Removal of Toxic Mercury from Petroleum Oil by Newly Synthesized Molecularly-Imprinted Polymer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Studies
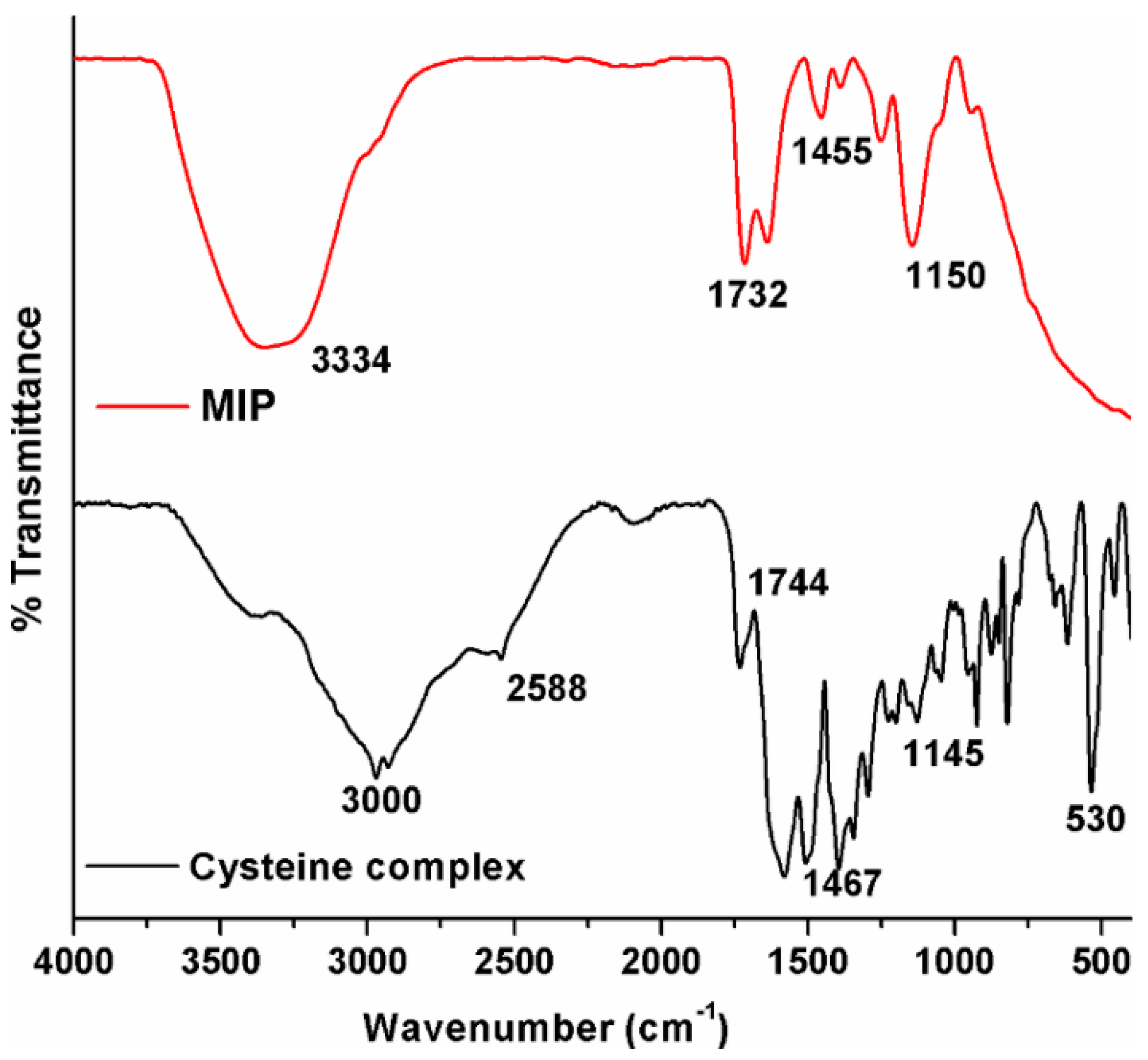
2.1.1. FTIR
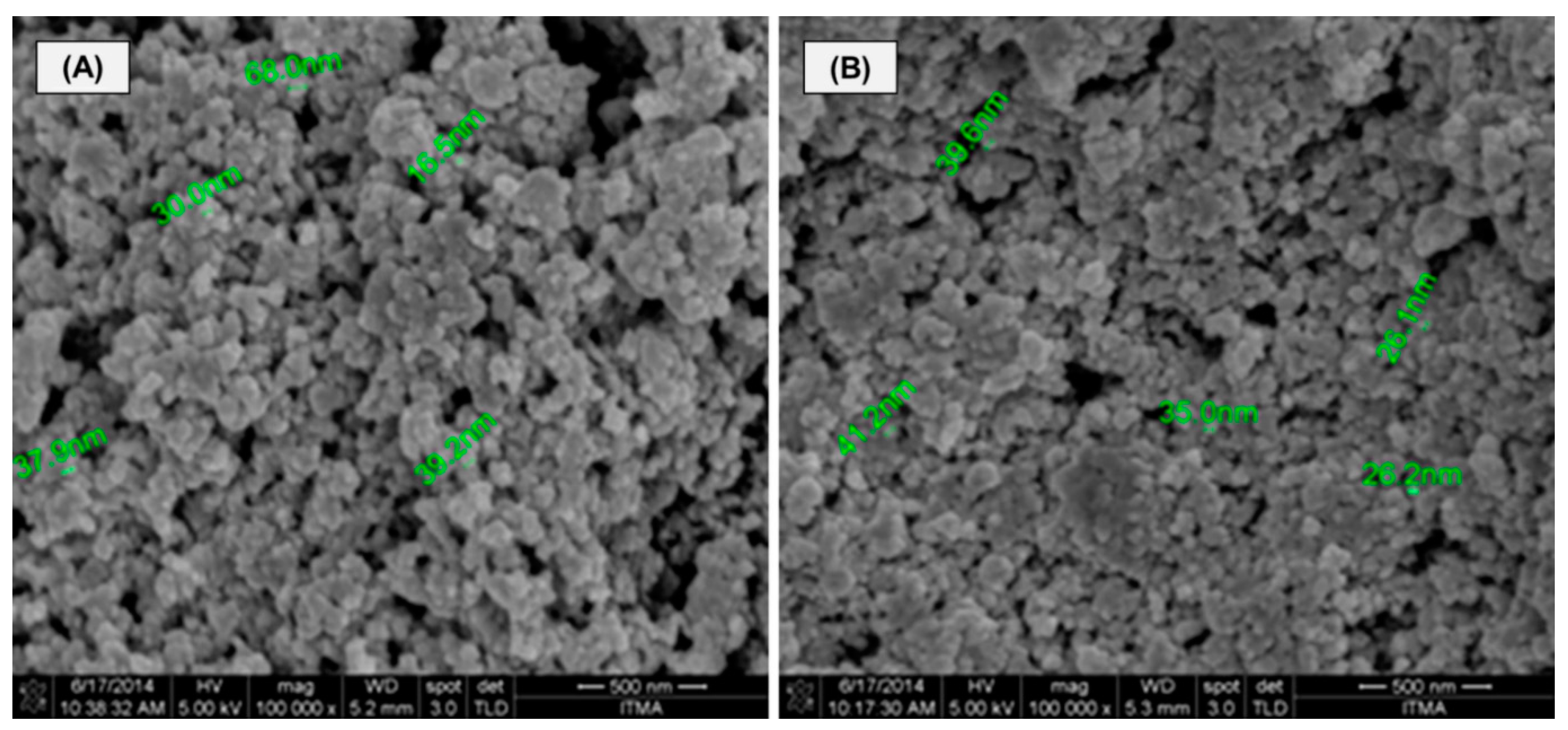
2.1.2. FE-SEM Analysis
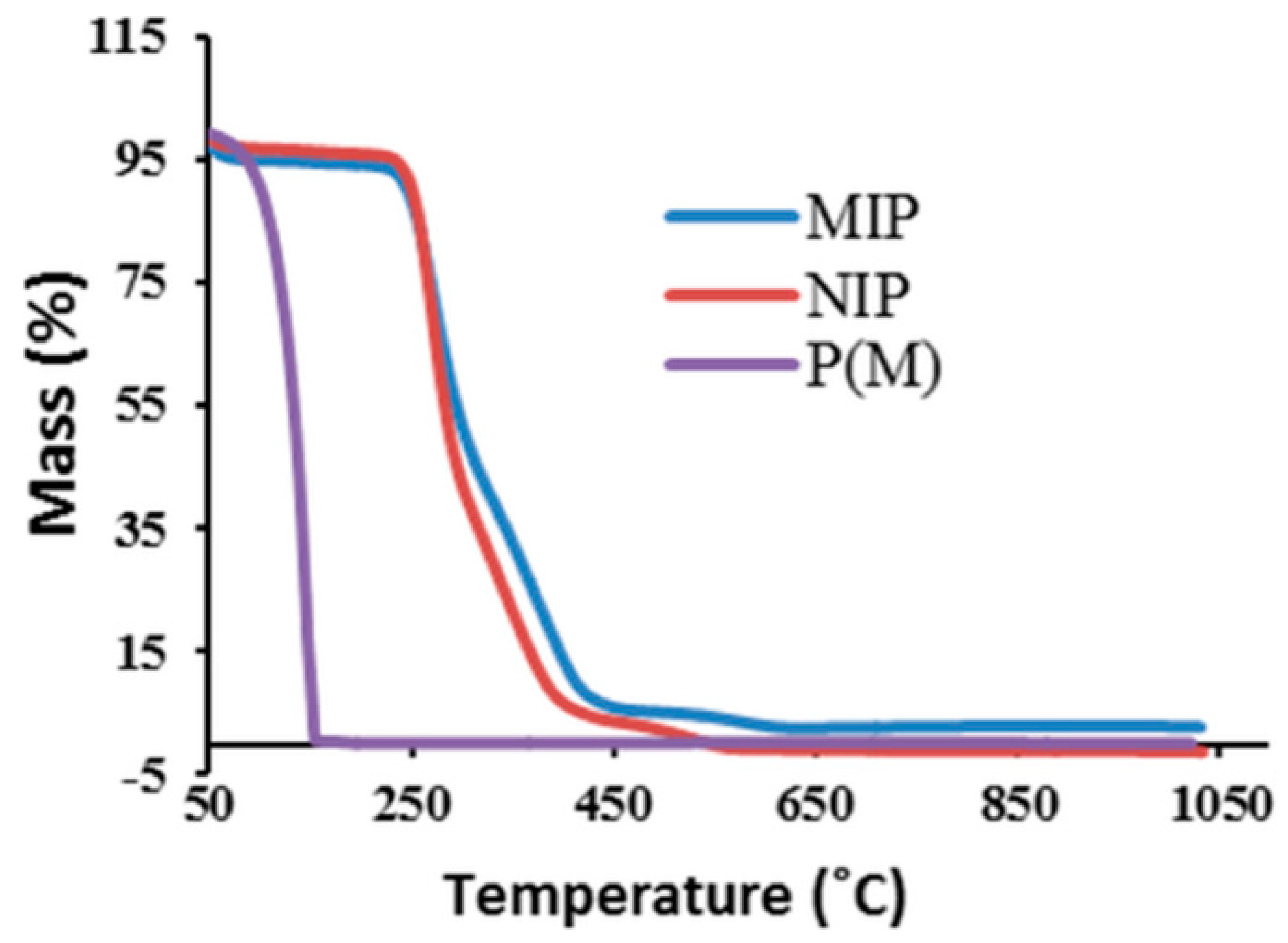
2.1.3. Thermal Stability
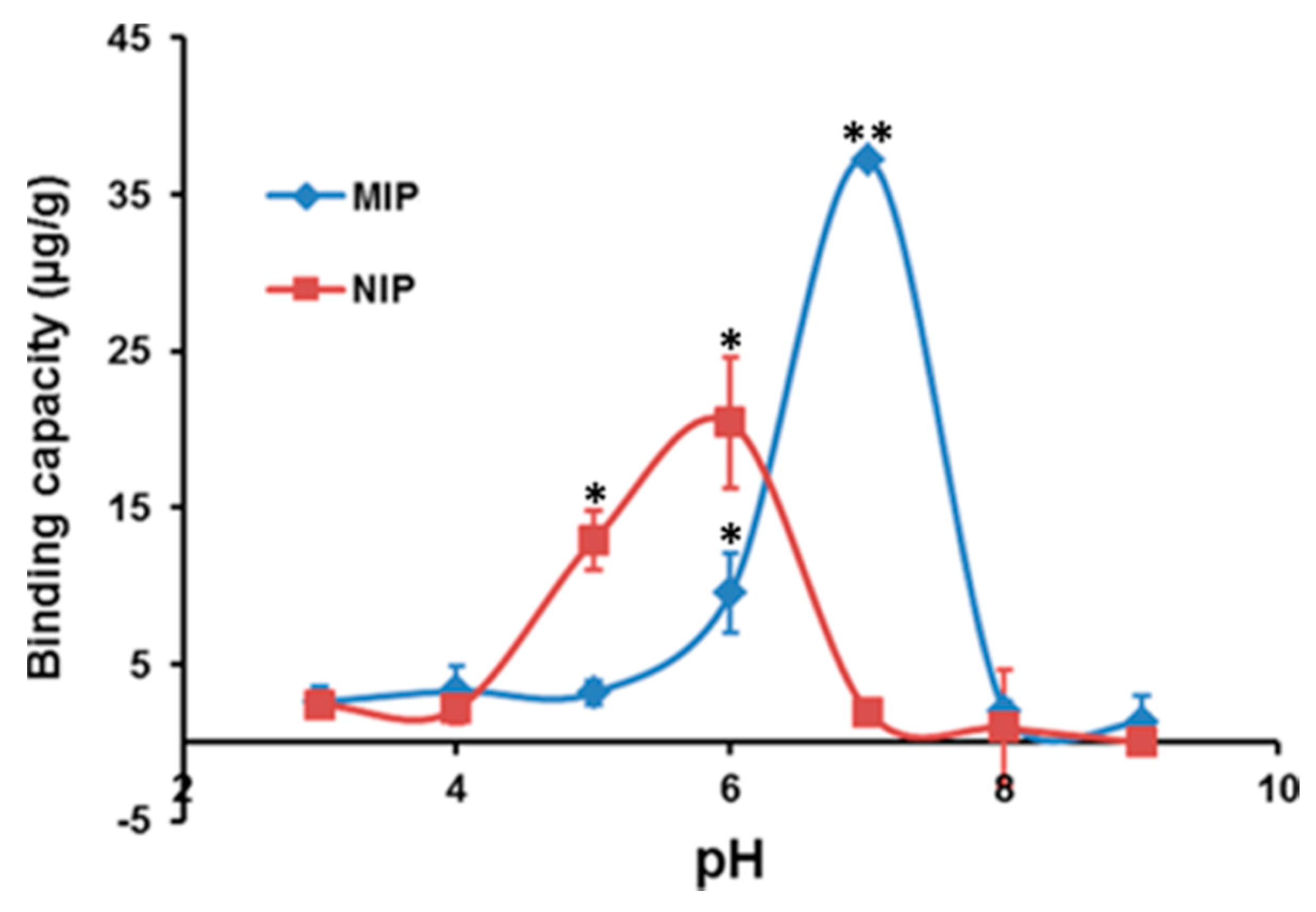
