25.1–434.1) | 82.0 (21.9–233.9) | 109.0 (46.7–275.9) | ||
| 8OHdG (nmol/mmol creatinine) | T | 2.4 (1.0–7.4) | 1.9 (0.7–6.8) | 2.5 (0.3–5.5) | −0.54 (−1.07–−0.01) 0.04 |
| P | 2.0 (0.8–10.4) | 2.3 (0.8–12.8) | 2.2 (1.1–8.8) | ||
| Lactate (µmol/L) | T | 833 (323–1540) | 1211 (744–4797) | 1257 (632–3347) | −44 (−347–258) 0.77 |
| P | 912 (467–2861) | 1568 (738–6787) | 1159(647–2092) | ||
| Pyruvate (µmol/L) | T | 48 (25–113) | 81 (50–242) | 86 (49–164) | 0.63 (−16.7–17.9) 0.94 |
| P | 52 (20–200) | 86 (49–275) | 75 (35–138) | ||
| Lactate/Pyruvate Ratio | T | 18 (12–29) | 16 (10–20) | 14 (10–20) | −1.10 (−2.33–0.12) 0.08 |
| P | 18 (9–33) | 14 (12–25) | 16 (10–22) | ||
| Group | Day 1 n/N (%) | Day 2 n/N (%) | Day 3 n/N (%) | Day 4 n/N (%) | Day 5 n/N (%) | * p-Value | |
|---|---|---|---|---|---|---|---|
| Nutrition Day Breakfast | |||||||
| All eaten | T | 57/77 (74) | 55/73 (75) | 65/75 (87) | 55/70 (79) | 48/57 (84) | 0.42 |
| P | 62/93 (67) | 73/93 (78) | 80/93 (86) | 61/77 (79) | 56/64 (87) | ||
| 50% Eaten | T | 12/77 (16) | 9/73 (12) | 7/75 (9) | 10/70 (14) | 5/57 (9) | |
| P | 7/93 (7) | 10/93 (11) | 8/93 (9) | 10/77 (13) | 5/64 (8) | ||
| 25% Eaten | T | 6/77 (8) | 5/73 (7) | 2/75 (3) | 4/70 (6) | 4/57 (7) | |
| P | 14/93(15) | 4/93 (4) | 4/93 (4) | 1/77 (1) | 2/64 (3) | ||
| Nothing | T | 2/77 (2) | 4/73 (6) | 1/75 (1) | 1/70 (1) | 0/57 (0) | |
| P | 10/93 (11) | 6/93 (7) | 1/93 (1) | 5/77 (7) | 1/64 (2) | ||
| Nutrition Day Lunch | |||||||
| All eaten | T | 48/73 (66) | 57/72 (79) | 52/68 (77) | 48/67 (72) | 36/44 (82) | 0.83 |
| P | 57/89 (64) | 67/87 (77) | 69/80 (86.5) | 54/69 (78) | 51/58 (88) | ||
| 50% Eaten | T | 15/73 (21) | 8/73 (11) | 9/68 (13) | 15/67 (22) | 5/44 (11) | |
| P | 16/89 (18) | 6/87 (7) | 5/80 (6) | 8/69 (12) | 4/58 (7) | ||
| 25% Eaten | T | 9/73 (12) | 5/72 (7) | 3/68 (4) | 2/67 (3) | 1/44 (2) | |
| P | 7/89 (8) | 8/87 (9) | 5/80 (6) | 3/69 (4) | 1/58 (2) | ||
| Nothing | T | 1/73 (1) | 2/72 (3) | 4/68 (6) | 2/67 (3) | 2/44 (5) | |
| P | 9/89 (10) | 6/87 (7) | 1/80 (1.5) | 4/69 (6) | 2/58 (3) | ||
| Nutrition Day Dinner | |||||||
| All eaten | T | 30/65 (46) | 33/62 (53) | 34/64 (53) | 29/58 (50) | 26/40 (65) | 0.38 |
| P | 29/74 (39) | 37/72 (51.5) | 45/71 (63) | 43/66 (65) | 31/48 (65) | ||
| 50% eaten | T | 17/65 (65) | 16/62 (26) | 16/64 (25) | 21/58 (36) | 5/40 (13) | |
| P | 27/74 (37) | 21/72 (29) | 19/71 (27) | 15/66 (23) | 12/48 (25) | ||
| 25% eaten | T | 15/65 (23) | 11/62 (18) | 12/64 (19) | 6/58 (10) | 8/40 (20) | |
