Insulin Induces Phosphorylation of Serine Residues of Translationally Controlled Tumor Protein in 293T Cells
Abstract
:1. Introduction
2. Results
2.1. Insulin Induces Phosphorylation of TCTP at Serine Residues and Promotes Its Translocalization to the Cytosol
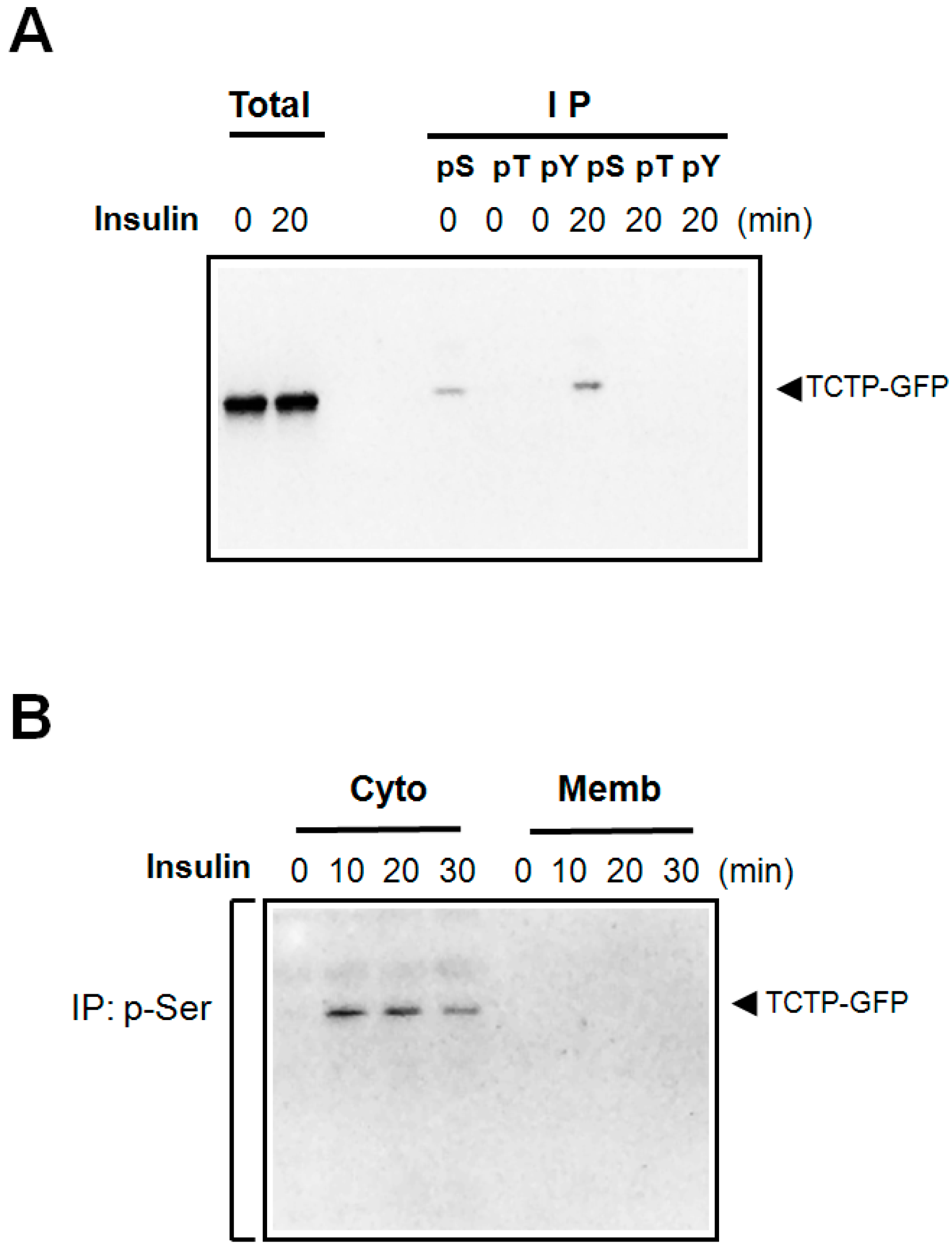
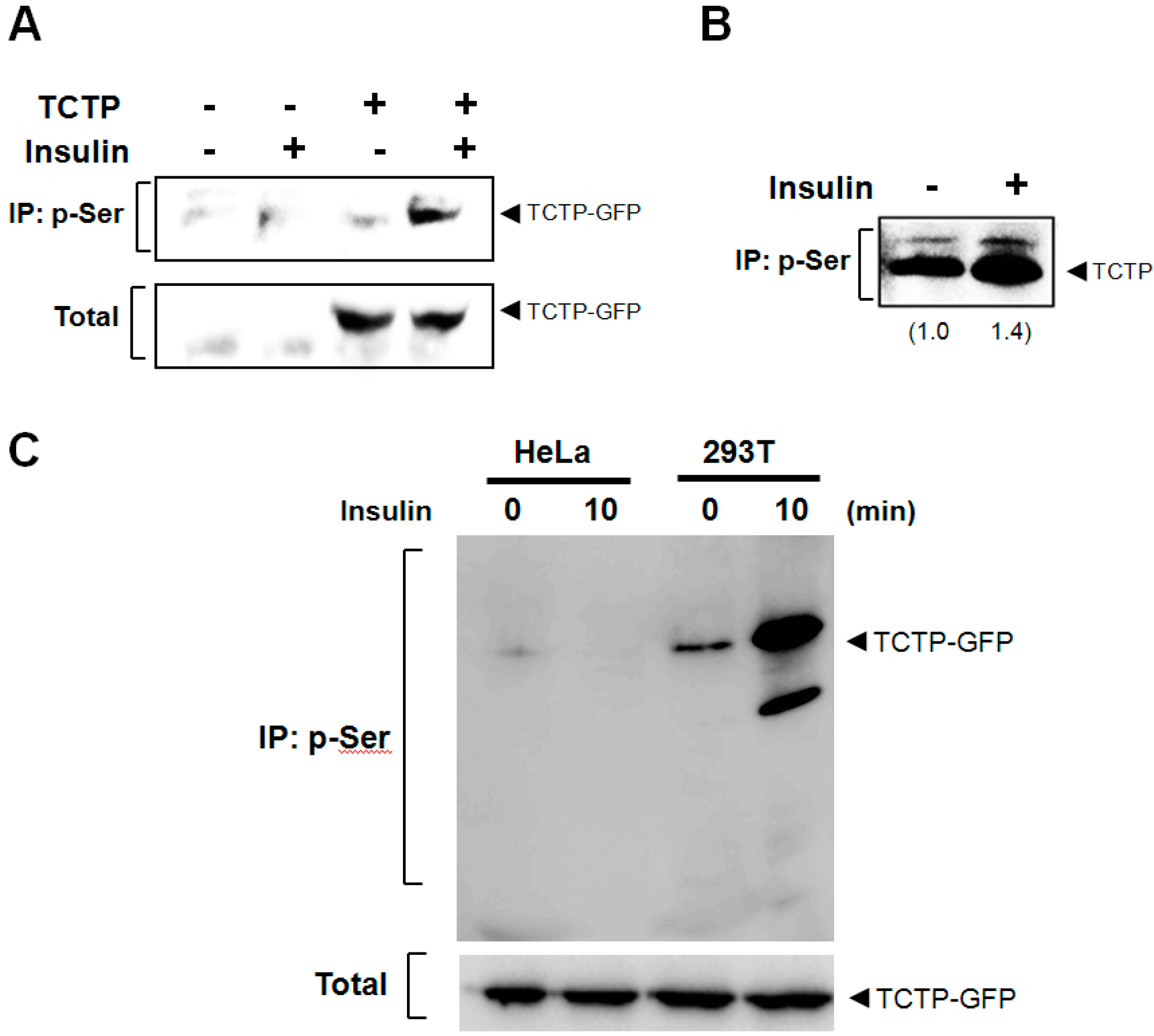
2.2. Insulin Induces the Phosphorylation of both Endogenous and Exogenous TCTP in 293T Cells
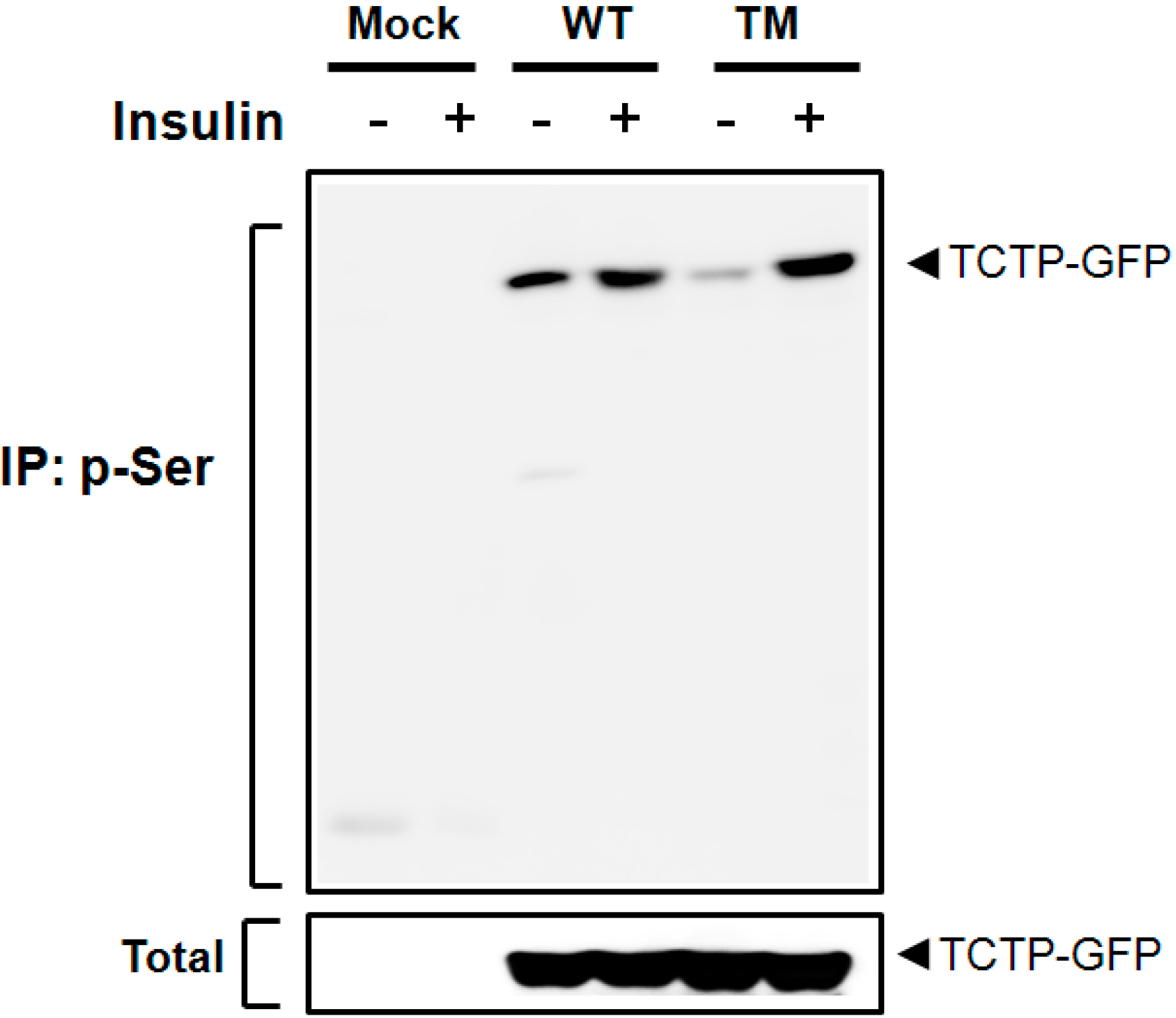
2.3. Insulin Phosphorylates TCTP at Ser-9 and -15 Residues

3. Discussion
4. Experimental Section
4.1. Cell Culture and Treatment
4.2. Generation of Ser to Ala Mutants of TCTP
4.3. Western Blotting
4.4. Preparation of Membrane and Cytosol Fractions
4.5. Detection of Phosphorylated Proteins in Cells
4.6. Prediction of Phosphorylation Sites in TCTP
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sweeney, G.; Klip, A. Regulation of the Na+/K+-ATPase by insulin: why and how? Mol. Cell Biochem. 1998, 182, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Galvan, A.Q.; Gastaldelli, A.; Camastra, S.; Sironi, A M.; Toschi, E.; Baldi, S.; Frascerra, S.; Monzani, F.; Antonelli, A.; et al. Insulin: new roles for an ancient hormone. Eur. J. Clin. Investig. 1999, 29, 842–852. [Google Scholar]