2.2. Effect of pH
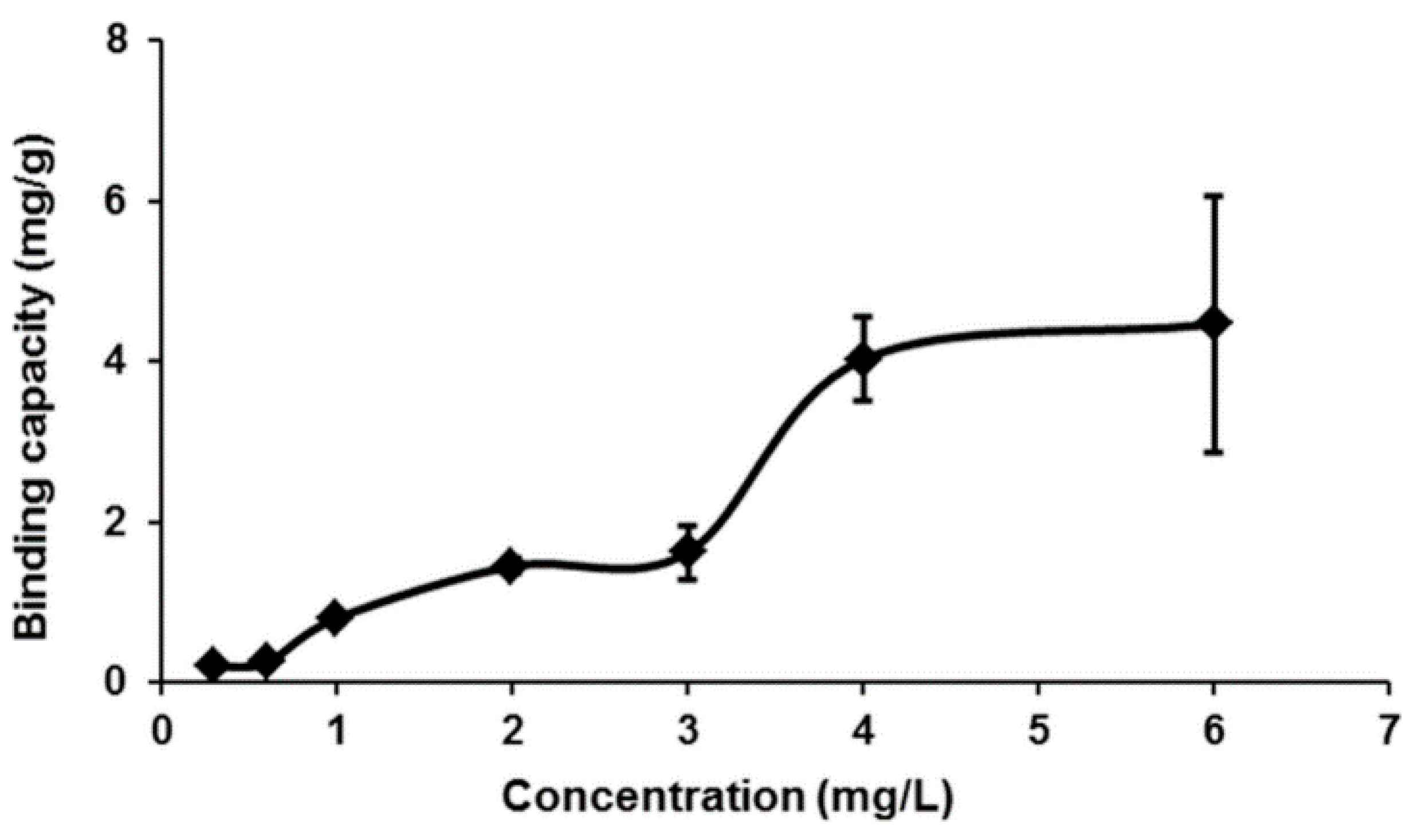
2.3. Effect of Dosage on MIP

2.4. Adsorption Isotherm
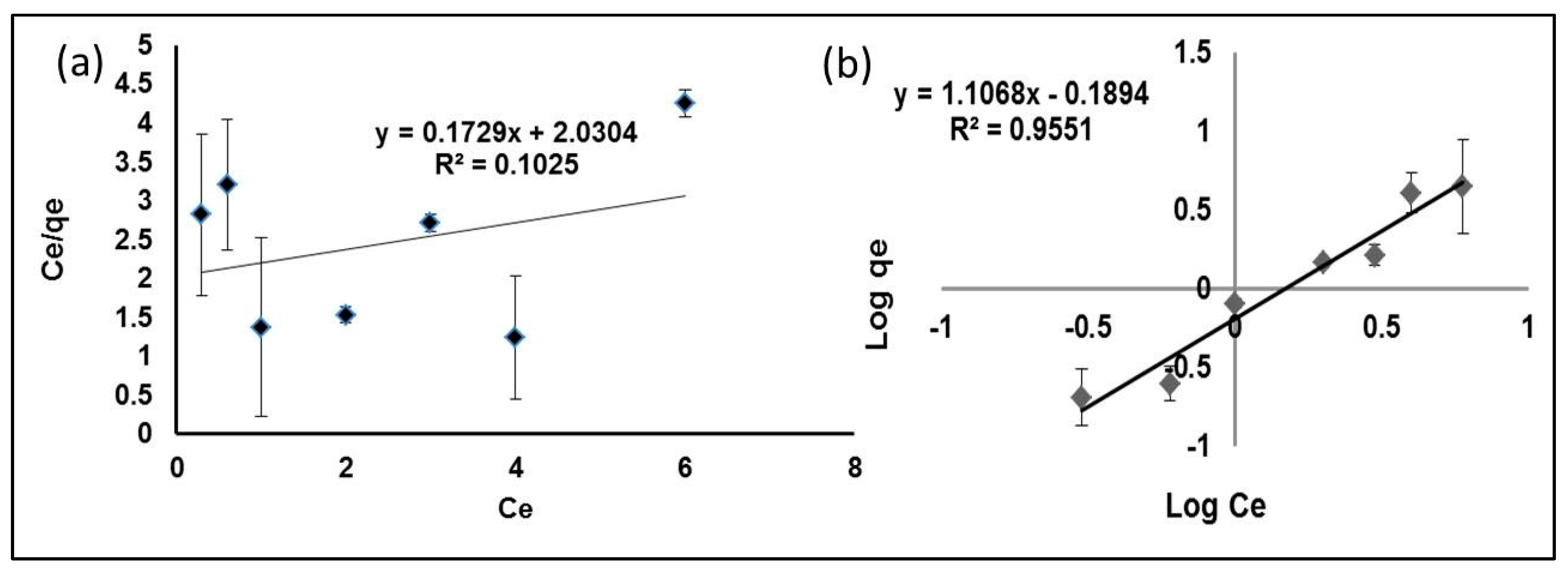
| Experimental (qe = mg/g) | Langmuir Constant | Freundlich Constant | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | bL (L/mg) | R2 | Kf (mg/g) | 1/n (L/mg) | R2 | |
| 4.46 | 5.7837 | 0.0505 | 0.1025 | 0.6465 | 1.1068 | 0.9551 |
