Blunt Snout Bream (Megalobrama amblycephala) MyD88 and TRAF6: Characterisation, Comparative Homology Modelling and Expression
Abstract
:1. Introduction
2. Results
2.1. Sequence Analysis
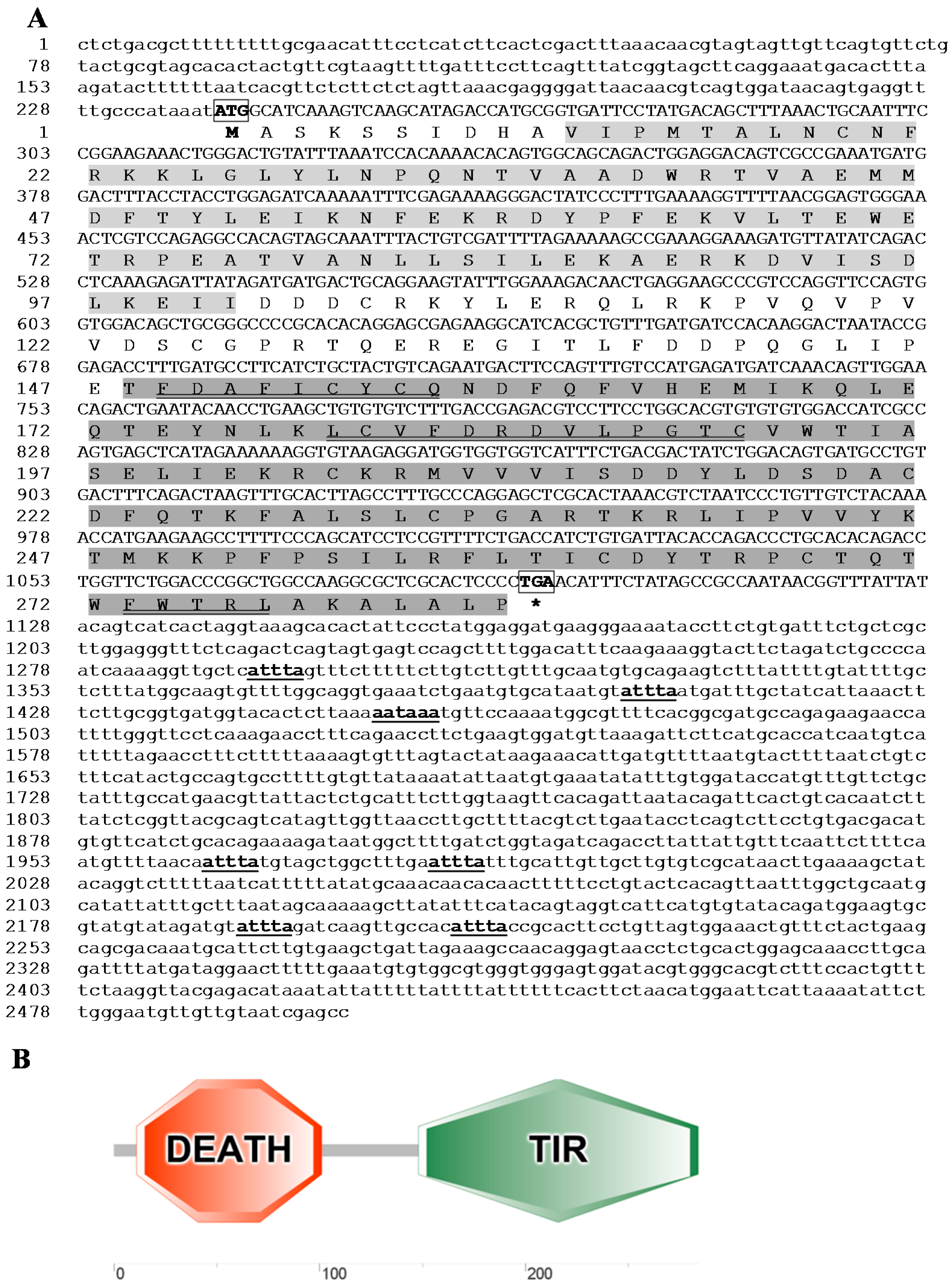



2.2. Genomic Organisation Analysis
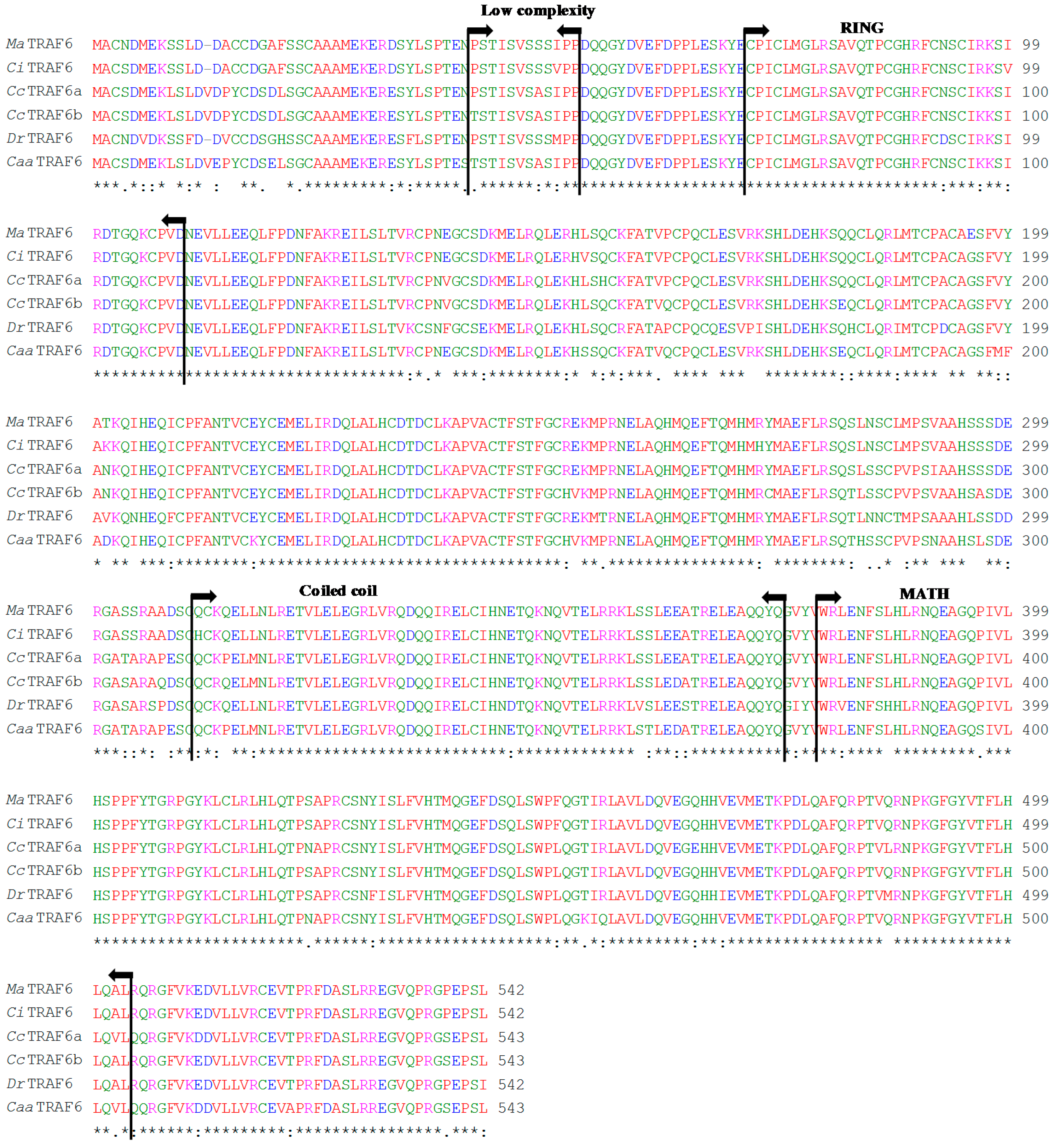
2.3. Phylogenetic Analysis


2.4. Physicochemical and Functional Characterisation
| Index | MaMyD88 | MaTRAF6 |
|---|---|---|
| No. of aa | 284 | 542 |
| Mol. Wt. | 33,027.3 | 61,798.3 |
| pI | 5.89 | 5.91 |
| −R | 41 | 71 |
| +R | 39 | 60 |
| EC * | 41,660/40,910 | 30,880/28,880 |
| II | 33.5 | 58.6 |
| AI | 87.18 | 72.14 |
| GRAVY | −0.241 | −0.491 |
2.5. Protein Structure Prediction and Model Validation
| Element | MaMyD88 | MaTRAF6 |
|---|---|---|
| Alpha helix | 44.37 | 40.96 |
| 310 helix | 0 | 0 |
| Pi helix | 0 | 0 |
| Beta bridge | 0 | 0 |
| Extended strand | 20.07 | 16.24 |
| Beta turn | 7.39 | 6.83 |
| Bend region | 0 | 0 |
| Random coil | 28.17 | 35.98 |
| Ambiguous states | 0 | 0 |
| Other states | 0 | 0 |
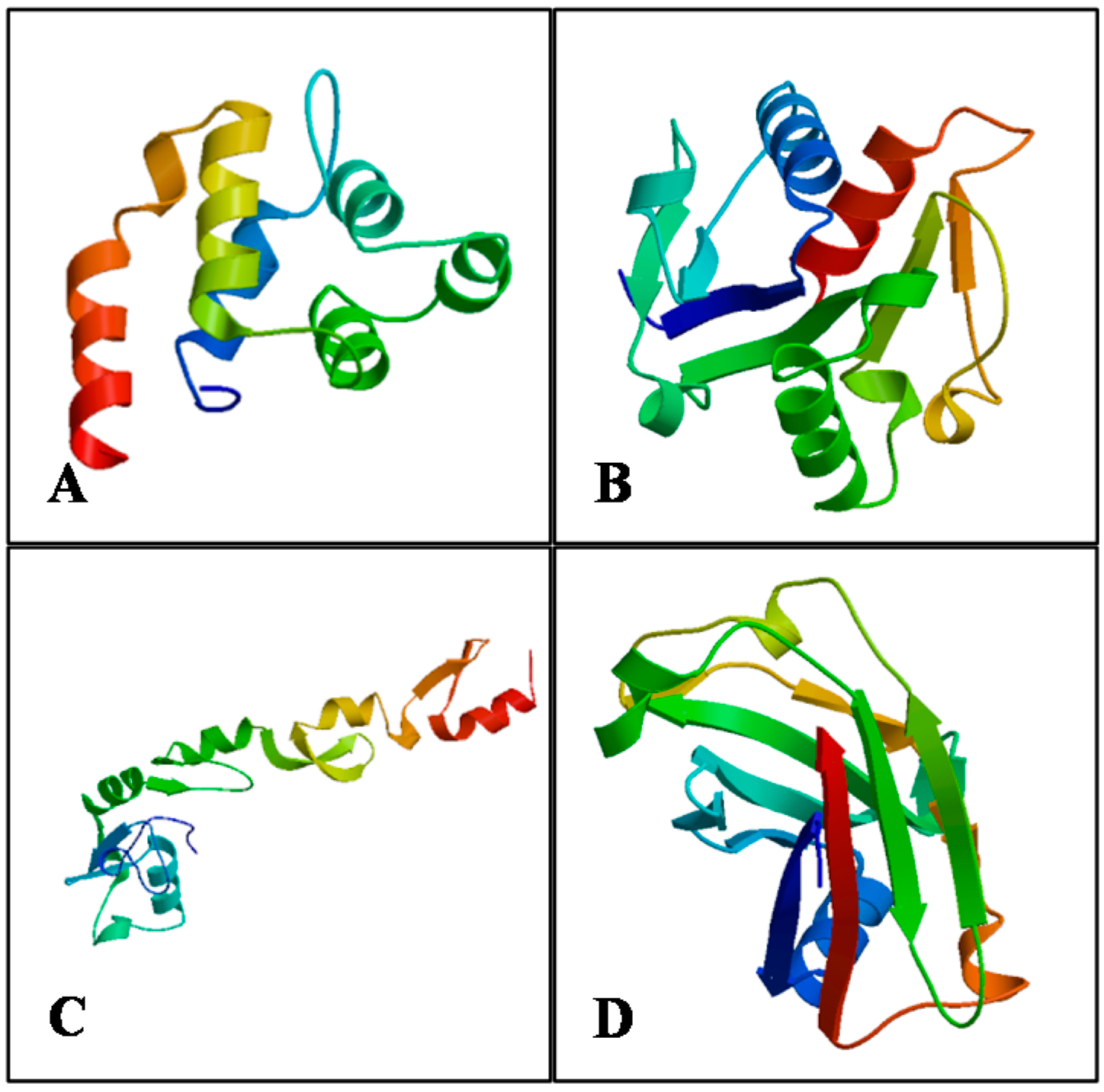
| Validation Index | MaMyD88 | MaTRAF6 | ||
|---|---|---|---|---|
| DEATH | TIR | RING | MATH | |
| Ramachandran plot | ||||
| Residues in most favoured regions | 71.4% | 92.2% | 87.8% | 90.8% |
| Residues in additional allowed regions | 25.3% | 7.8% | 11.5% | 7.7% |
| Residues in generously allowed regions | 2.2% | 0% | 0.7% | 0.8% |
| Residues in disallowed regions | 1.1% | 0% | 0% | 0.8% |
| Overall G-factor | −0.15 | 0.18 | 0.1 | 0.06 |
| ProQ | ||||
| Lgscore | 5.047 | 6.366 | 2.436 | 5.561 |
| MaxSub | 0.645 | 0.844 | 0.254 | 0.529 |
| ProSA | ||||
| Z-Score | −5.24 | −7.63 | −5.32 | −5.62 |
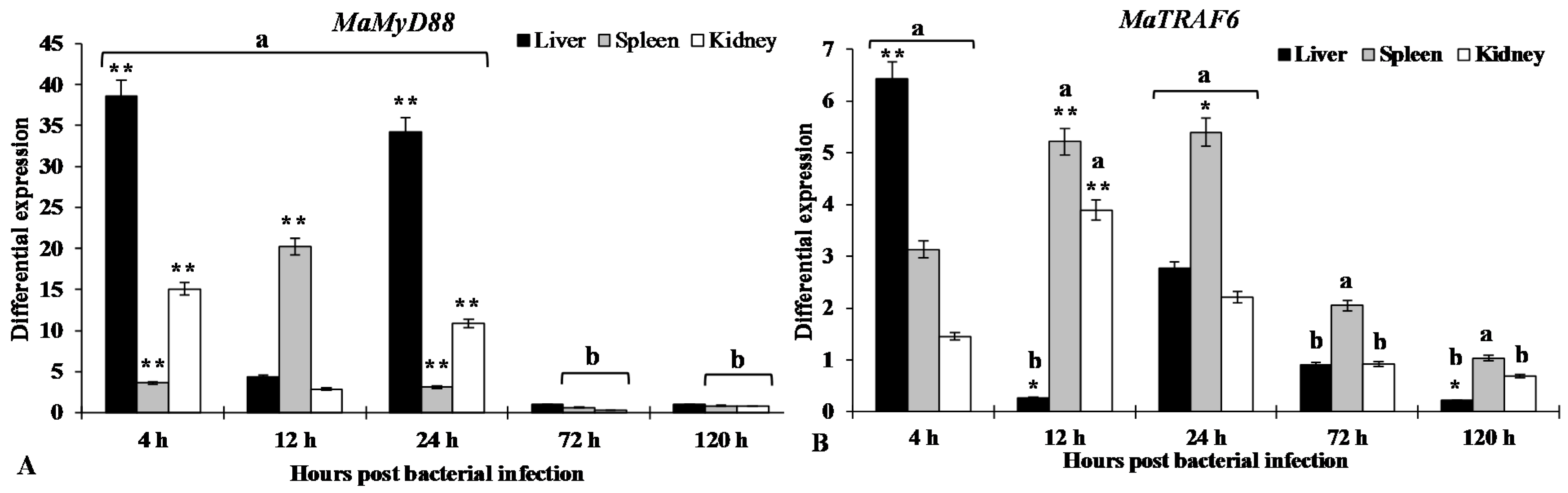
2.6. Expression of MaMyD88 and MaTRAF6 Transcripts after A. hydrophila Infection
