A Fusion Protein of RGD4C and β-Lactamase Has a Favorable Targeting Effect in Its Use in Antibody Directed Enzyme Prodrug Therapy
Abstract
:1. Introduction
2. Results and Discussion
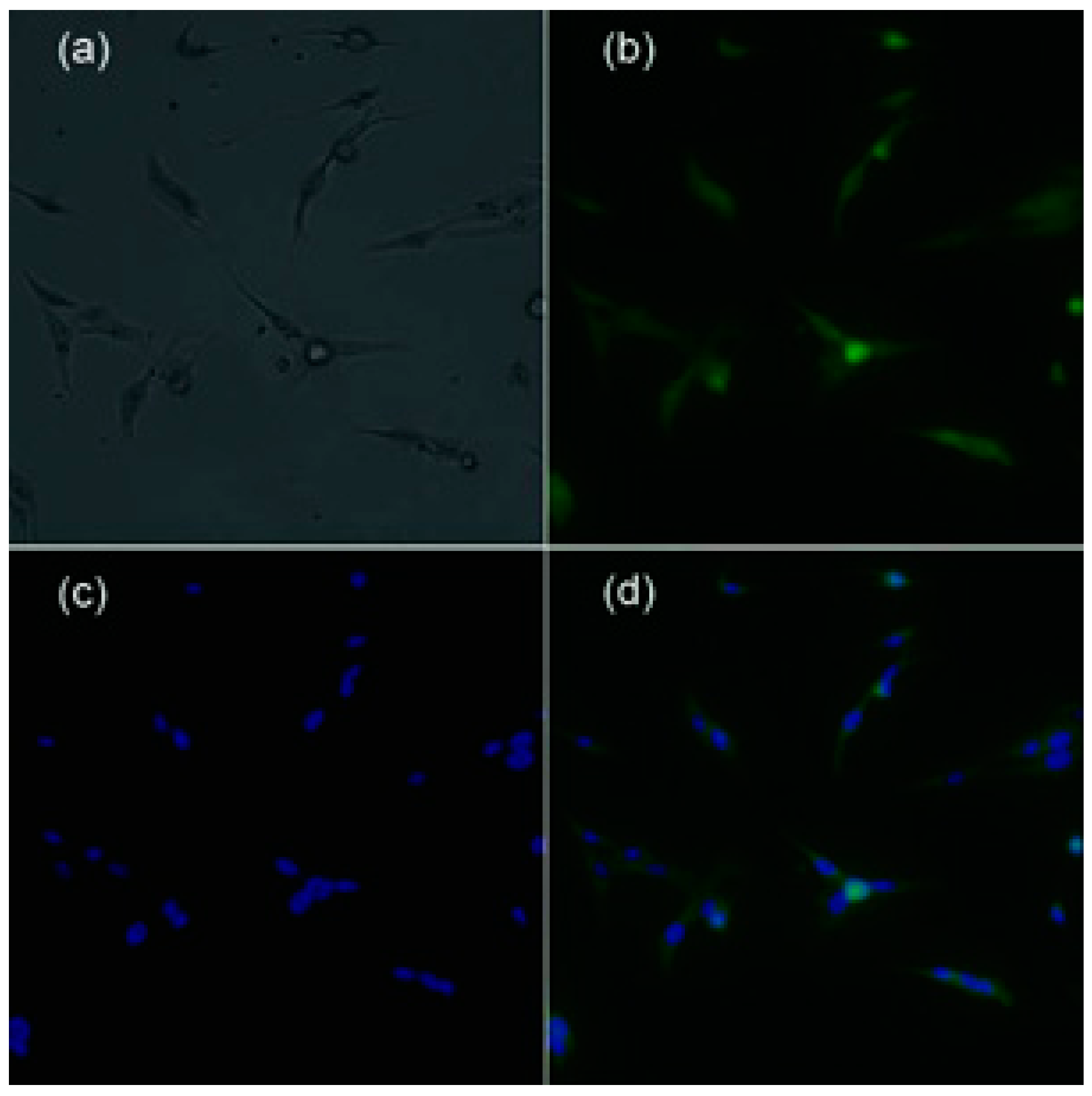
2.1. Immunofluorescent Staining
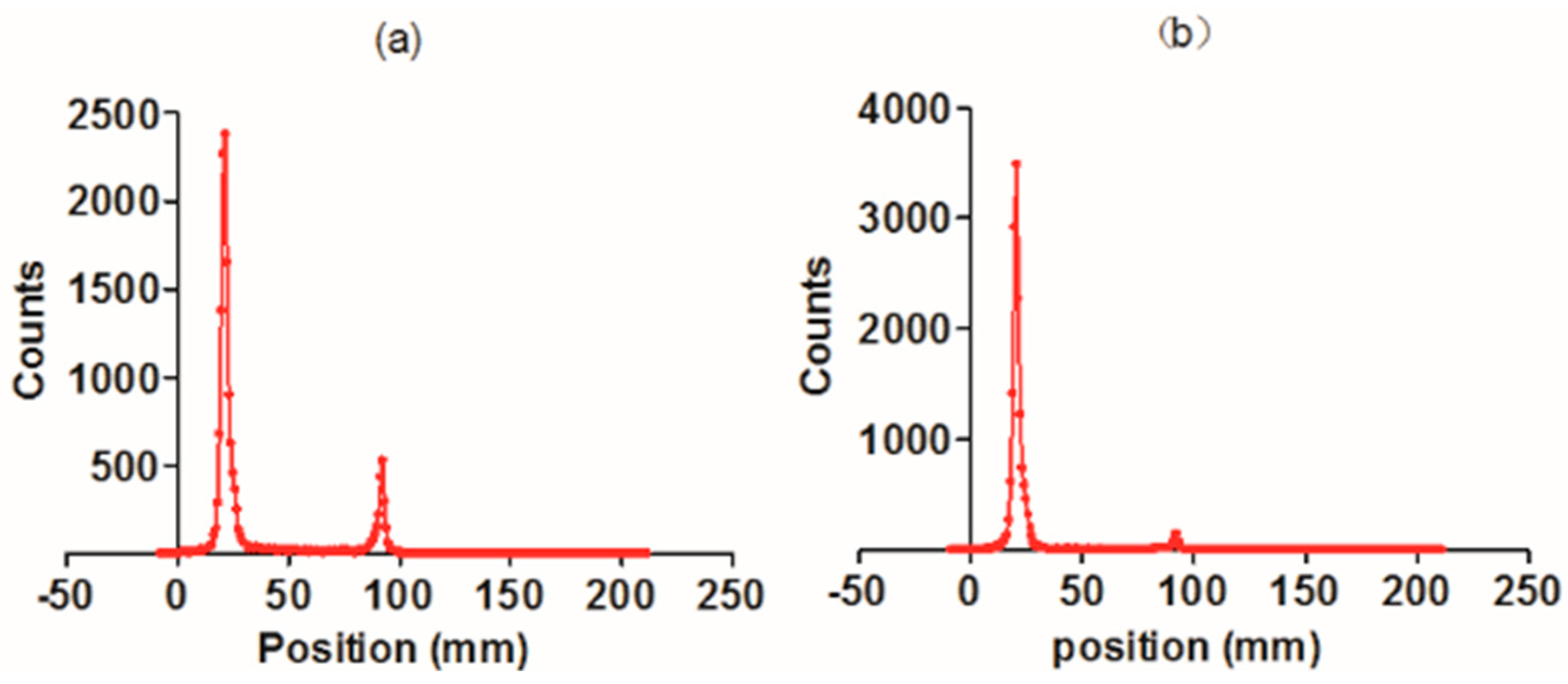
2.2. Radiolabeling and Radiochemical Purity
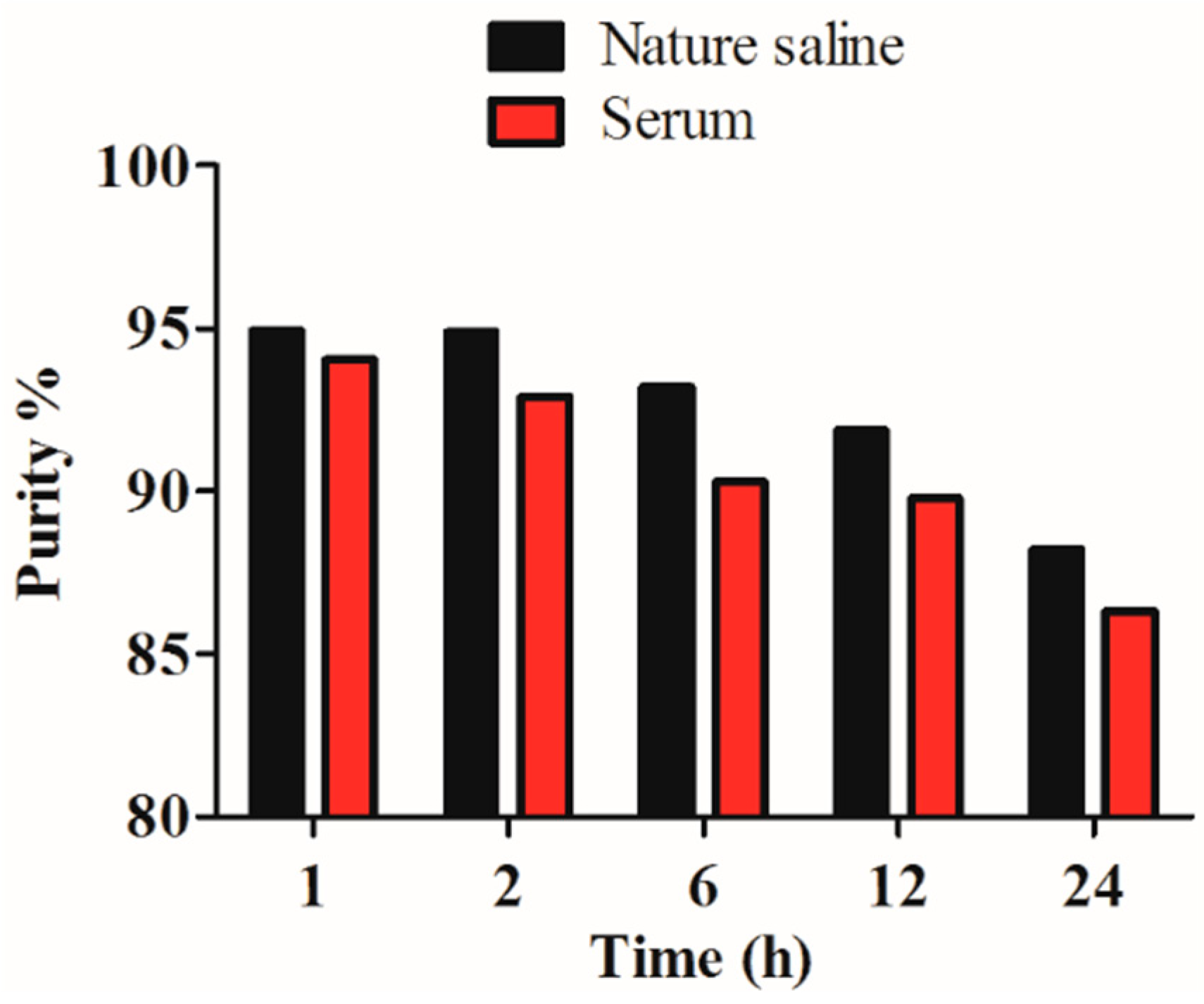
2.3. In Vitro Stability
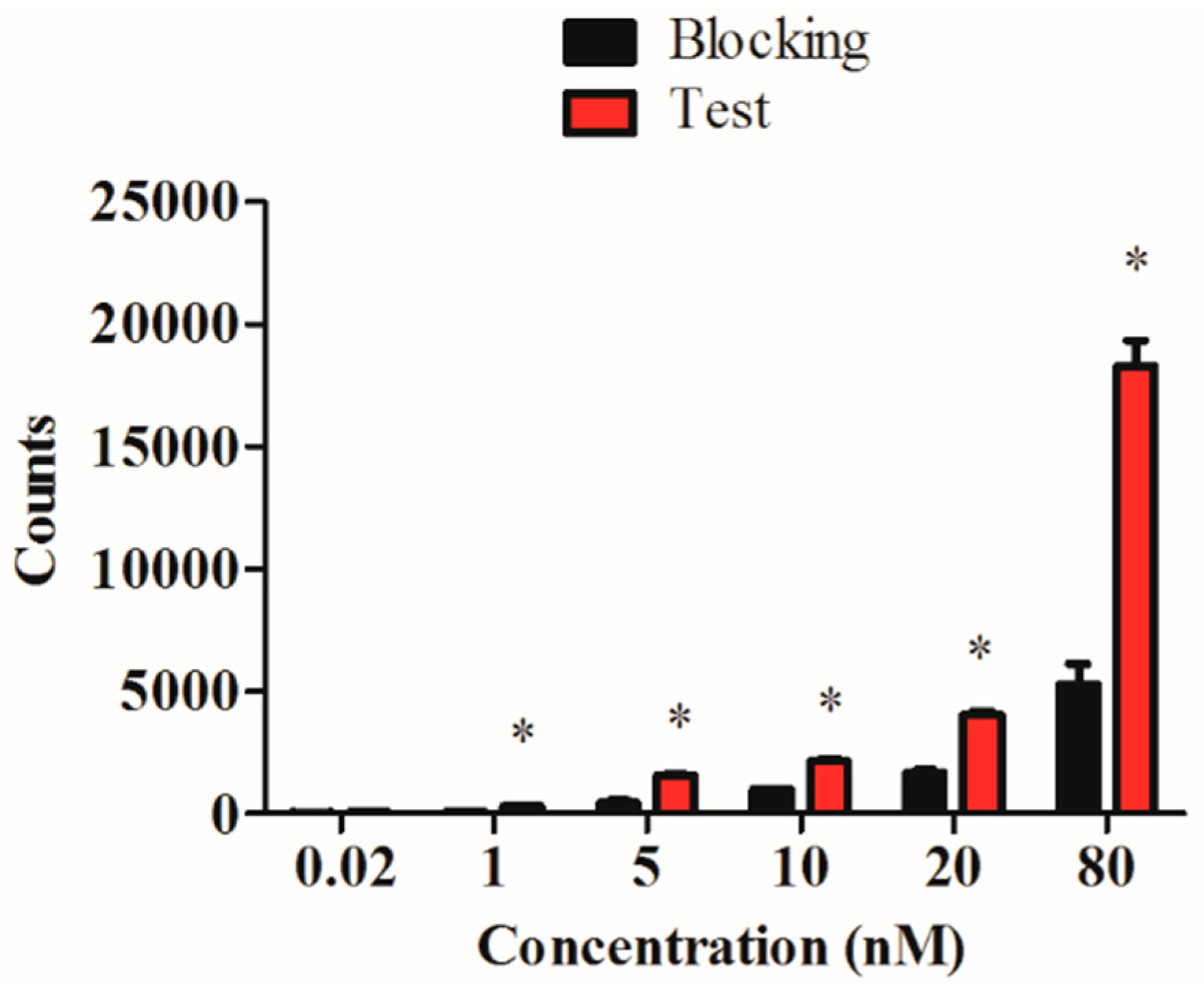
2.4. In Vitro Evaluation of the 99mTc-RGD4CβL
2.5. Blood Clearance of the 99mTc-RGD4CβL
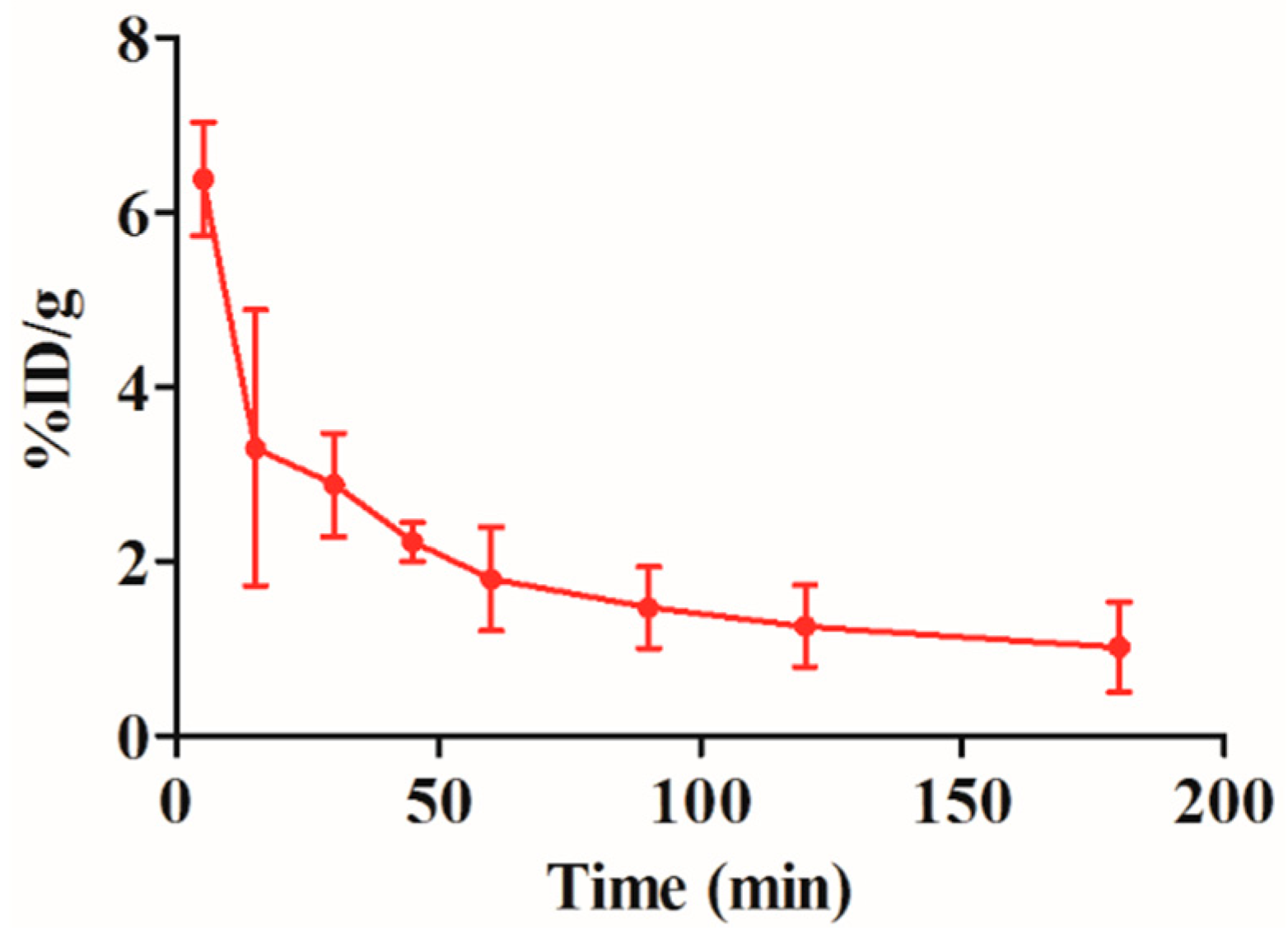
2.6. Biodistribution
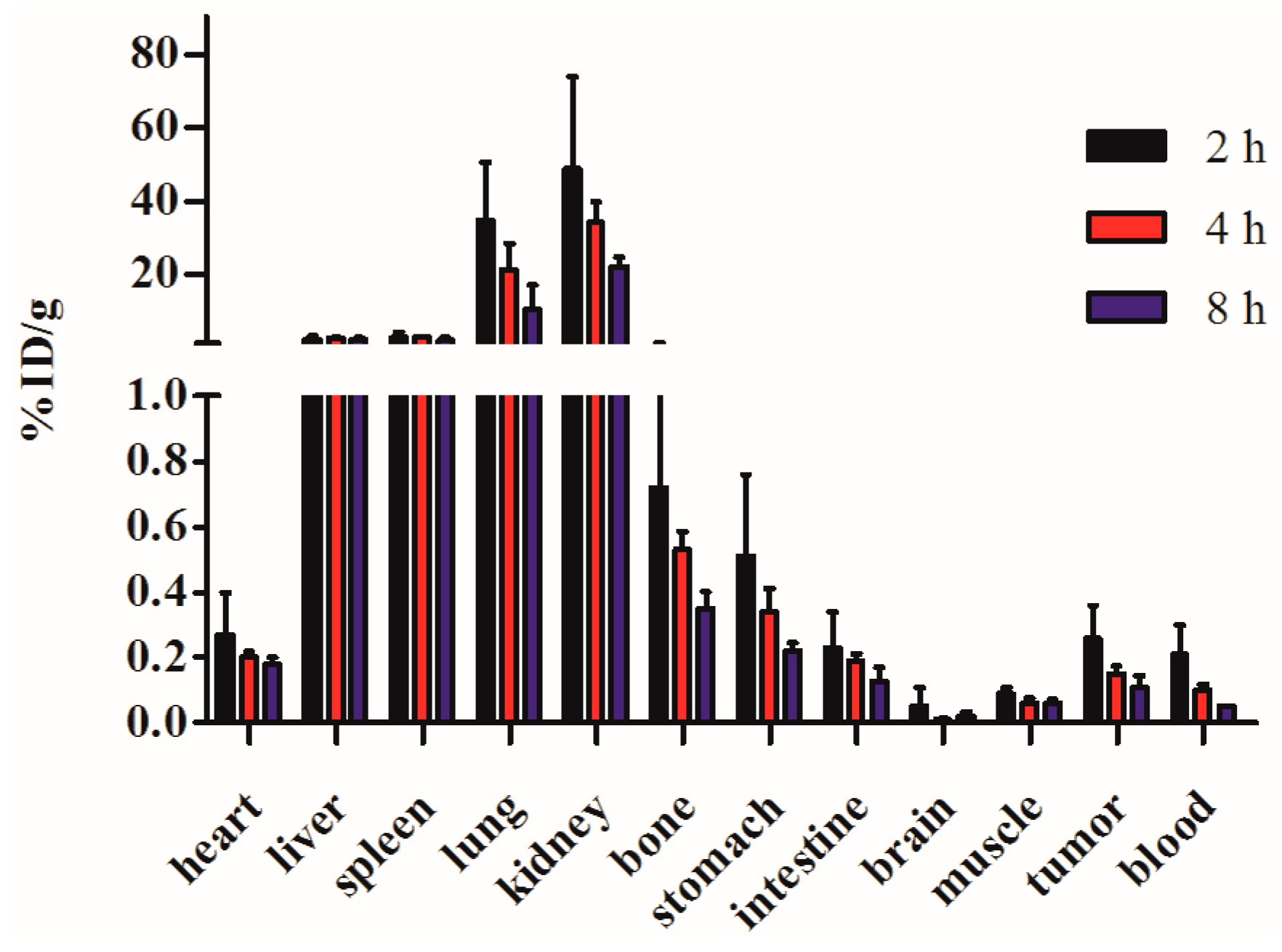
3. Experimental Section
3.1. Materials
3.2. RGD4C-β-Lactamase Conjugate
3.3. Cell Culture and Animals
3.4. Immunofluorescent Staining Assay
3.5. Radioactive Technetium Labeling
3.6. Purification and Radiochemical Purity
