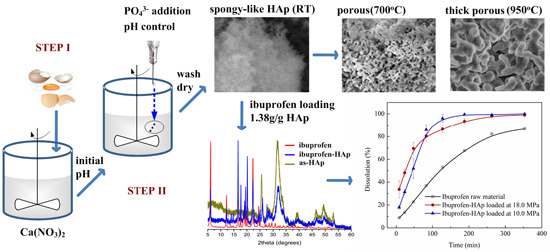
Synthesis of Spongy-Like Mesoporous Hydroxyapatite from Raw Waste Eggshells for Enhanced Dissolution of Ibuprofen Loaded via Supercritical CO2
Abstract
:1. Introduction
2. Results and Discussion
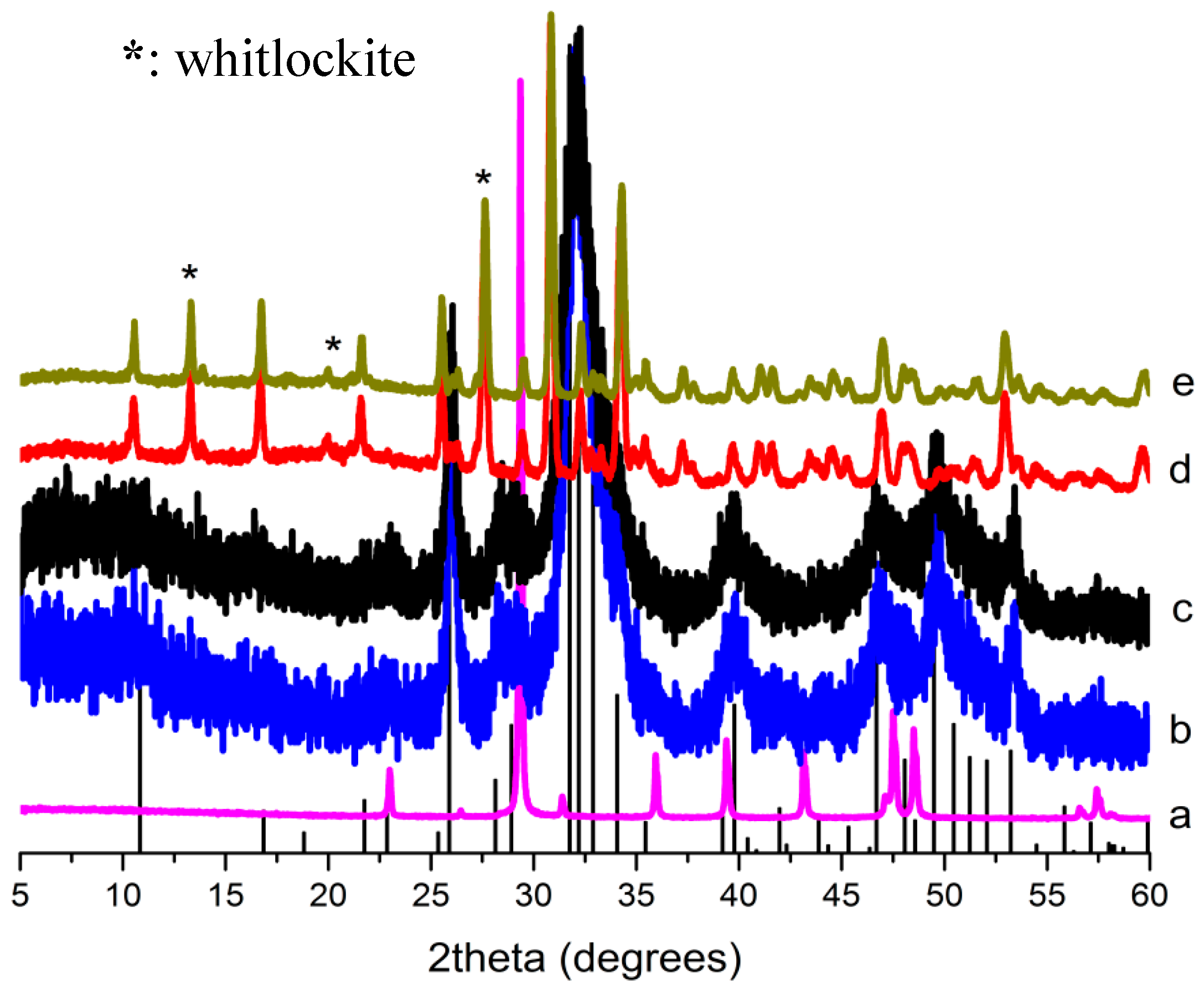
2.1. X-ray Diffraction (XRD) Analysis
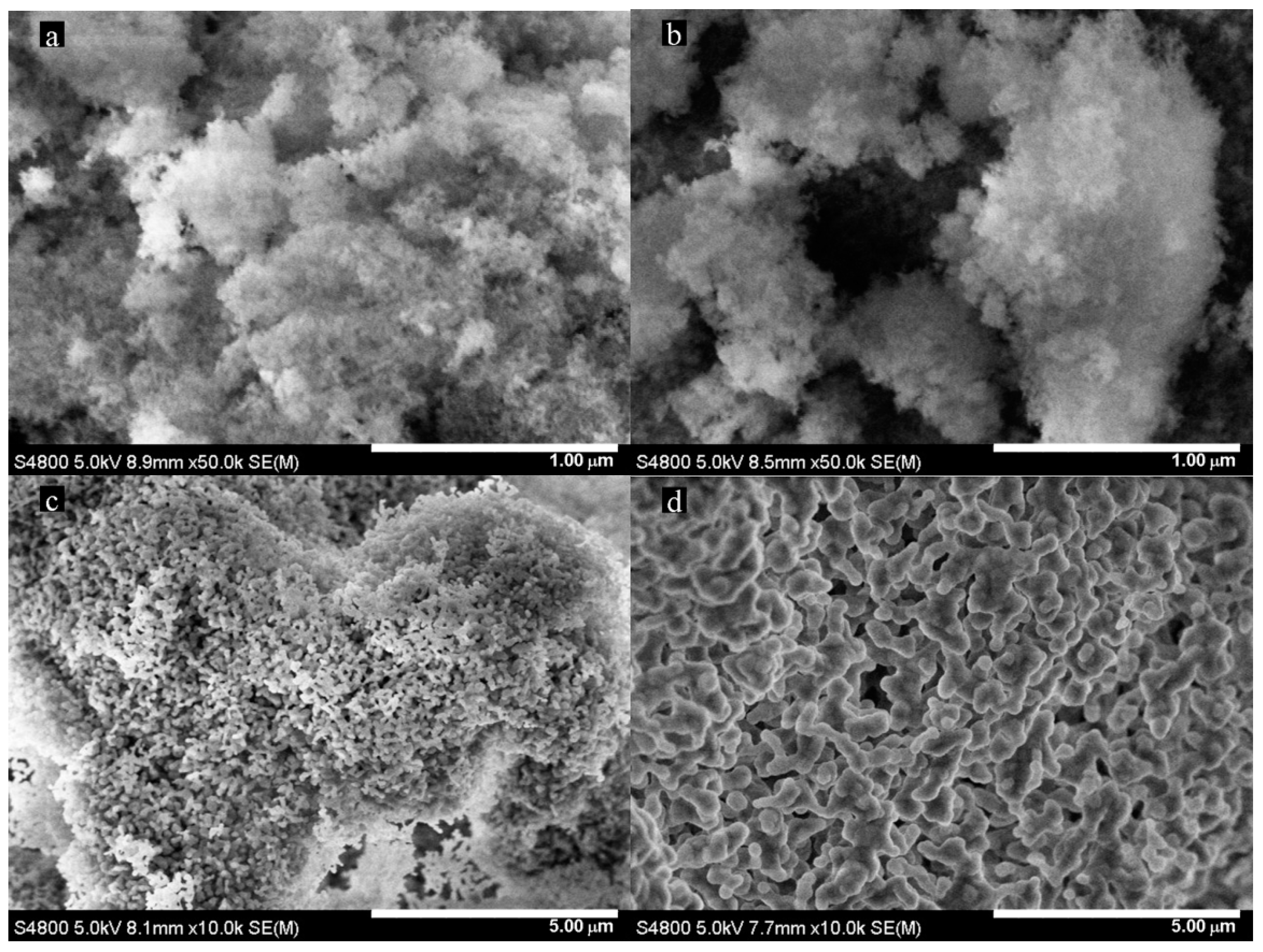
2.2. Scanning Electron Microscopy (SEM) Analysis
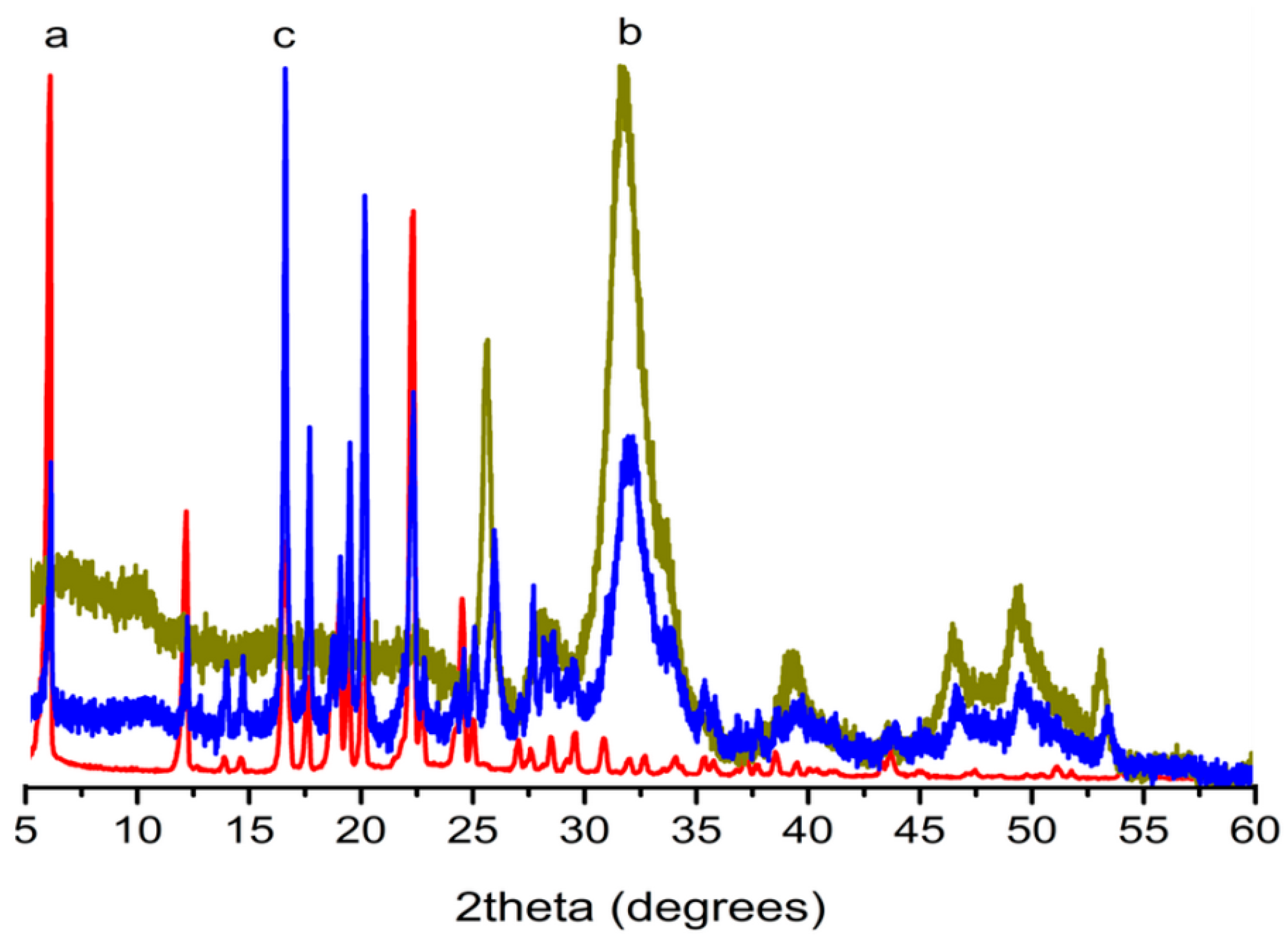
2.3. Functional Group Analysis
2.4. Brunner-Emmet-Teller (BET) Results
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | ACS a (nm) |
|---|---|---|---|---|
| Na2HPO4 | 205.0 | 0.71 | 12.4 | 9 |
| NaH2PO4 | 217.8 | 0.96 | 15.2 | 8 |
| (NH4)3PO4 | 226.9 | 0.85 | 13.0 | 7 |
| H3PO4(HAp284PA) | 284.1 | 1.4 | 17.2 | 7 |
| Calcined at 700 °C | 165.1 | 0.83 | 18.7 | 12 |
| Calcined at 950 °C | 57.1 | 0.47 | 20.2 | 46 |
2.5. Effect of Needle Size
| Needle Size (mm) | ND a | Lowest pH b | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | ACS (nm) |
|---|---|---|---|---|---|---|
| 0.45 × 16 | na c | 9.79 | 154.1 | 0.76 | 19.5 | 7 |
| 0.6 × 25 | na | 9.83 | 162.1 | 0.78 | 18.0 | 8 |
| 0.7 × 32 | 180 | 9.92 | 166.1 | 0.88 | 19.4 | 7 |
| 0.7 × 80 | 150 | 9.92 | 166.8 | 0.93 | 20.0 | 7 |