2.5. Adsorption Kinetics
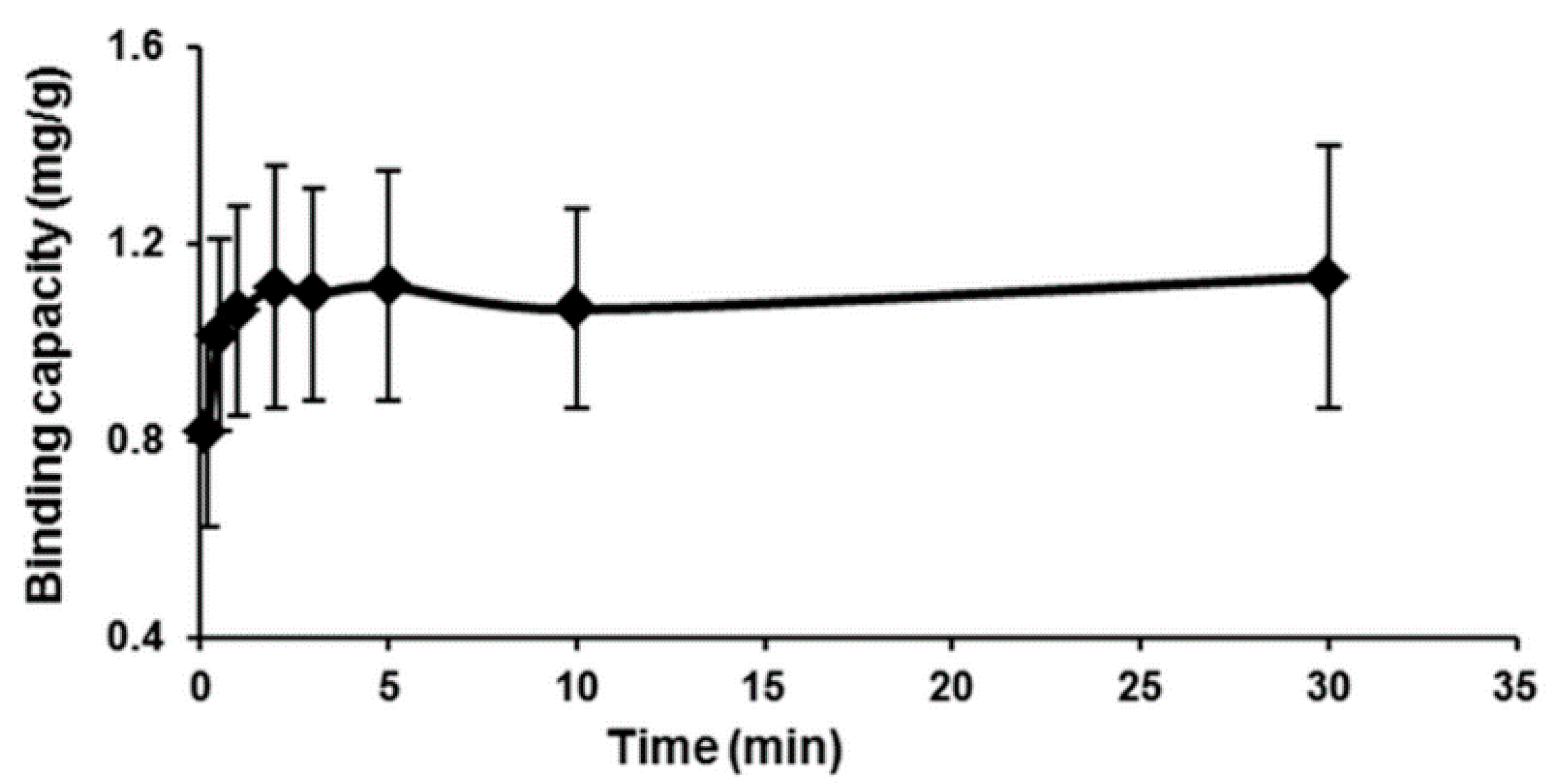

| Experimental qe (exp) | First Order | Second Order | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | qe (calc) (mg/g) | R2 | K2 (mg/g·min−1) | qe (calc) (mg/g) | R2 | |
| 1.132 | 0.0672 | 0.0496 | 0.0341 | 7.1577 | 1.074 | 0.9996 |
2.6. Selectivity Studies
| Metal Ion | MIP | NIP | K' | ||
|---|---|---|---|---|---|
