Chemical Conversion Pathways and Kinetic Modeling for the OH-Initiated Reaction of Triclosan in Gas-Phase
Abstract
:1. Introduction
2. Results and Discussion
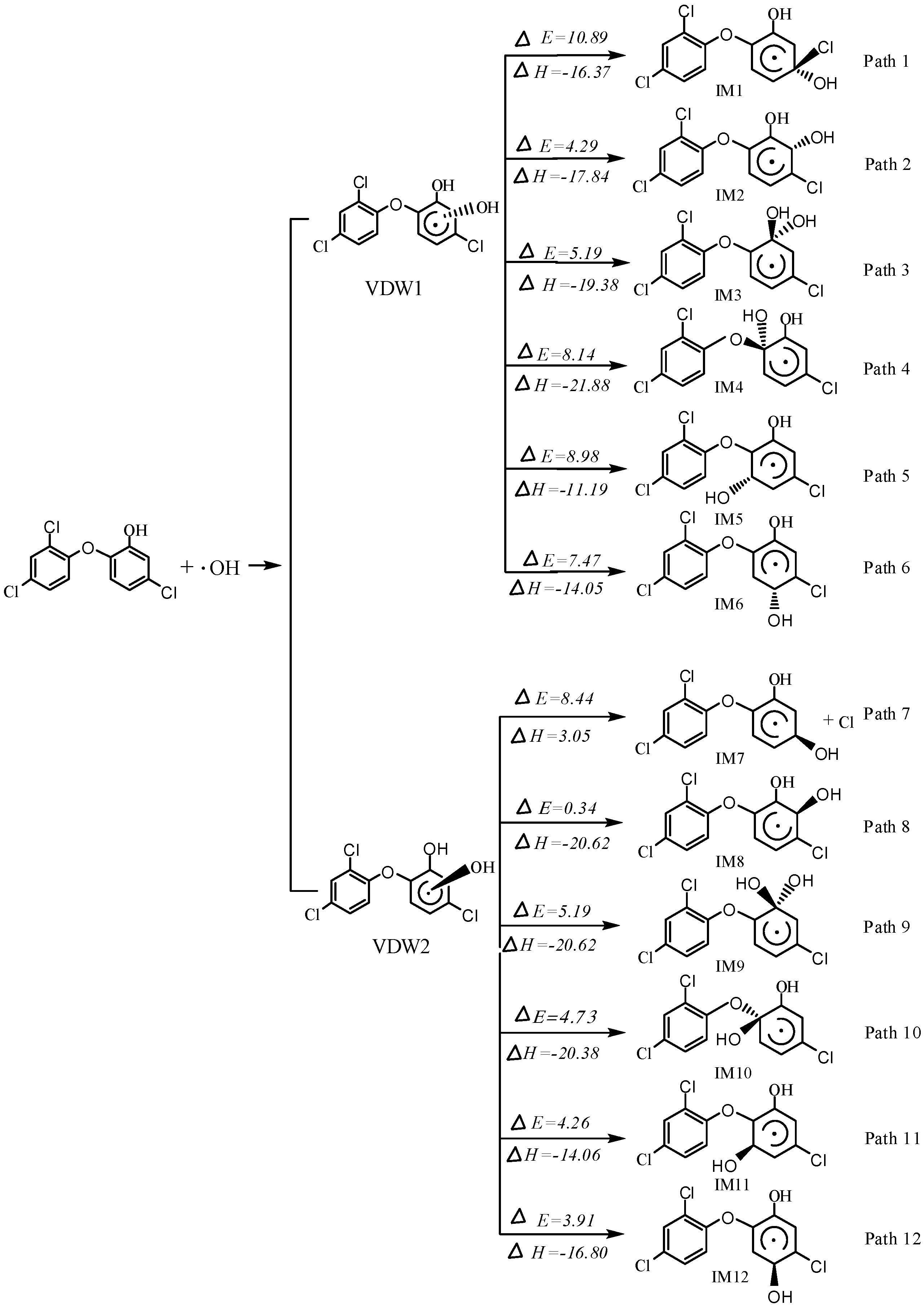
2.1. OH Addition Pathways
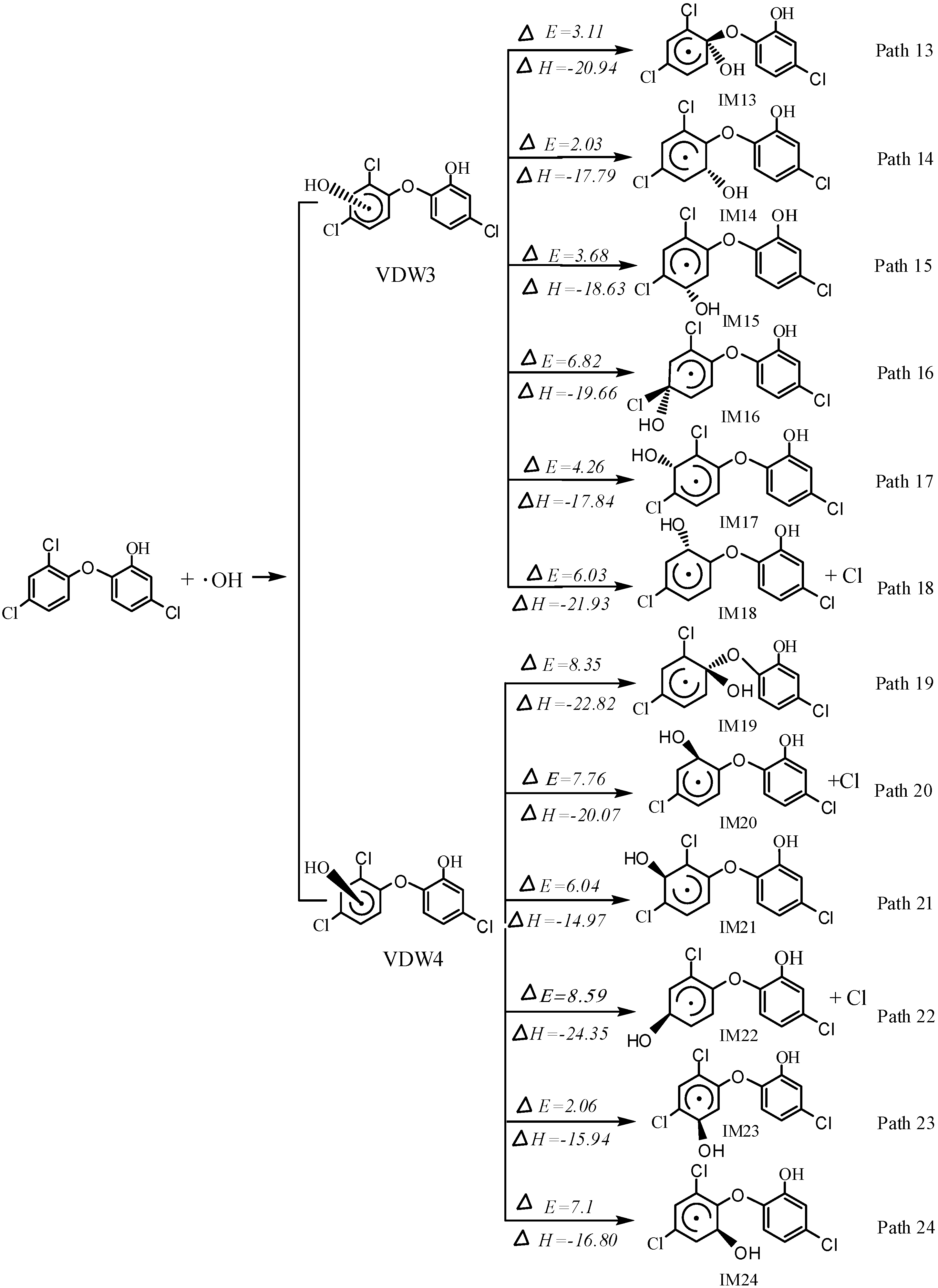

2.2. OH Abstraction


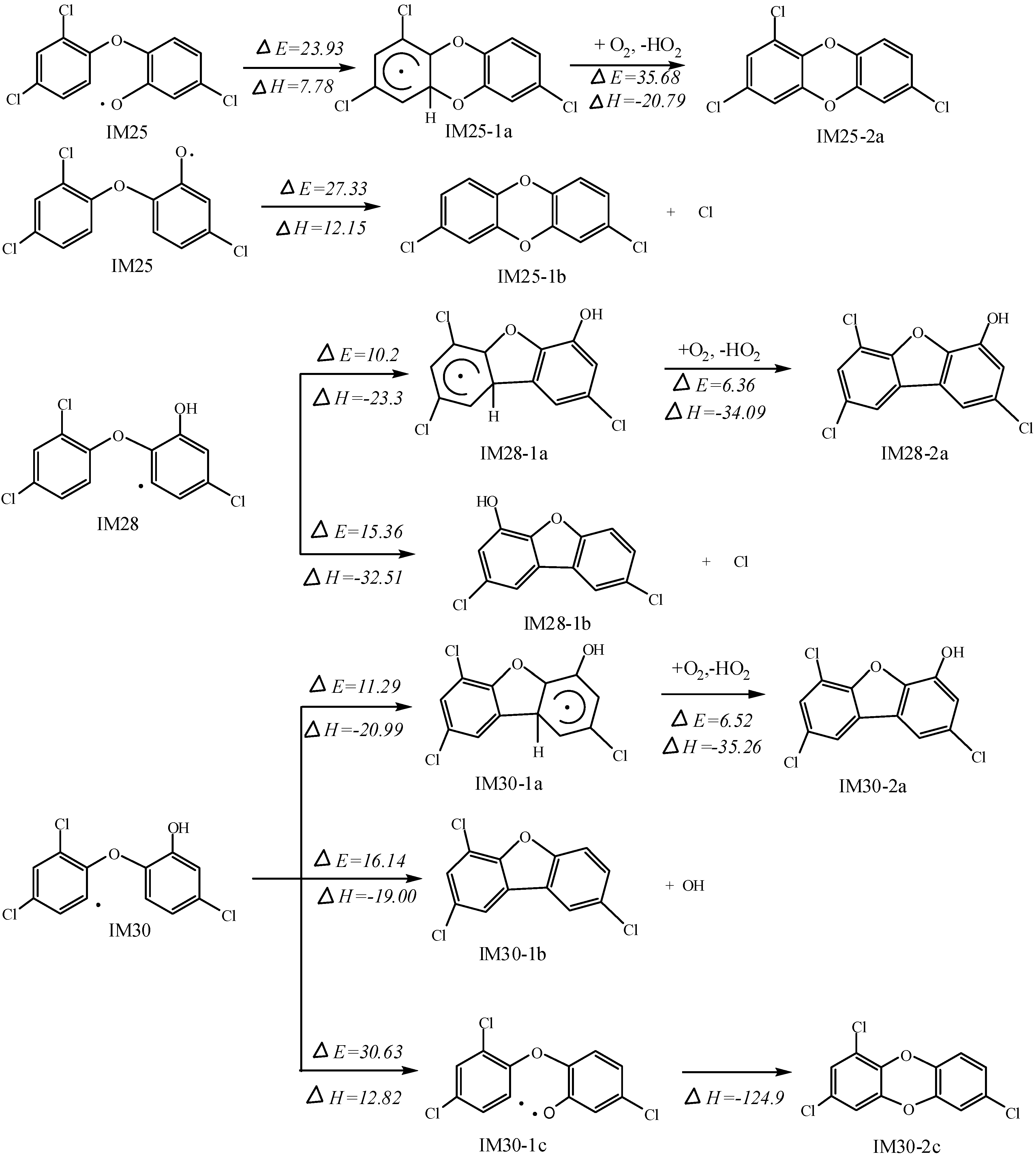
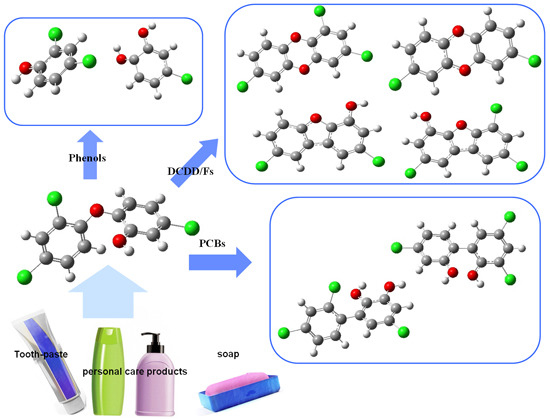
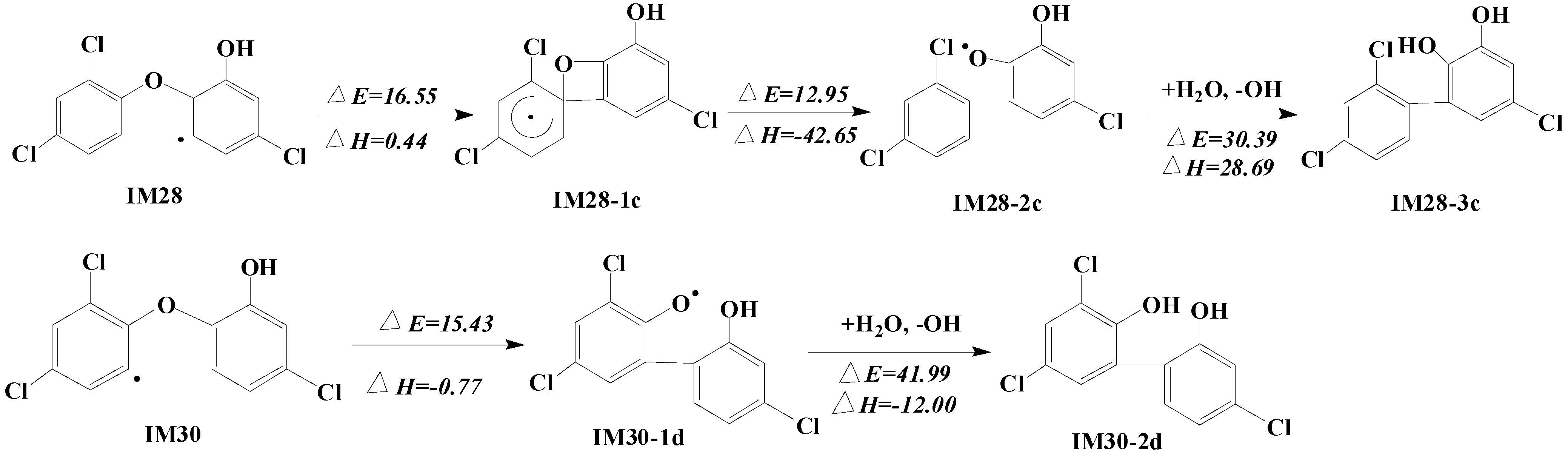
2.3. Formation of Polychlorinated Dibenzopdioxin and Furan (PCDD/Fs)
2.4. Formation of Polychlorinated Biphenyls (PCBs)
2.5. Kinetics
| Reactions | k298.15 K | Arrhenius Formulas |
|---|---|---|
| TCS + OH → IM1 | 1.09 × 10−18 | k(T) = 6.9 × 10−14 exp(−2902.9/T) |
| TCS + OH → IM2 | 4.19 × 10−18 | k(T) = 6.9 × 10−14 exp(−2902.9/T) |
| TCS + OH → IM3 | 6.68 × 10−16 | k(T) = 2.5 × 10−11 exp(−1075.8/T) |
| TCS + OH → IM4 | 8.08 × 10−15 | k(T) = 3.8 × 10−14 exp(−454.3/T) |
| TCS + OH → IM5 | 4.84 × 10−15 | k(T) = 1.3 × 10−13 exp(−971.5/T) |
| TCS + OH → IM6 | 4.51 × 10−14 | k(T) = 6.7 × 10−14 exp(−106.9/T) |
| TCS + OH → IM7 | 1.40 × 10−17 | k(T) = 5.0 × 10−14 exp(−2447.1/T) |
| TCS + OH → IM8 | 1.34 × 10−16 | k(T) = 2.8 × 10−14 exp(1597.7/T) |
| TCS + OH → IM9 | 2.54 × 10−17 | k(T) = 6.0 × 10−14 exp(−2321.3/T) |
| TCS + OH → IM10 | 2.60 × 10−16 | k(T) = 5.8 × 10−14 exp(−1611.7/T) |
| TCS + OH → IM11 | 5.33 × 10−15 | k(T) = 4.2 × 10−14 exp(−610.4/T) |
| TCS + OH → IM12 | 1.97 × 10−14 | k(T) = 3.6 × 10−14 exp(−174.1/T) |
| TCS + OH → IM13 | 9.14 × 10−16 | k(T) = 5.9 × 10-14 exp(−1242.2/T) |
| TCS + OH → IM14 | 7.38 × 10−17 | k(T) = 3.0 × 10−14 exp(−1789.7/T) |
| TCS + OH → IM15 | 8.85 × 10−15 | k(T) = 8.3 × 10−14 exp(−660.8/T) |
| TCS + OH → IM16 | 2.15 × 10−16 | k(T) = 5.3 × 10−14 exp(−1642.9/T) |
| TCS + OH → IM17 | 2.60 × 10−16 | k(T) = 2.0 × 10−14 exp(−1989.7/T) |