| 0.8 × 32 | 139 | 9.93 | 169.3 | 0.75 | 16.2 | 8 |
| 0.9 × 38 | 120 | 9.94 | 170.8 | 0.78 | 16.8 | 9 |
| 1.2 × 38 | 98 | 10.00 | 284.1 | 1.39 | 17.2 | 7 |
| 20 × 125 | 46 | 9.97 | 189.4 | 0.61 | 12.1 | 8 |
| 41 × 113 | 44 | 9.95 | 170.9 | 0.83 | 17.9 | 7 |
2.6. Thermal Stability
2.7. Loading Composition
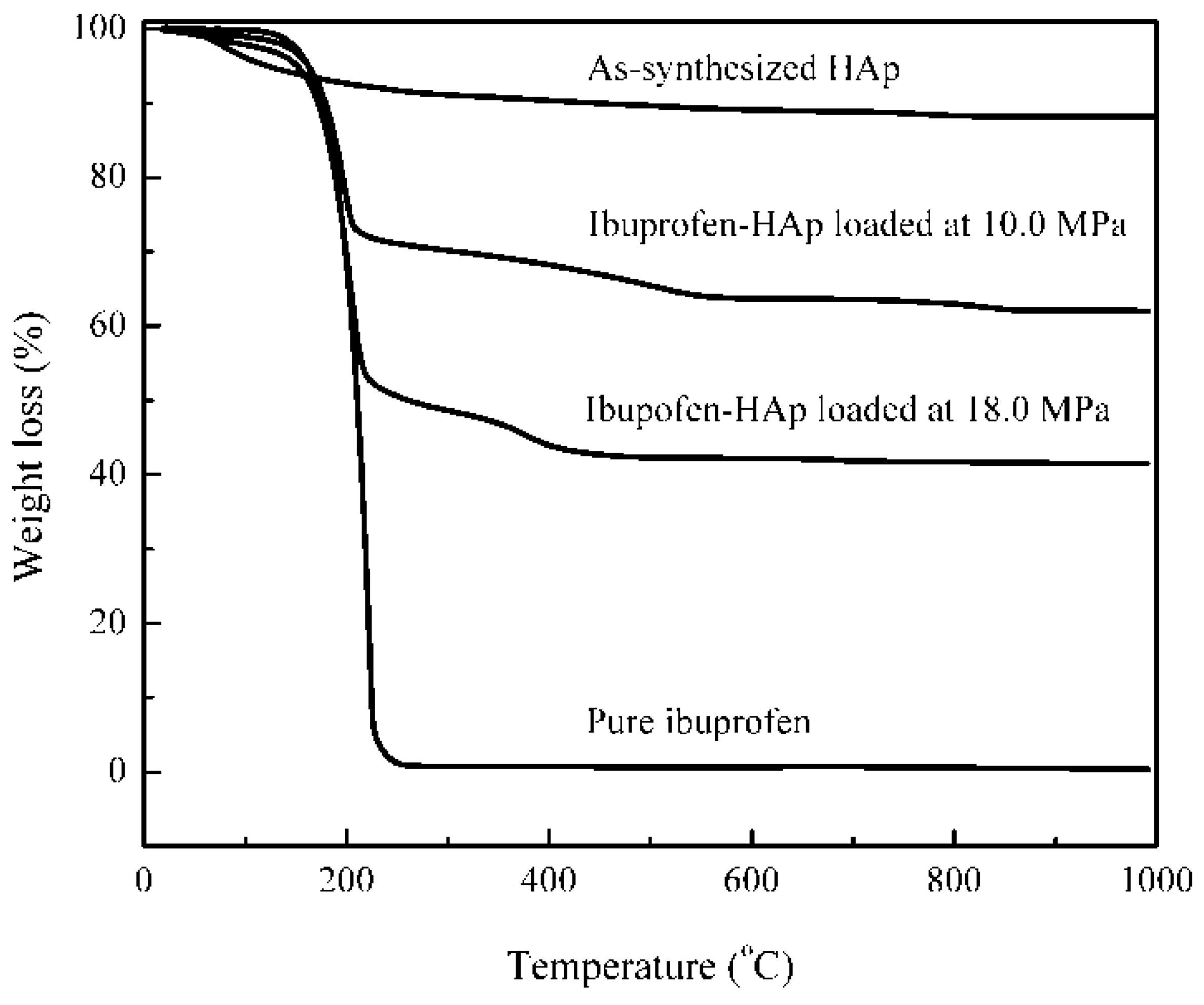
2.8. Dissolution Test
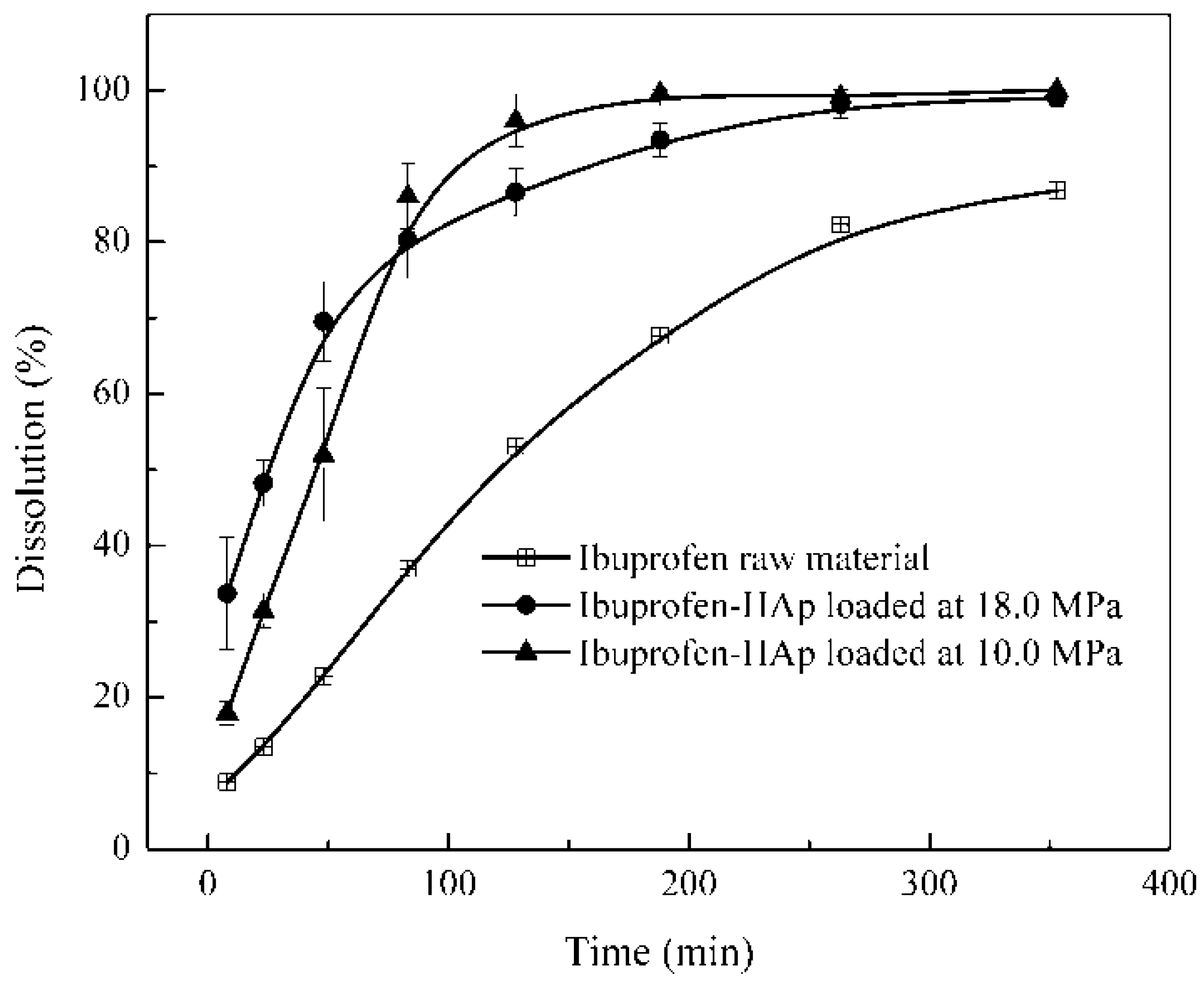
3. Experimental Section
3.1. Materials
3.2. Synthesis of HApNP

3.3. Characterization of HApNP
3.4. Loading of Ibuprofen
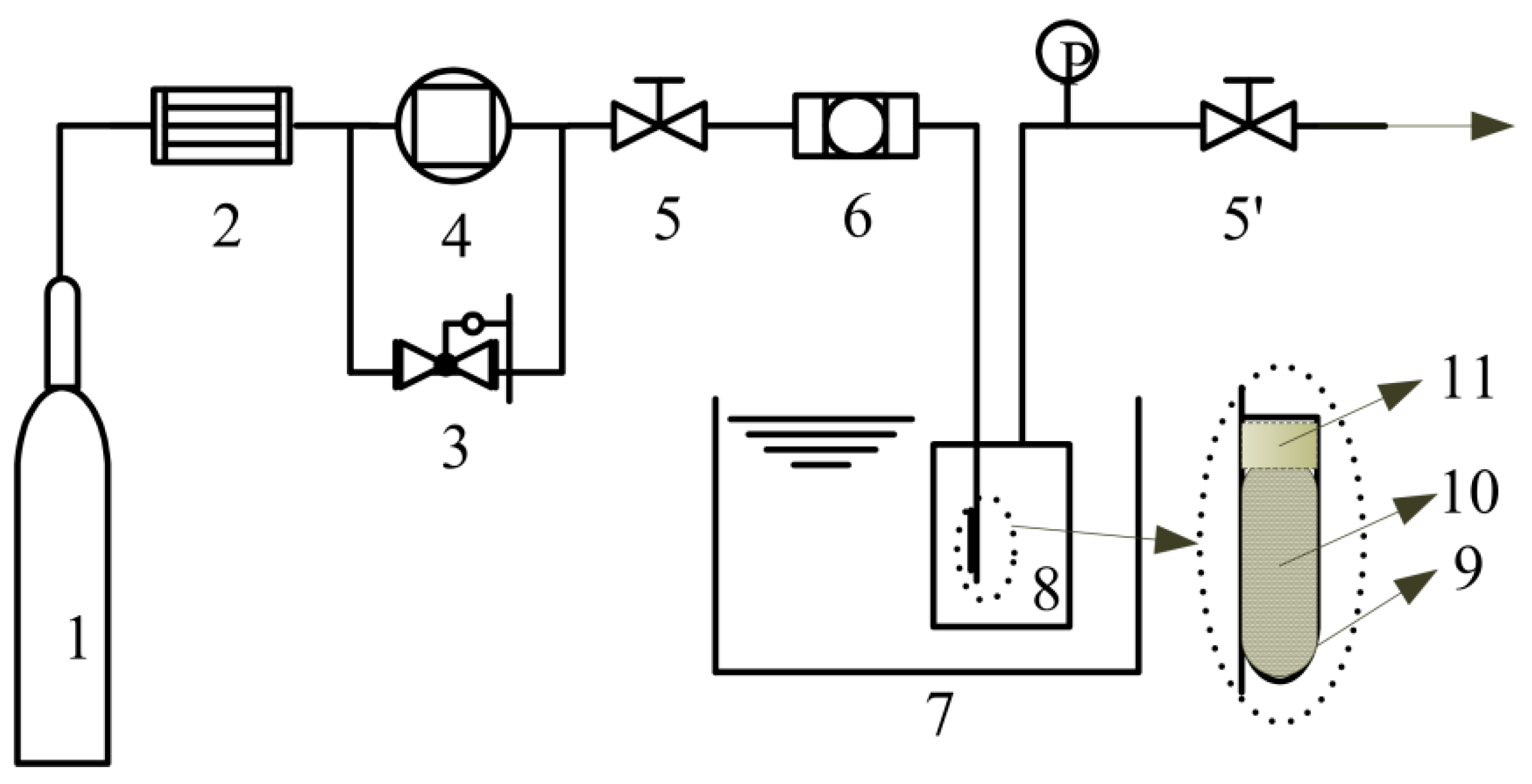
3.5. Composition Analysis
3.6. Dissolution Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Hui, P.; Meena, S.; Singh, G.; Agarawal, R.; Prakash, S. Synthesis of hydroxyapatite bio-ceramic powder by hydrothermal method. J. Miner. Mater. Charact. Eng. 2010, 9, 683. [Google Scholar]
- Feng, Y.; Gong, J.-L.; Zeng, G.-M.; Niu, Q.-Y.; Zhang, H.-Y.; Niu, C.-G.; Deng, J.-H.; Yan, M. Adsorption of Cd(II) and Zn(II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J. 2010, 162, 487–494. [Google Scholar] [CrossRef]