| Kd | K | Kd | K | ||
| Hg(II) | 0.87 | - | 0.66 | - | - |
| Zn(II) | 0.43 | 2.05 | 0.66 | 1.00 | 2.03 |
| Cd(II) | 0.54 | 1.60 | 0.62 | 1.07 | 1.50 |
| Pb(II) | 0.04 | 22.52 | 0.04 | 15.42 | 1.46 |
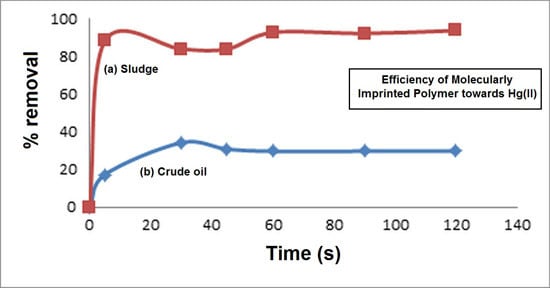
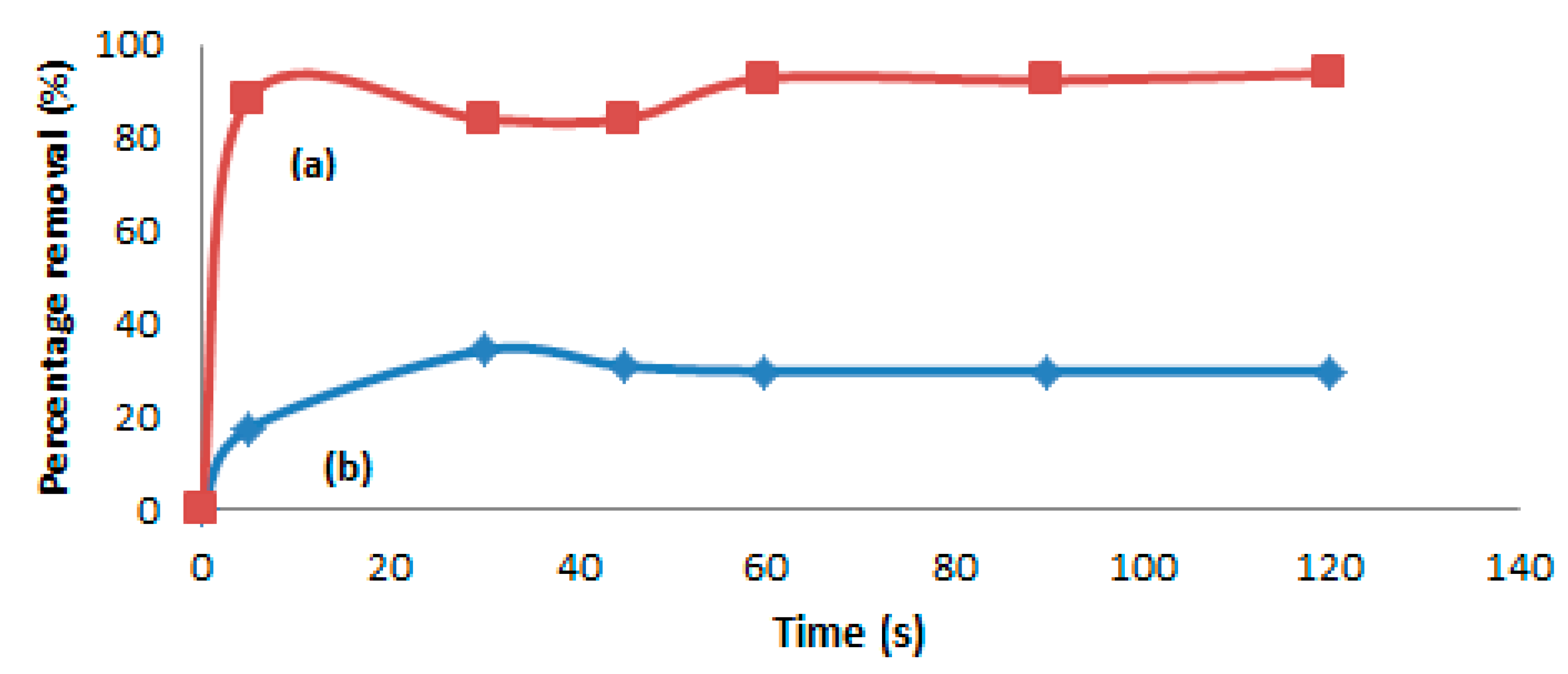
2.7. Sorption of Hg(II) from Petroleum Oil
3. Experimental Section
3.1. Materials
3.2. Preparation of Cysteine Complex
3.3. Preparation of Cysteine Complex-Hg-Imprinted and Non-Imprinted Polymer
3.4. Characterization of MIP and NIP
3.5. Adsorption Studies
3.6. Adsorption of Hg(II) Ions from Petroleum Oil Samples
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Risher, J.F.; Amler, S.N. Mercury exposure: evaluation and intervention the inappropriate use of chelating agents in the diagnosis and treatment of putative mercury poisoning. Neurotoxicology 2005, 26, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Díez, S. Human health effects of methylmercury exposure. Rev. Environ. Contam. Toxicol. 2009, 198, 111–132. [Google Scholar] [PubMed]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef] [PubMed]
- Sondreal, E.A.; Benson, S.A.; Pavlish, J.H.; Ralston, N.V.C. An overview of air quality III: Mercury, trace elements, and particulate matter. Fuel Process. Technol. 2004, 85, 425–440. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Mercury Study Report to Congress; EPA-452/R-97-003; Office of Air Quality Planning and Standards, Office of Research and Development, U.S. Government Printing Office: Washington, DC, USA, 1998.