| TCS + OH → IM18 | 4.05 × 10−16 | k(T) = 2.7 × 10−14 exp(−1254.0/T) |
| TCS + OH → IM19 | 1.20 × 10−18 | k(T) = 1.9 × 10−14 exp(−2887.7/T) |
| TCS + OH → IM20 | 3.42 × 10−17 | k(T) = 2.8 × 10−14 exp(−1998.8/T) |
| TCS + OH → IM21 | 1.26 × 10−16 | k(T) = 9.9 × 10−14 exp(−1990.4/T) |
| TCS + OH → IM22 | 2.03 × 10−16 | k(T) = 3.5 × 10−14 exp(−1535.9/T) |
| TCS + OH → IM23 | 9.77 × 10−16 | k(T) = 7.0 × 10−15 exp(−579.5/T) |
| TCS + OH → IM24 | 1.03 × 10−15 | k(T)=8.7 × 10−15 exp(−631.4/T) |
| TCS + OH → IM25 + H2O | 3.22 × 10−15 | k(T) = 6.7 × 10−14 exp(−699.7/T) |
| TCS + OH → IM26 + H2O | 1.19 × 10−15 | k(T) = 5.9 × 10−13 exp(−1159.1/T) |
| TCS + OH → IM27 + H2O | 1.31 × 10−19 | k(T) = 5.3 × 10−13 exp(−4558.9/T) |
| TCS + OH → IM28 + H2O | 1.18 × 10−16 | k(T) = 2.1 × 10−13 exp(−2243.9/T) |
| TCS + OH → IM29 + H2O | 3.94 × 10−19 | k(T) = 4.5 × 10−13 exp(−4176.5/T) |
| TCS + OH → IM30 + H2O | 2.28 × 10−17 | k(T) = 7.7 × 10−14 exp(−2429.8/T) |
| TCS + OH → IM31 + H2O | 3.03 × 10−17 | k(T) = 8.8 × 10−13 exp(−3077.9/T) |
3. Computational Methods
3.1. Density Functional Theory
3.2. Kinetic Calculation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Furia, T.E.; Schenkel, A.G. Recent development in methods for assessing performance of antibacterial toilet soaps. Soap Chem. Spec. 1968, 45, A472. [Google Scholar]
- Russell, A.D. Whither triclosan? J. Antimicrob. Chemother. 2004, 53, 693–695. [Google Scholar] [CrossRef] [PubMed]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.L.; Sylor, M.A.D.; Slocombe, A.J.; Lew, M.G. Triclosan occurrence in freshwater systems in the United States (1999–2012): A meta-analysis. Environ. Toxicol. Chem. 2013, 32, 1479–1487. [Google Scholar] [PubMed]
- Federle, T.W.; Kaiser, S.K.; Nuck, B.A. Fate and effects of triclosan in activated sludge. Environ. Toxicol. Chem. 2002, 21, 1330–1337. [Google Scholar]