| P | 12/74 (16) | 13/72 (18) | 5/71 (7) | 5/66 (8) | 3/48 (6) | ||
| Nothing | T | 3/65 (5) | 2/62 (3) | 2/64 (3) | 2/58 (4) | 1/40 (2) | |
| P | 6/74 (8) | 1/72 (1.5) | 2/71 (3) | 3/66 (4) | 2/48 (4) | ||
2.1.2. Postoperative Morbidity and Mortality
| Parameter | Taurine | Placebo | p-Value |
|---|---|---|---|
| Intake Supplement (grams) | 33.0 ± 9.7 | 33.3 ± 9.5 | 0.82 |
| Length of Hospital Stay (days) | 13 ± 10 | 13 ± 11 | 0.83 |
| In-Hospital Morbidity (quantity) | 1.0 ± 1.1 | 1.0 ± 1.1 | 0.95 |
| 0, n/N (%) | 45/110 (41) | 55/122 (45) | |
| 1, n/N (%) | 38/110 (34) | 30/122 (25) | |
| 2, n/N (%) | 15/110 (14) | 25/122 (20) | |
| ≥3, n/N (%) | 12/110 (11) | 12/122 (10) | |
| Infectious, n/N (%) | 11/110 (10) | 18/122 (15) | 0.54 |
| Cardiovascular event, n/N (%) | 5/110 (5) | 13/122 (11) | 0.12 |
| Cerebral vascular accident, n/N (%) | 1/110 (1) | 2/122 (2) | 0.62 |
| Delirium, n/N (%) | 26/110 (24) | 27/122 (22) | 0.79 |
| Blood transfusion, n/N (%) | 19/110 (17) | 20/122 (16) | 0.86 |
| Reoperation or surgery otherwise, n/N (%) | 6/110 (5) | 6/122 (5) | 0.91 |
| Other, n/N (%) | 40/110 (36) | 35/122 (29) | 0.33 |
| Barthel ADL index (max. 20 points) | 0.90 * | ||
| 3 months follow-up | 16 ± 4 | 17 ± 4 | 0.83 ‡ |
| 6 months follow-up | 17 ± 4 | 17 ± 3 | 0.95 ‡ |
| 9 months follow-up | 17 ± 3 | 17 ± 4 | 0.53 ‡ |
| 12 months follow-up | 17 ± 4 | 17 ± 4 | 0.58 ‡ |
| Medical Comorbidities in First Year (quantity) | 0.8 ± 0.9 | 0.8 ± 0.8 | 0.82 |
| 0, n/N (%) | 48/107 (44) | 51/117 (44) | |
| 1, n/N (%) | 36/107 (34) | 46/117 (39) | |
| 2, n/N (%) | 19/107 (18) | 14/117 (12) | |
| ≥3, n/N (%) | 4/107 (4) | 6/117 (5) | |
| Infectious, n/N (%) | 30/107 (28) | 29/117 (25) | 0.69 |
| Cardiovascular event, n/N (%) | 7/107 (7) | 8/117 (7) | 0.93 |
| Cerebral vascular accident, n/N (%) | 0/107 (0) | 2/117 (2) | 0.17 |
| Thromboembolic event, n/N (%) | 1/107 (1) | 2/117 (2) | 0.61 |
| Reoperation or surgery otherwise, n/N (%) | 16/107 (15) | 15/117 (13) | 0.64 |
| Anemia with medical intervention, n/N (%) | 2/107 (2) | 7/117 (6) | 0.12 |
| Cognitive impairment, n/N (%) | 7/107 (7) | 8/117 (7) | 0.93 |
| Other, n/N (%) | 24/107 (22) | 21/117 (18) | 0.40 |
| Mortality Overall, n/N (%) | 23/107 (21) | 27/116 (23) | 0.75 |
| In-hospital, n/N (%) | 11/111 (10) | 11/123 (9) | 0.80 |
| At 3 months follow-up, n/N (%) | 16/108 (15) | 16/119 (13) | 0.77 |
| At 6 months follow-up, n/N (%) | 20/108 (18) | 22/119 (18) | 0.99 |
| At 9 months follow-up, n/N (%) | 22/108 (20) | 23/119 (19) | 0.84 |
| At 12 months follow-up, n/N (%) | 23/107 (21) | 27/116 (23) | 0.75 |