- Ye, F.; Guo, H.; Zhang, H.; He, X. Polymeric micelle-templated synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system. Acta Biomater. 2010, 6, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Weng, W.; Han, G.; Du, P.; Shen, G.; Yang, J.; Ferreira, J.M. The effect of triethanolamine on the formation of sol–gel derived fluoroapatite/hydroxyapatite solid solution. Mater. Chem. Phys. 2003, 78, 767–771. [Google Scholar] [CrossRef]
- Ozyegin, L.S.; Oktar, F.N.; Goller, G.; Kayali, E.S.; Yazici, T. Plasma-sprayed bovine hydroxyapatite coatings. Mater. Lett. 2004, 58, 2605–2609. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Crooks, J.; Khoali, L.; Roden, L. The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials 2009, 30, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Balázsi, C.; Wéber, F.; Kövér, Z.; Horváth, E.; Németh, C. Preparation of calcium–phosphate bioceramics from natural resources. J. Eur. Ceram. Soc. 2007, 27, 1601–1606. [Google Scholar] [CrossRef]
- Rovenský, J.; Stancikova, M.; Masaryk, P.; Svík, K.; Istok, R. Eggshell calcium in the prevention and treatment of osteoporosis. Int. J. Clin. Pharmacol. Res. 2002, 23, 83–92. [Google Scholar]
- Woolsey, M.; Beckman, C.; Zhang, J. Peoples Republic of China poultry and products. In USDA Global Agricultural Information Network, Annual Report 2011, CH 11043; USDA: Washington, DC, USA, 2011. [Google Scholar]