- Rosta, K.; Tulassay, E.; Enzsoly, A.; Ronai, K.; Szantho, A.; Pandics, T.; Fekete, A.; Mandl, P.; Ver, A. Insulin induced translocation of Na+/K+-ATPase is decreased in the heart of streptozotocin diabetic rats. Acta Pharmacol. Sin. 2009, 30, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Feraille, E.; Carranza, M.L.; Gonin, S.; Beguin, P.; Pedemonte, C.; Rousselot, M.; Caverzasio, J.; Geering, K.; Martin, P.Y.; Favre, H. Insulin-induced stimulation of Na+,K(+)-ATPase activity in kidney proximal tubule cells depends on phosphorylation of the alpha-subunit at Tyr-10. Mol. Biol. Cell 1999, 10, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Feraille, E.; Rousselot, M.; Rajerison, R.; Favre, H. Effect of insulin on Na+,K(+)-ATPase in rat collecting duct. J. Physiol. 1995, 488, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.S.; Marette, A.; Mitsumoto, Y.; Ramlal, T.; Blostein, R.; Klip, A. Insulin induces translocation of the alpha 2 and beta 1 subunits of the Na+/K(+)-ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. J. Biol. Chem. 1992, 267, 5040–5043. [Google Scholar] [PubMed]
- Longo, N.; Scaglia, F.; Wang, Y. Insulin increases the turnover rate of Na+-K+-ATPase in human fibroblasts. Am. J. Physiol. Cell Physiol. 2001, 280, C912–C919. [Google Scholar] [PubMed]
- Lytton, J. Insulin affects the sodium affinity of the rat adipocyte (Na+,K+)-ATPase. J. Biol. Chem. 1985, 260, 10075–10080. [Google Scholar] [PubMed]
- Sargeant, R.J.; Liu, Z.; Klip, A. Action of insulin on Na(+)-K(+)-ATPase and the Na(+)-K(+)-2Cl− cotransporter in 3T3-L1 adipocytes. Am. J. Physiol. 1995, 269, C217–C225. [Google Scholar] [PubMed]