- Ying, G.G.; Kookana, R.S.; Kolpin, D.W. Occurrence and removal of pharmaceutically active compounds in sewage treatment plants with different technologies. J. Environ. Monit. 2009, 11, 1498–1505. [Google Scholar] [CrossRef]
- Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of triclocarban, triclosan and methyltriclosan during wastewater and biosolids treatment processes. Water Res. 2013, 47, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.; Müller, S.T.C.; Pillonel, L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ. Sci. Technol. 2002, 36, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- McClellan, K.; Halden, R.U. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. Water Res. 2010, 4, 658–668. [Google Scholar]
- Zhao, J.L.; Zhang, Q.Q.; Chen, F.; Wang, L.; Ying, G.G.; Liu, Y.S.; Yang, B.; Zhou, L.J.; Liu, S.; Su, H.C.; et al. Evaluation of triclosan and triclocarban at river basin scale using monitoring and modeling tools: Implications for controlling of urban domestic sewage discharge. Water Res. 2013, 47, 395–405. [Google Scholar]
- Orvos, D.R.; Versteeg, D.J.; Inauen, J.; Capdevielle, M.; Rothenstein, A.; Cunningham, V. Aquatic toxicity of triclosan. Environ. Toxicol. Chem. 2002, 21, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Francesco, P.; Luca, N. Assessing triclosan-induced ecological and trans-generational effects in natural phytoplankton communities: A trait-based field method. Ecotoxicology 2013, 22, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Rüde, H.; Böhmer, W.; Müller, M.; Fliedner, A.; Ricking, M.; Teubner, Diana.; Schröter-Kermani, C. Retrospective study of triclosan and methyl-triclosan residues in fish and suspended particulate matter: Results from the German Environmental Specimen Bank. Chemosphere 2013, 91, 1517–1524. [Google Scholar]
