Characterization of the Recognition Specificity of BH2, a Monoclonal Antibody Prepared against the HLA-B27 Heavy Chain
Abstract
:1. Introduction
2. Results
2.1. Recognition Specificity of BH2
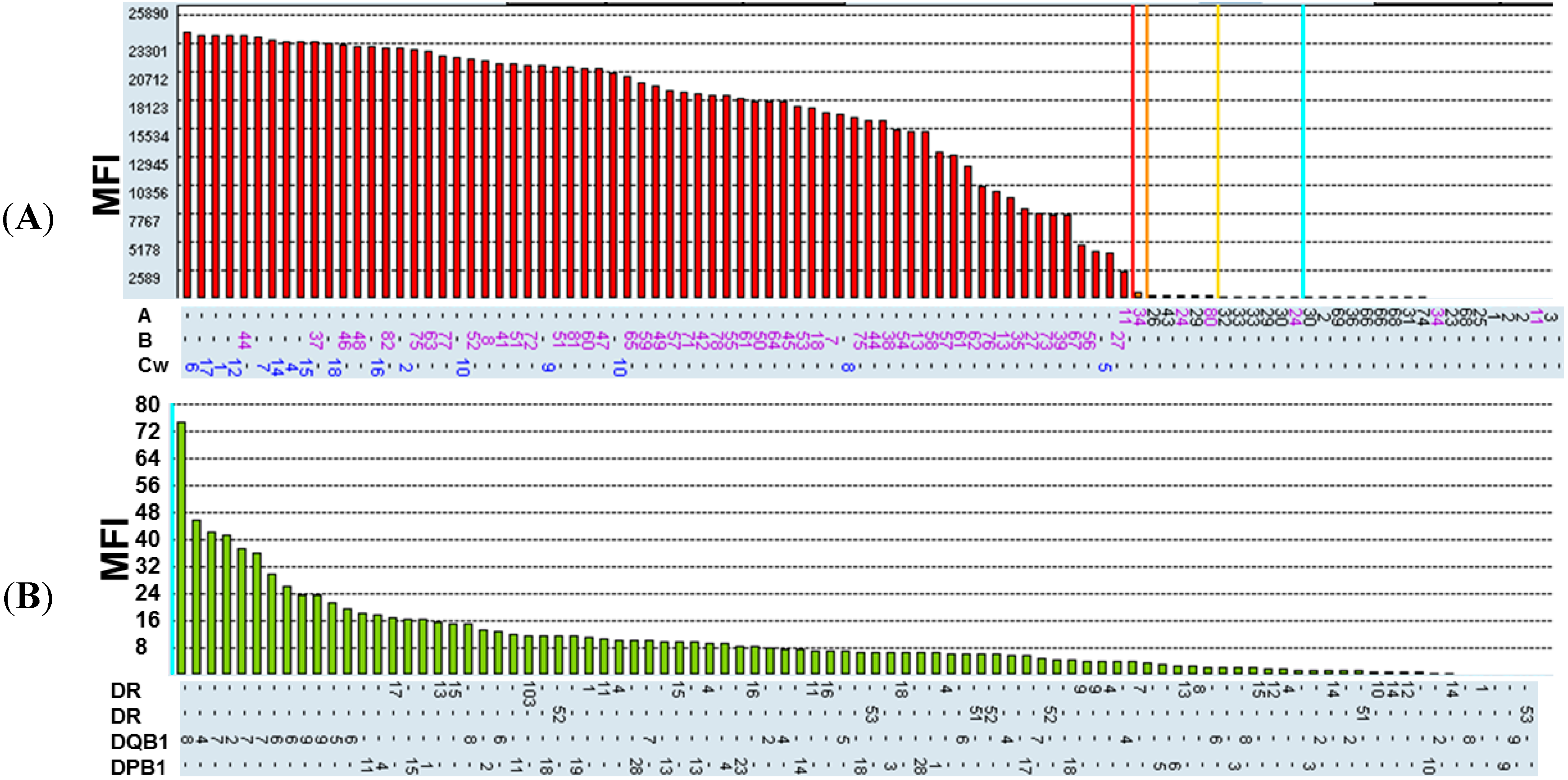
| Class I (Positive) | Class II (Positive) |
|---|---|
| Cw10, B27, B57, B51, B61, B13, B44 | |
| B75, B55, Cw4, Cw12, B49, B38, Cw1 | |
| B76, B54, Cw16, B8, B48, B35, Cw15 | |
| B42, Cw7, Cw9, B41, B7, B62, Cw6 | |
| B82, B37, Cw17, B60, B56, B65, B45, B81 | Negative |
| B59, B52, B39, Cw2, Cw14, B78, B46 | |
| Cw5, Cw8, B72, B58, B63, B77, B73 | |
| B71, B64, B53, B67, B47, B18, Cw18 | |
| B50, A11 |
2.2. BH2 Binds to the α2 Domain of HLA-B2704 HC
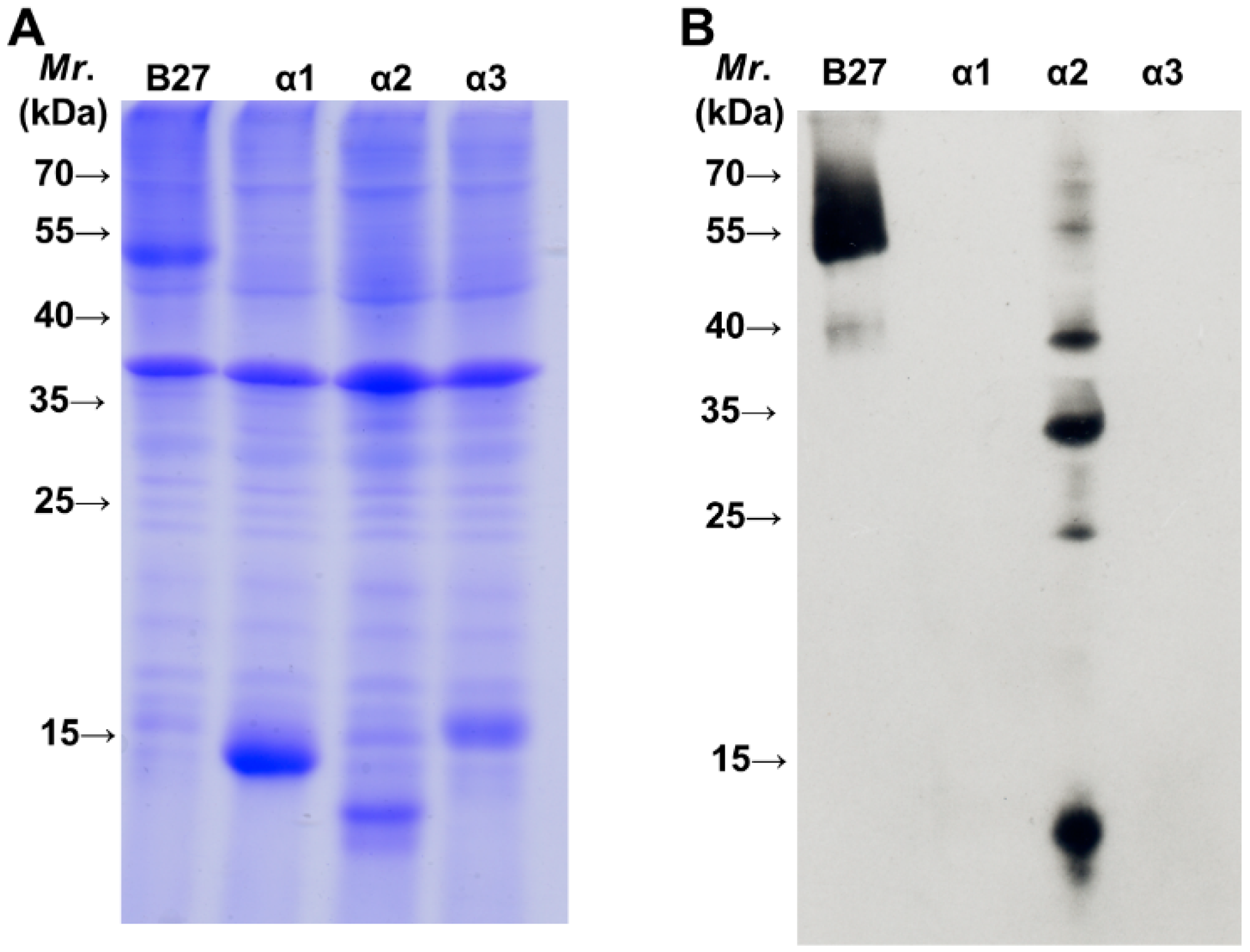

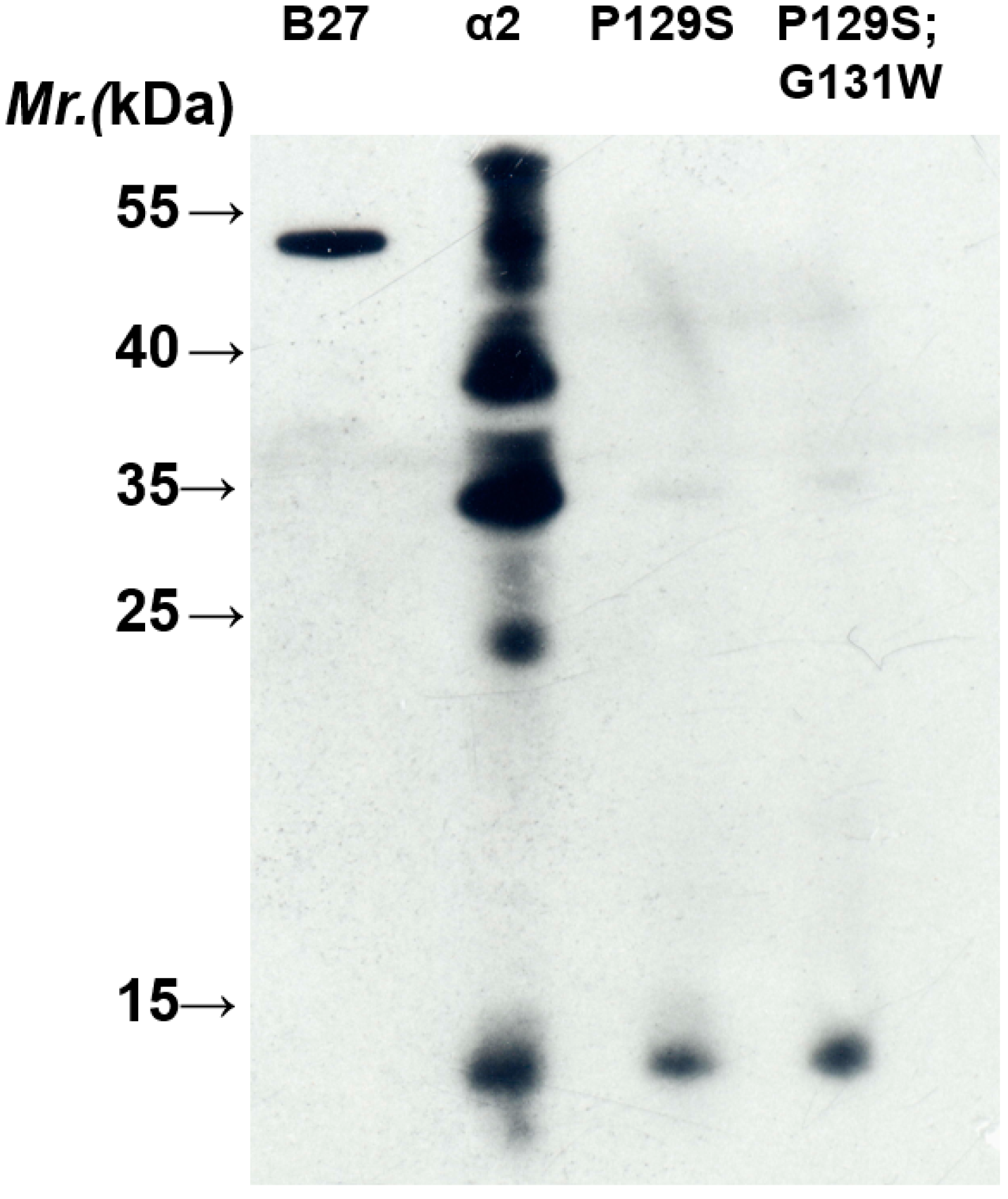
3. Experimental Section
3.1. Materials
3.2. HLA-Typing
3.3. Analysis of BH2-Binding Domain
- Alpha 1 primers: 5'-GGCGGCTCCCACTCCATGAGG-3' and 5'-GGCCTCGCTCTGGTTGATG-3'
- Alpha 2 primers: 5'-GGGTCTCACACCCTCCAGAA-3' and 5'-CGCGCGCTGCAGCGTCTC-3'
- Alpha 3 primers: 5'-GACCCCCCAAAGACACACG-3' and 5'-CCATCTCAGGGTGAGGGG-3'
3.4. Site-Directed Mutagenesis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ploegh, H.L.; Orr, H.T.; Strominger, J.L. Major histocompatibility antigens: The human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell 1981, 24, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, H.G.; Falk, K.; Rötzschke, O. Peptide naturally presented by MHC class I molecules. Annu. Rev. Immunol. 1993, 11, 213–244. [Google Scholar] [CrossRef] [PubMed]