- Stadelman, J.W. Eggs and egg products. In Encyclopedia of Food Science and Technology; Francis, F.J., Ed.; John Wiley and Sons: New York, NY, USA, 2000; pp. 593–599. [Google Scholar]
- Wu, S.C.; Tsou, H.K.; Hsu, H.C.; Hsu, S.K.; Liou, S.P.; Ho, W.F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Ibrahim, A.-R.; Wei, W.; Zhang, D.; Wang, H.; Li, J. Conversion of waste eggshells to mesoporous hydroxyapatite nanoparticles with high surface area. Mater. Lett. 2013, 110, 195–197. [Google Scholar] [CrossRef]
- Ibrahim, A.-R.; Zhou, Y.; Li, X.; Chen, L.; Hong, Y.; Su, Y.; Wang, H.; Li, J. Synthesis of rod-like hydroxyapatite with high surface area and pore volume from eggshells for effective adsorption of aqueous Pb(II). Mater. Res. Bull. 2015, 62, 132–141. [Google Scholar] [CrossRef]
- Wu, Y.; Bose, S. Nanocrystalline hydroxyapatite: Micelle templated synthesis and characterization. Langmuir 2005, 21, 3232–3234. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Imura, M.; Nemoto, Y.; Cheng, C.-H.; Yamauchi, Y. Block-copolymer-assisted synthesis of hydroxyapatite nanoparticles with high surface area and uniform size. Sci. Technol. Adv. Mater. 2011, 12, 045005. [Google Scholar] [CrossRef]