3.7. In Vitro Stability
3.8. In Vitro Evaluation of 99mTc-RGD4CβL
3.9. Blood Clearance of 99mTc-RGD4CβL
3.10. Biodistribution
3.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bagshawe, K.D. Targeting: The ADEPT story so far. Curr. Drug Targets 2009, 10, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Bagshawe, K.D.; Sharma, S.K.; Springer, C.J.; Rogers, G.T. Antibody directed enzyme prodrug therapy (ADEPT): A review of some theoretical, experimental and clinical aspects. Ann. Oncol. 1994, 5, 879–891. [Google Scholar] [PubMed]
- Tietze, L.F.; Schmuck, K. Prodrugs for targeted tumor therapies: Recent developments in ADEPT, GDEPT and PMT. Curr. Pharm. Des. 2011, 17, 3527–3547. [Google Scholar] [CrossRef] [PubMed]
- Paillard, F. Bystander effects in enzyme/prodrug gene therapy. Hum. Gene Ther. 1997, 8, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Capucha, V.; Mendes, E.; Francisco, A.P.; Perry, M.J. Development of triazene prodrugs for ADEPT strategy: New insights into drug delivery system based on carboxypeptidase G2 activation. Bioorg. Med. Chem. Lett. 2012, 22, 6903–6908. [Google Scholar] [CrossRef] [PubMed]
- Green, L.K.; Storey, M.A.; Williams, E.M.; Patterson, A.V.; Smaill, J.B.; Copp, J.N.; Ackerley, D.F. The flavin reductase MsuE is a novel nitroreductase that can efficiently activate two promising next-generation prodrugs for gene-directed enzyme prodrug therapy. Cancers (Basel) 2013, 5, 985–997. [Google Scholar] [CrossRef]
- Celik, A.; Yetis, G. An unusually cold active nitroreductase for prodrug activations. Bioorg. Med. Chem. 2012, 20, 3540–3550. [Google Scholar] [CrossRef] [PubMed]