- Androlewicz, M.J.; Ortmann, B.; van Endert, P.M.; Spies, T.; Cresswell, P. Characteristics of peptide and major histocompatibility complex class I/β2-microglobulin binding to the transporters associated with antigen processing (TAP1 and TAP2). Proc. Natl. Acad. Sci. USA 1994, 91, 12716–12720. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Seliger, B. The transporter associated with antigen processing (TAP): Structural integrity, expression, function, and its clinical relevance. Mol. Med. 2001, 7, 149–158. [Google Scholar] [PubMed]
- Jones, E. MHC class I and class II structures. Curr. Opin. Immunol. 1997, 9, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Garboczi, D.N.; Ghosh, P.; Utz, U.; Fan, Q.R.; Biddison, W.E.; Wiley, D.C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 1996, 384, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kostyu, D.D.; Hannick, L.I.; Traweek, J.L.; Ghanayem, M.; Heilpern, D.; Dawson, D.V. HLA class I polymorphism: Structure and function and still questions. Hum. Immunol. 1997, 57, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pamer, E.; Cresswell, P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998, 16, 323–358. [Google Scholar] [CrossRef] [PubMed]
- Colbert, R.A. HLA-B27 misfolding: A solution to the spondyloarthropathy conundrum? Mol. Med. Today 2000, 6, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Colbert, R.A.; DeLay, M.L.; Klenk, E.I.; Layh-Schmitt, G. From HLA-B27 to spondyloarthritis: A journey through the ER. Immunol. Rev. 2010, 233, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Melis, L.; Elewaut, D. Immunopathogenesis of spondykoarthritis: Which cells drive disease? Arthritis Res. Ther. 2009, 11, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A.; Marker-Hermann, E.; Colbert, R.A. Pathogenesis of ankylosing spondylitis: Current concepts. Best Pract. Res. Clin. Rheumatol. 2006, 20, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Tam, L.S.; Gu, J.; Yu, D. Pathogenesis of ankylosing spondylitis. Nat. Rev. Rheumatol. 2010, 6, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M.F.; James, D.C. Human lymphocyte antigen association in ankylosing spondylitis. Nature 1973, 242, 121. [Google Scholar] [CrossRef] [PubMed]
- Brewerton, D.A.; Hart, F.D.; Nicholls, A.; Caffrey, M.; James, D.C.O.; Sturrock, R.D. Ankylosing spondylitis and HLA-B27. Lancet 1973, 1, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.L.; O’Callaghan, C.A.; McMichael, A.J.; Bowness, P. HLA-B27 can form a novel β2-microglobulin-free heavy chain homodimer structure. J. Immunol. 1999, 62, 5045–5048. [Google Scholar]
- Kollnberger, S.; Bird, L.; Sun, M.Y.; Retiere, C.; Braud, V.M.; McMichael, A.; Bowness, P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheumatol. 2002, 6, 2972–2982. [Google Scholar] [CrossRef]
- Dangoria, N.; DeLay, M.L.; Kingsbury, D.J.; Mear, J.P.; Uchanska-Ziegler, B.; Ziegler, A; Colbert, R.A. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J. Biol. Chem. 2002, 77, 23459–23468. [Google Scholar]