- Bose, S.; Saha, S.K. Synthesis and characterization of hydroxyapatite nanopowders by emulsion technique. Chem. Mater. 2003, 15, 4464–4469. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.H.; Liang, Y.H.; Lin, F.H.; Wu, K.C.W. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. B 2013, 1, 2447–2450. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, S.; Zhu, L.; Tanyi, A.R.; Lan, H.; Hong, Y.; Su, Y.; Wang, H.; Li, J. Adsorption of 2-phenylethyl alcohol on silica aerogel from saturated solution in supercritical CO2. J. Supercrit. Fluids 2013, 79, 41–45. [Google Scholar] [CrossRef]
- Aghaei, H.; Nourbakhsh, A.A.; Karbasi, S.; JavadKalbasi, R.; Rafienia, M.; Nourbakhsh, N.; Bonakdar, S.; Mackenzie, K.J. Investigation on bioactivity and cytotoxicity of mesoporous nano-composite mcm-48/hydroxyapatite for ibuprofen drug delivery. Ceram. Int. 2014, 40, 7355–7362. [Google Scholar] [CrossRef]
- Ni, M.; Xu, Q.-Q.; Yin, J.-Z. Preparation of controlled release nanodrug ibuprofen supported on mesoporous silica using supercritical carbon dioxide. J. Mater. Res. 2012, 27, 2902–2910. [Google Scholar] [CrossRef]