- Adolfsson-Eric, M.; Pettersson, M.; Parkkonen, J.; Sturve, J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 2002, 46, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Foran, C.M.; Bennett, E.R.; Benson, W.H. Developmental evaluation of a potential non-steroidal estrogen: Triclosan. Mar. Environ. Res. 2000, 50, 153–156. [Google Scholar] [CrossRef]
- Matsumura, N.; Ishibashi, H.; Hirano, M.; Nagao, Y.; Watanabe, N.; Shiratsuchi, H.; Kai, T.; Nishimura, T.; Kashiwagi, A.; Arizono, K. Effects of nonylphenol and triclosan on production of plasma vitellogenin and testosterone in male South African clawed frogs (Xenopus laevis). Biol. Pharm. Bull. 2005, 28, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Cantonwine, D.E.; Rivera-González, L.O.; Ferguson, K.K.; Mukherjee, B.; Calafat, A.M.; Ye, X.; Toro, A.D.; Liza, V.; Crespo-Hernández, N.; et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ. Sci. Technol. 2013, 47, 3439–3447. [Google Scholar]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Urinary concentrations of triclosan in the US population: 2003–2004. Environ. Health Perspect. 2008, 116, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Gee, R.H.; Charles, A.; Taylor, N.; Darbre, P.D. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J. Appl. Toxicol. 2008, 28, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S. Endocrine disruptors and prostate cancer risk. Endocr. Relat. Cancer 2008, 15, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Kanetoshi, A.; Ogawa, H.; Katsura, E.; Kaneshima, H. Chlorination of irgasan DP300 and formation of dioxins from its chlorinated derivatives. J. Chromatogr. A 1987, 389, 139–153. [Google Scholar] [CrossRef]