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Novel sorbents for mercury removal from flue gas. Ind. Eng. Chem. Res. 2000, 39, 1020–1029. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Liang, L.; Kirchgessner, D. Identification and properties of mercury species in crude oil. Energ. Fuel 2006, 20, 180–186. [Google Scholar] [CrossRef]
- Shafeeq, A.; Muhammad, A.; Sarfraz, W.; Toqeer, A.; Rashid, S.; Rafiq, M.K. Mercury removal techniques for industrial waste water. World Acad. Sci. Eng. Technol. 2012, 6, 12–26. [Google Scholar]
- Reddy, K.S.; Shoaibi, A.A.; Srinivasakannan, C. Elemental mercury adsorption on sulfur-impregnated porous carbon—A review. Environ. Technol. 2014, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Bloom, N. Mercury in petroleum. Fuel Process. Technol. 2000, 63, 1–27. [Google Scholar] [CrossRef]
- Brüggemann, O.; Haupt, K.; Ye, L.; Yilmaz, E.; Mosbach, K. New configurations and applications of molecularly imprinted polymers. J. Chromatogr. A 2000, 889, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pichon, V.; Chapuis-Hugon, F. Role of molecularly imprinted polymers for selective determination of environmental pollutants—A review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Koesdjojo, M.T.; Tennico, Y.H.; Remcho, V.T. Molecularly imprinted polymers as sorbents for separation and extractions. In Separation Science and Technology: HPLC Method Development for Pharmaceuticals; Ahuja, S., Rasmussen, H., Eds.; Academic Press: New Jersey, NJ, USA, 2007; Volume 8, pp. 479–503. [Google Scholar]
- Owens, P.K.; Karlsson, L.; Lutz, E.S.M.; Andersson, L.I. Molecular imprinting for bio- and pharmaceutical analysis. Trends Anal. Chem. 1999, 18, 146–154. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Ma, D.; Shi, Z.; Ma, S. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 7. [Google Scholar]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic gold nanoshells: Stepwise changing of magnetism through stepwise biofunctionalization. J. Phys. Chem. Lett. 2010, 1, 3141–3146. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.A.; Rahman, S.K.A.; Hussein, M.A.; Ibrahim, N.A. Preparation and characterization of molecularly imprinted polymer as SPE sorbent for melamine isolation. Polymers 2013, 5, 1215–1228. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, X.; Yang, D.; Guo, Y.; Meng, S. Highly selective determination of inorganic mercury(II) after preconcentration with Hg(II)-imprinted diazoaminobenzene-vinylpyridine copolymers. Anal. Chim. Acta 2005, 538, 85–91. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Z.; Wang, J.; He, C. Hierarchically imprinted organic-inorganic hybrid sorbent for selective separation of mercury ion from aqueous solution. Anal. Chim. Acta 2007, 582, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Dekhil, A.B.; Hannachi, Y.; Ghorbel, A.; Baubaker, T. Comparative study of the removal of cadmium from aqueos solution by low cost adsorbents. J. Environ. Sci. Technol. 2011, 4, 520–533. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, D.H.K.K.; Venkateswarlu, P.; Seshaiah, K. Removal of mercury from aqueous solutions using activated carbon prepared from agricultural by-product/waste. J. Environ. Manag. 2009, 90, 634–643. [Google Scholar] [CrossRef]
- Umpleby, R.J.; Baxter, S.C.; Rampey, A.M.; Rushton, G.T.; Chen, Y.; Shimizu, K.D. Characterization of the heterogeneous binding site affinity distributions in molecularly imprinted polymers. J. Chromatogr. 2004, 804, 141–149. [Google Scholar]
- García-Calzón, J.A.; Díaz-García, M.E. Characterization of binding sites in molecularly imprinted polymers. Sens. Actuators B 2007, 123, 1180–1194. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Dhedan, S.K. Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilib. 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Tuzen, M.; Sari, A.; Mendil, D.; Soylak, M. Biosorptive removal of mercury(II) from aqueous solution using lichen (Xanthoparmelia conspersa) biomass: Kinetic and equilibrium studies. J. Hazard. Mater. 2009, 169, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Arica, M.Y. Synthesis of Cr(VI)-imprinted poly(4-vinyl pyridine-co-hydroxyethyl methacrylate) particles: Its adsorption propensity to Cr(VI). J. Hazard. Mater. 2011, 187, 213–221. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairi, N.A.S.; Yusof, N.A.; Abdullah, A.H.; Mohammad, F. Removal of Toxic Mercury from Petroleum Oil by Newly Synthesized Molecularly-Imprinted Polymer. Int. J. Mol. Sci. 2015, 16, 10562-10577. https://doi.org/10.3390/ijms160510562
Khairi NAS, Yusof NA, Abdullah AH, Mohammad F. Removal of Toxic Mercury from Petroleum Oil by Newly Synthesized Molecularly-Imprinted Polymer. International Journal of Molecular Sciences. 2015; 16(5):10562-10577. https://doi.org/10.3390/ijms160510562
Chicago/Turabian StyleKhairi, Nor Ain Shahera, Nor Azah Yusof, Abdul Halim Abdullah, and Faruq Mohammad. 2015. "Removal of Toxic Mercury from Petroleum Oil by Newly Synthesized Molecularly-Imprinted Polymer" International Journal of Molecular Sciences 16, no. 5: 10562-10577. https://doi.org/10.3390/ijms160510562
APA StyleKhairi, N. A. S., Yusof, N. A., Abdullah, A. H., & Mohammad, F. (2015). Removal of Toxic Mercury from Petroleum Oil by Newly Synthesized Molecularly-Imprinted Polymer. International Journal of Molecular Sciences, 16(5), 10562-10577. https://doi.org/10.3390/ijms160510562