- Sweeney, G.; Niu, W.; Canfield, V.A.; Levenson, R.; Klip, A. Insulin increases plasma membrane content and reduces phosphorylation of Na(+)-K(+) pump alpha(1)-subunit in HEK-293 cells. Am. J. Physiol. Cell Physiol. 2001, 281, C1797–C1803. [Google Scholar] [PubMed]
- McGill, D.L.; Guidotti, G. Insulin stimulates both the alpha 1 and the alpha 2 isoforms of the rat adipocyte (Na+,K+) ATPase. Two mechanisms of stimulation. J. Biol. Chem. 1991, 266, 15824–15831. [Google Scholar]
- Bommer, U.A.; Thiele, B.J. The translationally controlled tumour protein (TCTP). Int. J. Biochem. Cell Biol. 2004, 36, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, M.; Kim, M.J.; Kim, J.; Moon, J.; Lim, J.S.; Lee, K. Translationally controlled tumor protein interacts with the third cytoplasmic domain of Na,K-ATPase alpha subunit and inhibits the pump activity in HeLa cells. J. Biol. Chem. 2004, 279, 49868–49875. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kwon, J.S.; Suh, S.H.; Suh, J.K.; Jung, J.; Lee, S.N.; Kim, Y.H.; Cho, M.C.; Oh, G.T.; Lee, K. Transgenic overexpression of translationally controlled tumor protein induces systemic hypertension via repression of Na+,K+-ATPase. J. Mol. Cell Cardiol. 2008, 44, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Siddle, K. Signalling by insulin and IGF receptors: Supporting acts and new players. J. Mol. Endocrinol. 