- Van Rite, B.D.; Krais, J.J.; Cherry, M.; Sikavitsas, V.I.; Kurkjian, C.; Harrison, R.G. Antitumor activity of an enzyme prodrug therapy targeted to the breast tumor vasculature. Cancer Investig. 2013, 31, 505–510. [Google Scholar] [CrossRef]
- Guillen, K.P.; Kurkjian, C.; Harrison, R.G. Targeted enzyme prodrug therapy for metastatic prostate cancer—A comparative study of l-methioninase, purine nucleoside phosphorylase, and cytosine deaminase. J. Biomed. Sci. 2014, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Pereira, B.; Medina, C.; Camacho, E.M.; Flores, A.; Santero, E. Improved cytotoxic effects of Salmonella-producing cytosine deaminase in tumour cells. Microb. Biotechnol. 2014, 8, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Valdes, G.; Schulte, R.W.; Ostermeier, M.; Iwamoto, K.S. The high-affinity maltose switch MBP317–347 has low affinity for glucose: Implications for targeting tumors with metabolically directed enzyme prodrug therapy. Chem. Biol. Drug Des. 2014, 83, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Harding, F.A.; Liu, A.D.; Stickler, M.; Razo, O.J.; Chin, R.; Faravashi, N.; Viola, W.; Graycar, T.; Yeung, V.P.; Aehle, W.; et al. A β-lactamase with reduced immunogenicity for the targeted delivery of chemotherapeutics using antibody-directed enzyme prodrug therapy. Mol. Cancer Ther. 2005, 4, 1791–1800. [Google Scholar]
- Majhen, D.; Richardson, J.; Vukelic, B.; Dodig, I.; Cindric, M.; Benihoud, K.; Ambriovic-Ristov, A. The disulfide bond of an RGD4C motif inserted within the Hi loop of the adenovirus type 5 fiber protein is critical for retargeting to αv-integrins. J. Gene Med. 2012, 14, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Tang, W.; Chen, H.; Lin, X.; Todd, T.; Wang, G.; Cowger, T.; Chen, X.; Xie, J. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano 2013, 7, 4830–4837. [Google Scholar] [CrossRef] [PubMed]