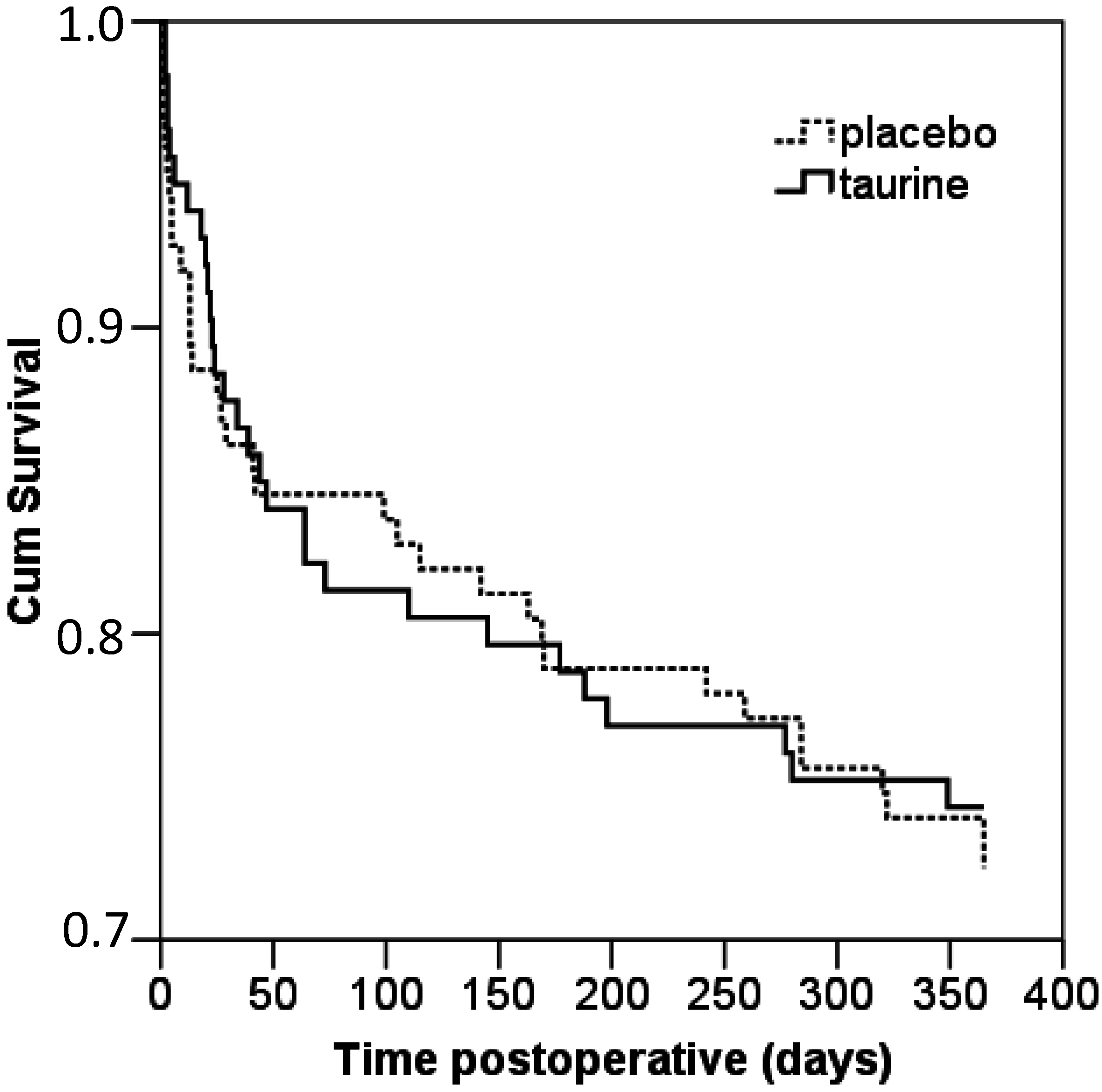
2.1.3. Postoperative Surgical Stress Response
2.2. Discussion
3. Materials and Methods
3.1. Patients
3.2. Randomization, Blinding and Nutritional Intervention
3.3. Study Outcomes
3.4. Laboratory Measurements
3.4.1. Inflammatory Parameters
3.4.2. Antioxidant/Oxidant Parameters
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Appendix

Conflicts of Interest
References
- Franzo, A.; Francescutti, C.; Simon, G. Risk factors correlated with post-operative mortality for hip fracture surgery in the elderly: A population-based approach. Eur. J. Epidemiol. 2005, 20, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Partanen, J.; Syrjala, H.; Vahanikkila, H.; Jalovaara, P. Impact of deep infection after hip fracture surgery on function and mortality. J. Hosp. Infect. 2006, 62, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.G.; Beck, S.J.; Hood, K.; Johansen, A. Using dietetic assistants to improve the outcome of hip fracture: A randomised controlled trial of nutritional support in an acute trauma ward. Age Ageing 2006, 35, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Eneroth, M.; Olsson, U.B.; Thorngren, K.G. Nutritional supplementation decreases hip fracture-related complications. Clin. Orthop. Relat. Res. 2006, 451, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.T.; Kucukakin, B.; Lykkesfeldt, J.; Rosenberg, J.; Gogenur, I. Oxidative stress response after laparoscopic versus conventional sigmoid resection: A randomized, double-blind clinical trial. Surg. Laparosc. Endosc. Percutan Tech. 2012, 22, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Anlasik, T.; Sies, H.; Griffiths, H.R.; Mecocci, P.; Stahl, W.; Polidori, M.C. Dietary habits are major determinants of the plasma antioxidant status in healthy elderly subjects. Br. J. Nutr. 2005, 94, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Haber, C.A.; Lam, T.K.; Yu, Z.; Gupta, N.; Goh, T.; Bogdanovic, E.; Giacca, A.; Fantus, I.G. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: Possible role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E744–E753. [Google Scholar] [CrossRef] [PubMed]
- Milei, J.; Ferreira, R.; Llesuy, S.; Forcada, P.; Covarrubias, J.; Boveris, A. Reduction of reperfusion injury with preoperative rapid intravenous infusion of taurine during myocardial revascularization. Am. Heart J. 1992, 123, 339–345. [Google Scholar] [CrossRef]
- Jeevanandam, M.; Young, D.H.; Ramias, L.; Schiller, W.R. Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am. J. Clin. Nutr. 1990, 51, 1040–1045. [Google Scholar] [PubMed]