2011, 47, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Gnad, F.; Ren, S.; Cox, J.; Olsen, J.V.; Macek, B.; Oroshi, M.; Mann, M. PHOSIDA (phosphorylation site database): Management, structural and evolutionary investigation, and prediction of phosphosites. Genome Biol. 2007, 8, R250. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Yarm, F.R. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol. Cell. Biol. 2002, 22, 6209–6221. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.E.; Boustead, J.N.; Chapman, S.C.; Svitek, C.A.; Oeser, J.K.; Robey, R.B.; O'Brien, R.M. Insulin-mediated activation of activator protein-1 through the mitogen-activated protein kinase pathway stimulates collagenase-1 gene transcription in the MES 13 mesangial cell line. J. Mol. Endocrinol. 2004, 33, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; de Meyts, P. Molecular mechanisms of differential intracellular signaling from the insulin receptor. Vitam. Horm. 2009, 80, 51–75. [Google Scholar] [PubMed]
- Bommer, U.A. Cellular function and regulation of the translationally controlled tumor protein TCTP. Open Allergy J. 2012, 5, 19–32. [Google Scholar] [CrossRef]
- Cheng, H.C.; Qi, R.Z.; Paudel, H.; Zhu, H.J. Regulation and function of protein kinases and phosphatases. Enzym. Res. 2011, 2011, 794089. [Google Scholar] [CrossRef]