3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. Fish and Challenge Experiment
4.3. Total RNA Preparation and cDNA Synthesis
4.4. Cloning of Full-Length cDNAs and Bioinformatics Analyses
4.5. Protein Physicochemical and Functional Characterisation
4.6. Protein Structure Prediction
4.7. Quantitative Real-Time PCR (RT-qPCR) and Statistics
| Gene | Primer Sequence (5'–3') |
|---|---|
| Primers used for RACE-PCR | |
| MaMyD88 3'-RACE | GAGTCTGAGAAACCCTCCAAGCGA |
| MaMyD88 5'-RACE | AGGTGTAAGAGGATGGTGGTGGTC |
| MaTRAF6 3'-RACE | CAGTGACGTTGCGCTGATGTTTG |
| MaTRAF6 5'-RACE | GTCCCTGATGGACTTCCTGATGC |
| Primers used for RT-qPCR | |
| MaMyD88-F | GACAACAGGGATTAGACG |
| MaMyD88-R | TGGAACAGACTGAATACAAC |
| MaTRAF6-F | CGAGCGAAGACCCATTAGAC |
| MaTRAF6-R | ATCTGAGCCCGACAGAGAAC |
| 18S rRNA-F | CGGAGGTTCGAAGACGATCA |
| 18S rRNA-R | GGGTCGGCATCGTTTACG |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunología 2003, 22, 277–286. [Google Scholar]
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish Shellfish Immun. 2007, 23, 1127–1151. [Google Scholar] [CrossRef]
- Basu, M.; Swai, B.; Maiti, N.K.; Routray, P.; Samanta, M. Inductive expression of toll-like receptor 5 (TLR5) and associated downstream signaling molecules following ligand exposure and bacterial infection in the Indian major carp, mrigal (Cirrhinus mrigala). Fish Shellfish Immun. 2012, 32, 121–131. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, J.C. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, J.; Selvarajoo, K.; Tsuchiya, M.; Lee, G.; Choi, S. Toll-like receptor signal transduction. Exp. Mol. Med. 2007, 39, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Lord, K.A.; Hoffman-Liebermann, B.; Liebermann, D.A. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene 1990, 5, 1095–1097. [Google Scholar] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-κB by toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Muhlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wesche, H.; Henzel, W.J.; Shillinglaw, W.; Li, S.; Cao, Z. MyD88: An adapter that recruits IRAK to the IL-1 receptor complex. Immunity 1997, 7, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Deepika, A.; Sreedharan, K.; Paria, A.; Makesh, M.; Rajendran, K.V. Toll-pathway in tiger shrimp (Penaeus monodon) responds to white spot syndrome virus infection: Evidence through molecular characterisation and expression profiles of MyD88, TRAF6 and TLR genes. Fish Shellfish Immun. 2014, 41, 441–454. [Google Scholar] [CrossRef]
- Ye, H.; Arron, J.R.; Lamothe, B.; Cirilli, M.; Kobayashi, T.; Shevde, N.K.; Segal, D.; Dzivenu, O.; Vologodskaia, M.; Yim, M.; et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 2002, 418, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Song, L.; Yu, Y.; Xu, W.; Ni, D.; Zhang, Q. Identification and characterization of a myeloid differentiation factor 88 (MyD88) cDNA from Zhikong scallop Chlamys farreri. Fish Shellfish Immun. 2007, 23, 614–623. [Google Scholar] [CrossRef]
- Kongchum, P.; Hallerman, E.M.; Hulata, G.; David, L.; Palti, Y. Molecular cloning, characterization and expression analysis of TLR9, MyD88 and TRAF6 genes in common carp (Cyprinus carpio). Fish Shellfish Immun. 2011, 30, 361–371. [Google Scholar] [CrossRef]
- Tang, D.; Gao, Y.; Wang, R.; Sun, Y.; Xu, T. Characterization, genomic organization, and expression profiles of MyD88, a key adaptor molecule in the TLR signaling pathways in miiuy croaker (Miichthys miiuy). Fish Physiol. Biochem. 2012, 38, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.Z.; Yan, H.; Qiu, W.; Chen, Y.G.; Wang, P.H.; Weng, S.P.; He, J.G. Identification and function of Myeloid Differentiation Factor 88 (MyD88) in Litopenaeus vannamei. PLoS ONE 2012, 7, e47038. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Li, F.; Sun, Z.; Li, S.; Xiang, J. Shrimp MyD88 responsive to bacteria and white spot syndrome virus. Fish Shellfish Immun. 2013, 34, 574–581. [Google Scholar] [CrossRef]
- Phelan, P.E.; Mellon, M.T.; Kim, C.H. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio). Mol. Immunol. 2005, 42, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Li, X.; Xiao, X.X.; Zhao, F.; Luo, X.C.; Dan, X.M.; Li, A.X. Molecular characterization and functional analysis of TRAF6 in orange-spotted grouper (Epinephelus coioides). Dev. Comp. Immunol. 2014, 44, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Wan, D.H.; Gu, Z.H.; Deng, X.X.; Weng, S.P.; Yu, X.Q.; He, J.G. Litopenaeus vannamei tumor necrosis factor receptor-associated factor 6 (TRAF6) responds to Vibrio alginolyticus and white spot syndrome virus (WSSV) infection and activates antimicrobial peptide genes. Dev. Comp. Immunol. 2011, 35, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, Y.W.; Pan, H.J.; Wu, S.Q.; Shi, C.B.; Luo, X.C.; Li, A.X. Grass carp (Ctenopharyngodon idella) TRAF6 and TAK1: Molecular cloning and expression analysis after Ichthyophthirius multifiliis infection. Fish Shellfish Immun. 2013, 34, 1514–1523. [Google Scholar] [CrossRef]
- Guruprasad, K.; Reddy, B.V.P.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Prot. Eng. 1990, 4, 155–164. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, H.C.; Wei, J.G.; Huang, Y.H.; Huang, X.H.; Qin, Q.W. Functional genomic studies on an immune- and antiviral-related gene of MyD88 in orange-spotted grouper, Epinephelus coioides. Chin. Sci. Bull. 2012, 57, 3277–3287. [Google Scholar] [CrossRef]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465(7300), 885–890. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.A.; Cirl, C.; Jiang, J.; Chen, K.; Waldhuber, A.; Smith, P.; Römmler, F.; Snyder, N.; Fresquez, T.; Dürr, S.; et al. Molecular mechanisms for the subversion of MyD88 signaling by TcpC from virulent uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2013, 110, 6985–6990. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Lin, S.C.; Lamothe, B.; Lu, M.; Lo, Y.; Hura, G.; Zheng, L.; Rich, R.L.; Campos, A.D.; Myszka, D.G.; et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat. Struct. Mol. Biol. 2009, 16, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tao, X.; Shen, B.; Horng, T.; Medzhitov, R.; Manley, J.L.; Tong, L. Structural basis for signal transduction by the toll/interleukin-1 receptor domains. Nature 2000, 408, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Cristobal, S.; Zemla, A.; Fischer, D.; Rychlewski, L.; Elofsson, A. A study of quality measures for protein threading models. BMC Bioinform. 2001, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.H.; Vincenz, C. The death domain superfamily: A tale of two interfaces? Trends Biochem. Sci. 2001, 26, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Beyaert, R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 2003, 11, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.L.; Schooley, K.; Bonnert, T.P.; Mitcham, J.L.; Qwarnstrom, E.E.; Sims, J.E.; Dower, S.K. Identification of two major sites in the type I Interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways. J. Biol. Chem. 2000, 275, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Sachs, A.B. Messenger RNA degradation in eukaryotes. Cell 1993, 74, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.; Sun, S.; Tang, Y.; Zhang, B.; Diao, L.; Wang, C. The coiled-coil domain of TRAF6 is essential for its auto-ubiquitination. Biochem. Biophys. Res. Commun. 