- Kollnberger, S.; Bird, L.A.; Roddis, M.; Hacquard-Bouder, C.; Kubagawa, H.; Bodmer, H.C.; Breban, M.; McMichael, A.J.; Bowness, P. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J. Immunol. 2004, 73, 1699–1710. [Google Scholar] [CrossRef]
- Chan, A.T.; Kollnberger, S.D.; Wedderburn, L.R.; Bowness, P. Expansion and enchanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 inspondylarthritis. Arthritis. Rheumatol. 2005, 52, 3586–3595. [Google Scholar] [CrossRef]
- Bowness, P.; Ridley, A.; Shaw, J.; Chan, A.T.; Wong-Baeza, I.; Fleming, M.; Cummings, F.; McMichael, A.; Kollnberger, S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. 2011, 186, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Lu, M.C.; Li, C.; Huang, H.L.; Huang, K.Y.; Liu, S.Q.; Lai, N.S.; Huang, H.B. Targeted delivery of an antigenic peptide to the endoplasmic reticulum: application for development of a peptide therapy for ankylosing spondylitis. PLoS ONE 2013, 8, e77451. [Google Scholar] [CrossRef] [PubMed]
- Stam, N.J.; Spits, H.; Ploegh, H.L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol. 1986, 137, 2299–2306. [Google Scholar] [PubMed]
- Tran, T.M.; Satumtira, N.; Dorris, M.L.; May, E.; Wang, A.; Furuta, E.; Taurog, J.D. HLAB27 in transgenic rats forms disulfide-linked heavy chain oligomers and multimers that bind to the chaperone BiP. J. Immunol. 2004, 172, 5110–5119. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.B.; Haworth, S.E.; Poli, F.; Nocco, A.; Puglisi, G.; Innocente, A.; Serafini, M.; Messa, P.; Mario, M. Luminex technology for anti-HLA antibody screening: Evaluation of performance and of impact on laboratory routine. Cytometry 2007, 72B, 465–471. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-C.; Huang, K.-Y.; Lu, M.-C.; Huang, H.-L.; Liu, W.-T.; Lee, W.-C.; Liu, S.-Q.; Huang, H.-B.; Lai, N.-S. Characterization of the Recognition Specificity of BH2, a Monoclonal Antibody Prepared against the HLA-B27 Heavy Chain. Int. J. Mol. Sci. 2015, 16, 8142-8150. https://doi.org/10.3390/ijms16048142
Yu H-C, Huang K-Y, Lu M-C, Huang H-L, Liu W-T, Lee W-C, Liu S-Q, Huang H-B, Lai N-S. Characterization of the Recognition Specificity of BH2, a Monoclonal Antibody Prepared against the HLA-B27 Heavy Chain. International Journal of Molecular Sciences. 2015; 16(4):8142-8150. https://doi.org/10.3390/ijms16048142
Chicago/Turabian StyleYu, Hui-Chun, Kuang-Yung Huang, Ming-Chi Lu, Hsien-Lu Huang, Wei-Ting Liu, Wen-Chien Lee, Su-Qin Liu, Hsien-Bin Huang, and Ning-Sheng Lai. 2015. "Characterization of the Recognition Specificity of BH2, a Monoclonal Antibody Prepared against the HLA-B27 Heavy Chain" International Journal of Molecular Sciences 16, no. 4: 8142-8150. https://doi.org/10.3390/ijms16048142
APA StyleYu, H.-C., Huang, K.-Y., Lu, M.-C., Huang, H.-L., Liu, W.-T., Lee, W.-C., Liu, S.-Q., Huang, H.-B., & Lai, N.-S. (2015). Characterization of the Recognition Specificity of BH2, a Monoclonal Antibody Prepared against the HLA-B27 Heavy Chain. International Journal of Molecular Sciences, 16(4), 8142-8150. https://doi.org/10.3390/ijms16048142