- Hamadouche, M.; Sedel, L. Ceramics in orthopaedics. J. Bone Jt. Surg. Br. 2000, 82, 1095–1099. [Google Scholar] [CrossRef]
- Lagier, R.; Baud, C.-A. Magnesium whitlockite, a calcium phosphate crystal of special interest in pathology. Pathol. Res. Pract. 2003, 199, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Pankaew, P.; Hoonnivath, E.; Limsuwan, P.; Naemchanth, K. Temperature effect on calcium phosphate synthesized from chicken eggshells and ammonium phosphate. J. Appl. Sci. 2010, 10, 3337–3342. [Google Scholar] [CrossRef]
- Kong, L.; Ma, J.; Boey, F. Nanosized hydroxyapatite powders derived from coprecipitation process. J. Mater. Sci. 2002, 37, 1131–1134. [Google Scholar] [CrossRef]
- Shum, H.C.; Bandyopadhyay, A.; Bose, S.; Weitz, D.A. Double emulsion droplets as microreactors for synthesis of mesoporous hydroxyapatite. Chem. Mater. 2009, 21, 5548–5555. [Google Scholar] [CrossRef]
- Hoffmann, C.; Zollfrank, C.; Ziegler, G. Enzyme-catalyzed synthesis of calcium phosphates. J. Mater. Sci. Mater. Med. 2008, 19, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T.; Hsien, K.-J.; Hsu, H.-C.; Lin, C.-M.; Lin, K.-Y.; Chiu, C.-H. Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresour. Technol. 