- Houdijk, A.P.; Rijnsburger, E.R.; Jansen, J.; Wesdorp, R.I.; Weiss, J.K.; McCamish, M.A.; Teerlink, T.; Meuwissen, S.G.; Haarman, H.J.; Thijs, L.G.; et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 1998, 352, 772–776. [Google Scholar] [CrossRef]
- Boelens, P.G.; Houdijk, A.P.; Fonk, J.C.; Puyana, J.C.; Haarman, H.J.; von Blomberg-van der Flier, M.E.; van Leeuwen, P.A. Glutamine-enriched enteral nutrition increases in vitro interferon-gamma production but does not influence the in vivo specific antibody response to KLH after severe trauma. A prospective, double blind, randomized clinical study. Clin. Nutr. 2004, 23, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Boelens, P.G.; Houdijk, A.P.; de Thouars, H.N.; Teerlink, T.; van Engeland, M.I.; Haarman, H.J.; van Leeuwen, P.A. Plasma taurine concentrations increase after enteral glutamine supplementation in trauma patients and stressed rats. Am. J. Clin. Nutr. 2003, 77, 250–256. [Google Scholar] [PubMed]
- Symeonidis, P.D.; Clark, D. Assessment of malnutrition in hip fracture patients: Effects on surgical delay, hospital stay and mortality. Acta Orthop. Belg. 2006, 72, 420–427. [Google Scholar] [PubMed]
- Holt, G.; Smith, R.; Duncan, K.; Finlayson, D.F.; Gregori, A. Early mortality after surgical fixation of hip fractures in the elderly: An analysis of data from the scottish hip fracture audit. J. Bone Jt. Surg. Br. 2008, 90, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, N.; Boulton, C.; Morris, C.; Moran, C. Hip fracture audit: The Nottingham experience. Osteoporos. Int. 2010, 21, S647–S653. [Google Scholar] [CrossRef] [PubMed]
- Avenell, A.; Handoll, H.H. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst. Rev. 2010, 18, CD001880. [Google Scholar]
- Botella-Carretero, J.I.; Iglesias, B.; Balsa, J.A.; Arrieta, F.; Zamarron, I.; Vazquez, C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: A randomized clinical trial. Clin. Nutr. 2010, 29, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Gerstorfer, I.; Thaler, H.W.; Stundner, H.; Biswas, P.; Elmadfa, I. Nutritional supplementation affects postoperative oxidative stress and duration of hospitalization in patients with hip fracture. Wien. Klin. Wochenschr. 2011, 123, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Myint, M.W.; Wu, J.; Wong, E.; Chan, S.P.; To, T.S.; Chau, M.W.; Ting, K.H.; Fung, P.M.; Au, K.S. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: A single blind randomised controlled trial. Age Ageing 2013, 42, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Hamid, N.A.; Latiff, A.A.; Zakaria, Z.; Mazlan, M.; Yusof, Y.A.; Karim, A.A.; Ibahim, J.; Hamid, Z.; Ngah, W.Z. Reduction of DNA damage in older healthy adults by Tri E Tocotrienol supplementation. Nutrition 2008, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Traustadottir, T.; Davies, S.S.; Stock, A.A.; Su, Y.; Heward, C.B.; Roberts, L.J.; Harman, S.M. Tart cherry juice decreases oxidative stress in healthy older men and women. J. Nutr. 2009, 139, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P.; Ko, S.H.; Cho, B.; Yoo, S.H.; Choi, S.W.; Ye, S.K.; Kasai, H.; Chung, M.-H. Neopterin, a marker of immune response, and 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress, correlate at high age as determined by automated simultaneous high-performance liquid chromatography analysis of human urine. Anal. Biochem. 2008, 383, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Landow, L. Splanchnic lactate production in cardiac surgery patients. Crit. Care Med. 1993, 21, S84–S91. [Google Scholar] [CrossRef] [PubMed]
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Grosman, B.; Frishman, S.; Salai, M.; Beloosesky, Y. The relationship between nutritional status of hip fracture operated elderly patients and their functioning, comorbidity and outcome. Clin. Nutr. 2012, 31, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.J.; Bauer, J.D.; Capra, S.; Pulle, R.C. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients—Results of a pragmatic intervention. Clin. Nutr. 2014, 33, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Loizzo, A.; Ghirlanda, G.; Seghieri, G. Taurine supplementation and diabetes mellitus. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ando, K.; Noda, H.; Ito, Y.; Sato, Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987, 75, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.W.; Yang, C.S. An isocratic high-performance liquid chromatography method for the simultaneous analysis of plasma retinol, alpha-tocopherol, and various carotenoids. Anal. Biochem. 1985, 145, 21–26. [Google Scholar] [CrossRef]