2004, 324, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Arora, N.; Murty, U.S.N. Analyzing a potential drug target N-Myristoyltransferase of Plasmodium falciparum through in silico approaches. J. Glob. Infect. Dis. 2012, 4, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Singh, B.R. Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Hogg, P.J. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 2003, 8, 210–214. [Google Scholar] [CrossRef]
- Yao, C.L.; Kong, P.; Wang, Z.Y.; Ji, P.F.; Liu, X.D.; Cai, M.Y.; Han, X.Z. Molecular cloning and expression of MyD88 in large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immun. 2009, 26, 249–255. [Google Scholar] [CrossRef]
- Blast assembled genome. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 November 2014).
- ORF Finder (Open Reading Frame Finder). Available online: http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi (accessed on 20 November 2014).
- SignalP 4.1 Server. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 20 November 2014).
- The GENESCAN Web Server at MIT. Available online: http://genes.mit.edu/GENSCAN.html (accessed on 20 November 2014).
- SMART (Simple Modular Architecture Research Tool). Available online: http://smart.embl-heidelberg.de/ (accessed on 20 November 2014).
- ClustalW2. Available online: http://www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 20 November 2014).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Expasy’s ProtParam Tool. Available online: http://web.expasy.org/protparam/ (accessed on 20 November 2014).
- Hirokawa, T.; Boon-Chieng, S.; Mitaku, S. SOSUI: Classification and secondary structure prediction system for membrane proteins. Bioinformatics 1998, 14, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Softberry. Available online: http://linux1.softberry.com/ (accessed on 20 November 2014).