- Latch, D.E.; Packer, J.L.; Stender, B.L.; VanOverbeke, J.; Arnold, W.A.; McNeill, K. Aqueous photochemistry of triclosan: Ormation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and ligomerization products. Environ. Toxicol. Chem. 2005, 24, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sires, I.; Oturan, N.; Oturan, M.A.; Rodriguez, R.; Garrido, J.A.; Brillas, E. Electro-Fenton degradation of antimicrobials triclosan and triclocarban. Electrochim. Acta 2007, 52, 5493–5503. [Google Scholar]
- Yang, B.; Ying, G.; Zhao, J.L.; Zhang, L.J.; Fang, Y.X.; Nghiem, L.D. oxidation of triclosan by ferrate: Reaction kinetics, products identification and toxicity evaluation. J. Hazard. Mater. 2011, 186, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Triclosan breakdown by Fenton-like oxidation. Chem. Eng. J. 2012, 198–199, 275–281. [Google Scholar]
- Son, H.S.; Ko, G.; Zoh, K.D. Kinetics and mechanism of photolysis and TiO2 photocatalysis of triclosan. J. Hazard. Mater. 2009, 166, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Latch, D.E.; Packer, J.L.; Arnold, W.A.; McNeill, K. Photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueoussolution. J. Photochem. Photobiol. A 2003, 158, 63–66. [Google Scholar] [CrossRef]
- Stamatics, N.; Antonopoulou, M.; Hela, D.; Konstantinou, L. Photocatalytic degradation kinetics and mechanisms of antibacterial triclosan in aqueous TiO2 suspensions under simulated solar irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1145–1154. [Google Scholar] [CrossRef]
- Kazushi, A.; James, W. Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 2007, 66, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Llompart, M.; Lores, M.; Fernandez-Alvarez, M.; Garcia, J.C.; Cela, R. Further research on the photo-SPME of triclosan. Anal. Bioanal. Chem. 2006, 384, 1548–1557. [Google Scholar] [CrossRef]