- Temming, K.; Schiffelers, R.M.; Molema, G.; Kok, R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updat. 2005, 8, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, H.; Shi, P.; Meng, A.M. Characterization of a fusion protein of RGD4C and the β-lactamase variant for antibody-directed enzyme prodrug therapy. Onco Targets Ther. 2014, 7, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, P.J.; Wu, M.F.; Li, N.; Zhou, X.L.; Fan, F.Y. Construction, expression and functional characterization of the β-lactamase with αv-integrin ligands. Protein Pept. Lett. 2010, 17, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Schellmann, N.; Deckert, P.M.; Bachran, D.; Fuchs, H.; Bachran, C. Targeted enzyme prodrug therapies. Mini Rev. Med. Chem. 2010, 10, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Gainkam, L.O.; Caveliers, V.; Devoogdt, N.; Vanhove, C.; Xavier, C.; Boerman, O.; Muyldermans, S.; Bossuyt, A.; Lahoutte, T. Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice. Contrast Media Mol. Imaging 2011, 6, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Devoogdt, N.; Hernot, S.; Vaneycken, I.; D’Huyvetter, M.; de Vos, J.; Massa, S.; Lahoutte, T.; Caveliers, V. Site-specific labeling of His-tagged Nanobodies with 99mTc: A practical guide. Methods Mol. Biol. 2012, 911, 485–490. [Google Scholar] [PubMed]
- Gainkam, L.O.; Huang, L.; Caveliers, V.; Keyaerts, M.; Hernot, S.; Vaneycken, I.; Vanhove, C.; Revets, H.; de Baetselier, P.; Lahoutte, T. Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. J. Nucl. Med. 2008, 49, 788–795. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhou, X.-L.; Long, W.; Liu, J.-J.; Fan, F.-Y. A Fusion Protein of RGD4C and β-Lactamase Has a Favorable Targeting Effect in Its Use in Antibody Directed Enzyme Prodrug Therapy. Int. J. Mol. Sci. 2015, 16, 9625-9634. https://doi.org/10.3390/ijms16059625
Wang H, Zhou X-L, Long W, Liu J-J, Fan F-Y. A Fusion Protein of RGD4C and β-Lactamase Has a Favorable Targeting Effect in Its Use in Antibody Directed Enzyme Prodrug Therapy. International Journal of Molecular Sciences. 2015; 16(5):9625-9634. https://doi.org/10.3390/ijms16059625
Chicago/Turabian StyleWang, Hao, Xiao-Liang Zhou, Wei Long, Jin-Jian Liu, and Fei-Yue Fan. 2015. "A Fusion Protein of RGD4C and β-Lactamase Has a Favorable Targeting Effect in Its Use in Antibody Directed Enzyme Prodrug Therapy" International Journal of Molecular Sciences 16, no. 5: 9625-9634. https://doi.org/10.3390/ijms16059625