2008, 99, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Gergely, G.; Wéber, F.; Lukács, I.; Tóth, A.L.; Horváth, Z.E.; Mihály, J.; Balázsi, C. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.; Pakan, I.; Hofstede, G.; Muskiet, F.; van der Veer, E.; de Vries, P. Mineral, amino acid, and hormonal composition of chicken eggshell powder and the evaluation of its use in human nutrition. Poult. Sci. 2000, 79, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Toro, P.; Quijada, R.; Yazdani-Pedram, M.; Arias, J.L. Eggshell, a new bio-filler for polypropylene composites. Mater. Lett. 2007, 61, 4347–4350. [Google Scholar] [CrossRef]
- Yoo, S.; Hsieh, J.S.; Zou, P.; Kokoszka, J. Utilization of calcium carbonate particles from eggshell waste as coating pigments for ink-jet printing paper. Bioresour. Technol. 2009, 100, 6416–6421. [Google Scholar] [CrossRef] [PubMed]
- Raihana, M.; Hamdi, M.; Ramesh, S. Novel chemical conversion of eggshell to hydroxyapatite powder. In Proceedings of the 4th Kuala Lumpur International Conference on Biomedical Engineering, Kuala Lumpur, Malaysia, 25–28 June 2008; Springer: Berlin, Germany, 2008; pp. 333–336. [Google Scholar]