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [PubMed]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 13, 3381–3385. [Google Scholar] [CrossRef]
- Fiser, A. Template-based protein structure modeling. Methods Mol. Biol. 2010, 673, 73–94. [Google Scholar] [PubMed]
- Ramachandran, G.N.; Ramakrishnan, C.; Sasisekhran, V. Stereochemistry of polypeptide chain configuarations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Rullmannn, J.A.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Colovos, V.C.; Yeates, T.O. Verification of protein structures: Patterns of non-bonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, J.; Wen, J.F.; Liu, H.; Wang, W.M.; Gao, Z.X. Molecular cloning and expression analysis of major histocompatibility complex class I, IIA and IIB genes of blunt snout bream (Megalobrama amblycephala). Dev. Comp. Immunol. 2014, 42, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.T.; Liu, H.; Jakovlić, I.; Wang, W.-M. Blunt Snout Bream (Megalobrama amblycephala) MyD88 and TRAF6: Characterisation, Comparative Homology Modelling and Expression. Int. J. Mol. Sci. 2015, 16, 7077-7097. https://doi.org/10.3390/ijms16047077
Tran NT, Liu H, Jakovlić I, Wang W-M. Blunt Snout Bream (Megalobrama amblycephala) MyD88 and TRAF6: Characterisation, Comparative Homology Modelling and Expression. International Journal of Molecular Sciences. 2015; 16(4):7077-7097. https://doi.org/10.3390/ijms16047077
Chicago/Turabian StyleTran, Ngoc Tuan, Han Liu, Ivan Jakovlić, and Wei-Min Wang. 2015. "Blunt Snout Bream (Megalobrama amblycephala) MyD88 and TRAF6: Characterisation, Comparative Homology Modelling and Expression" International Journal of Molecular Sciences 16, no. 4: 7077-7097. https://doi.org/10.3390/ijms16047077