- Van der Zwan, L.P.; Teerlink, T.; Dekker, J.M.; Henry, R.M.; Stehouwer, C.D.; Jakobs, C.; Heine, R.J.; Scheffer, P.G. Circulating oxidized LDL: Determinants and association with brachial flow-mediated dilation. J. Lipid Res. 2009, 50, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Van de Kerkhof, J.; Schalkwijk, C.G.; Konings, C.J.; Cheriex, E.C.; van der Sande, F.M.; Scheffer, P.G.; Ter Wee, P.M.; Leunissen, K.M.; Kooman, J.P. Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine and vascular cell adhesion molecule-1 (VCAM-1) in relation to peritoneal glucose prescription and residual renal function; A study in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2004, 19, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.G.; Perez, R.S.; Nouta, J.; Zuurmond, W.W.; Scheffer, P.G. Oxidative Stress in complex regional pain syndrome (CRPS): No systemically elevated levels of malondialdehyde, F2-Isoprostanes and 8OHdG in a selected sample of patients. Int. J. Mol. Sci. 2013, 14, 7784–7794. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T.; van Leeuwen, P.A.; Houdijk, A. Plasma amino acids determined by liquid chromatography within 17 min. Clin. Chem. 1994, 40, 245–249. [Google Scholar]
- Twisk, J.W.R. Applied Longitudinal Data Analysis for Epidemiology A Practical Guide; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Stijn, M.F.M.; Bruins, A.A.; Vermeulen, M.A.R.; Witlox, J.; Teerlink, T.; Schoorl, M.G.; De Bandt, J.P.; Twisk, J.W.R.; Van Leeuwen, P.A.M.; Houdijk, A.P.J. Effect of Oral Taurine on Morbidity and Mortality in Elderly Hip Fracture Patients: A Randomized Trial. Int. J. Mol. Sci. 2015, 16, 12288-12306. https://doi.org/10.3390/ijms160612288
Van Stijn MFM, Bruins AA, Vermeulen MAR, Witlox J, Teerlink T, Schoorl MG, De Bandt JP, Twisk JWR, Van Leeuwen PAM, Houdijk APJ. Effect of Oral Taurine on Morbidity and Mortality in Elderly Hip Fracture Patients: A Randomized Trial. International Journal of Molecular Sciences. 2015; 16(6):12288-12306. https://doi.org/10.3390/ijms160612288
Chicago/Turabian StyleVan Stijn, Mireille F. M., Arnoud A. Bruins, Mechteld A. R. Vermeulen, Joost Witlox, Tom Teerlink, Margreet G. Schoorl, Jean Pascal De Bandt, Jos W. R. Twisk, Paul A. M. Van Leeuwen, and Alexander P. J. Houdijk. 2015. "Effect of Oral Taurine on Morbidity and Mortality in Elderly Hip Fracture Patients: A Randomized Trial" International Journal of Molecular Sciences 16, no. 6: 12288-12306. https://doi.org/10.3390/ijms160612288
APA StyleVan Stijn, M. F. M., Bruins, A. A., Vermeulen, M. A. R., Witlox, J., Teerlink, T., Schoorl, M. G., De Bandt, J. P., Twisk, J. W. R., Van Leeuwen, P. A. M., & Houdijk, A. P. J. (2015). Effect of Oral Taurine on Morbidity and Mortality in Elderly Hip Fracture Patients: A Randomized Trial. International Journal of Molecular Sciences, 16(6), 12288-12306. https://doi.org/10.3390/ijms160612288