- Chen, W.; Hu, X.; Hong, Y.; Su, Y.; Wang, H.; Li, J. Ibuprofen nanoparticles prepared by a pgss™-based method. Powder Technol. 2013, 245, 241–250. [Google Scholar] [CrossRef]
- Krishna, D.S.R.; Siddharthan, A.; Seshadri, S.; Kumar, T.S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.-R.; Li, X.; Zhou, Y.; Huang, Y.; Chen, W.; Wang, H.; Li, J. Synthesis of Spongy-Like Mesoporous Hydroxyapatite from Raw Waste Eggshells for Enhanced Dissolution of Ibuprofen Loaded via Supercritical CO2. Int. J. Mol. Sci. 2015, 16, 7960-7975. https://doi.org/10.3390/ijms16047960
Ibrahim A-R, Li X, Zhou Y, Huang Y, Chen W, Wang H, Li J. Synthesis of Spongy-Like Mesoporous Hydroxyapatite from Raw Waste Eggshells for Enhanced Dissolution of Ibuprofen Loaded via Supercritical CO2. International Journal of Molecular Sciences. 2015; 16(4):7960-7975. https://doi.org/10.3390/ijms16047960
Chicago/Turabian StyleIbrahim, Abdul-Rauf, Xiangyun Li, Yulan Zhou, Yan Huang, Wenwen Chen, Hongtao Wang, and Jun Li. 2015. "Synthesis of Spongy-Like Mesoporous Hydroxyapatite from Raw Waste Eggshells for Enhanced Dissolution of Ibuprofen Loaded via Supercritical CO2" International Journal of Molecular Sciences 16, no. 4: 7960-7975. https://doi.org/10.3390/ijms16047960