- Sun, X.M.; Zhang, C.X.; Zhao, Y.Y.; Bai, J.; Zhang, Q.Z.; Wang, W.X. Atmospheric chemical reactions of 2,3,7,8-tetrachlorinated dibenzofuran initiated by an OH radical: Mechanism and kinetics study. Environ. Sci. Technol. 2012, 46, 8148–8155. [Google Scholar]
- Zhang, C.X.; Sun, T.L.; Sun, X.M. Mechanism for OH-Initiated Degradation of 2,3,7,8-tetrachlorinated dibenzo-p-dioxins in the presence of O2 and NO/H2O. Environ. Sci. Technol. 2011, 45, 4756–4762. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Kwong, T.Y.; Luo, Q.; Cai, Z. Photocatalytic oxidation of triclosan. Chemosphere 2006, 65, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Zhao, Y.Y.; Bai, J.; Gong, C.; Sun, X.M. Mechanism and kinetic study on the OH-initiated degradation of 2,3,7,8-tetrachlorinated dibenzofuran in atmosphere. Sci. Total Environ. 2012, 435–436, 53–60. [Google Scholar]
- Wong-Wah-Chung, P.; Rafqah, S.; Voyard, G.; Sarakha, M. Photochemical behaviour of triclosan in aqueous solutions: Kinetic and analytical studies. J. Photochem. Photobiol. A 2007, 191, 201–208. [Google Scholar] [CrossRef]
- Guan, B.; Wan, P. Photochemistry of dibenzo-1,4-dioxins: Intramolecular rearrangement-reduction through observable 2,2'-biphenylquinones. J. Photochem. Photobiol. A Chem. 1994, 80, 199–210. [Google Scholar] [CrossRef]
- Kliegman, S.; Eustis, S.N.; Arnold, W.A.; McNeill, K. Experimental and theoretical insights into the involvement of radicals in triclosan phototransformation. Environ. Sci. Technol. 2013, 47, 6756–6763. [Google Scholar] [PubMed]
- Zhao, Y.; Truhlar, D.G. Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: The MPW1B95 and MPWB1K models and comparative assessments forhydrogen bonding and van der Waals interactions. J. Phys. Chem. A Chem. 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Sun, X.M.; Bai, J.; Zhao, Y.Y.; Zhang, C.; Wang, Y.; Hu, J.T. Chemical mechanism and kinetics study on the ocimene ozonolysis reaction in atmosphere. Atmos. Environ. 2011, 45, 6197–6203. [Google Scholar] [CrossRef]