- Kim, M.; Min, H.J.; Won, H.Y.; Park, H.; Lee, J.C.; Park, H.W.; Chung, J.; Hwang, E.S.; Lee, K. Dimerization of translationally controlled tumor protein is essential for its cytokine-like activity. PLoS ONE 2009, 4, e6464. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, H.Y.; Maeng, J.; Kim, M.; Shin, D.H.; Lee, K. Interaction of translationally controlled tumor protein with Apaf-1 is involved in the development of chemoresistance in HeLa cells. BMC Cancer 2014, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- NetPhos 2.0 Server. Available online: http://www.cbs.dtu.dk/services/NetPhos/ (accessed on 31 March 2015).
- PHOSIDA database. Available online: http://www.phosida.com/ (accessed on 31 March 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeng, J.; Kim, M.; Lee, H.; Lee, K. Insulin Induces Phosphorylation of Serine Residues of Translationally Controlled Tumor Protein in 293T Cells. Int. J. Mol. Sci. 2015, 16, 7565-7576. https://doi.org/10.3390/ijms16047565
Maeng J, Kim M, Lee H, Lee K. Insulin Induces Phosphorylation of Serine Residues of Translationally Controlled Tumor Protein in 293T Cells. International Journal of Molecular Sciences. 2015; 16(4):7565-7576. https://doi.org/10.3390/ijms16047565
Chicago/Turabian StyleMaeng, Jeehye, Miyoung Kim, Hyukjin Lee, and Kyunglim Lee. 2015. "Insulin Induces Phosphorylation of Serine Residues of Translationally Controlled Tumor Protein in 293T Cells" International Journal of Molecular Sciences 16, no. 4: 7565-7576. https://doi.org/10.3390/ijms16047565