- Baldridge, M.S.; Gordon, R.; Steckler, R.; Truhlar, D.G. Ab initio reaction paths and direct dynamics calculations. J. Phys. Chem. 1989, 93, 5107–5119. [Google Scholar] [CrossRef]
- Gonzalez-Lafont, A.; Truong, T.N.; Truhlar, D.G. Interpolated variational transition-state theory: Practical methods for estimating variational transition-state properties and tunneling contributions to chemical reaction rates from electronic structure calculations. J. Chem. Phys. 1991, 95, 8875–8894. [Google Scholar] [CrossRef]
- Liu, Y.P.; Lynch, G.C.; Truong, T.N.; Lu, D.H.; Truhlar, D.G.; Garrett, B.C. Molecular modeling of the kinetic isotope effect for the (1,5)-sigmatropic rearrangement of cis-1,3-pentadiene. J. Am. Chem. Soc. 1993, 115, 2408–2415. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, C.; Sun, X.; Kang, L.; Zhao, Y. Chemical Conversion Pathways and Kinetic Modeling for the OH-Initiated Reaction of Triclosan in Gas-Phase. Int. J. Mol. Sci. 2015, 16, 8128-8141. https://doi.org/10.3390/ijms16048128
Zhang X, Zhang C, Sun X, Kang L, Zhao Y. Chemical Conversion Pathways and Kinetic Modeling for the OH-Initiated Reaction of Triclosan in Gas-Phase. International Journal of Molecular Sciences. 2015; 16(4):8128-8141. https://doi.org/10.3390/ijms16048128
Chicago/Turabian StyleZhang, Xue, Chenxi Zhang, Xiaomin Sun, Lingyan Kang, and Yan Zhao. 2015. "Chemical Conversion Pathways and Kinetic Modeling for the OH-Initiated Reaction of Triclosan in Gas-Phase" International Journal of Molecular Sciences 16, no. 4: 8128-8141. https://doi.org/10.3390/ijms16048128