Nano SiO2 and MgO Improve the Properties of Porous β-TCP Scaffolds via Advanced Manufacturing Technology
Abstract
:1. Introduction
2. Results and Discussion
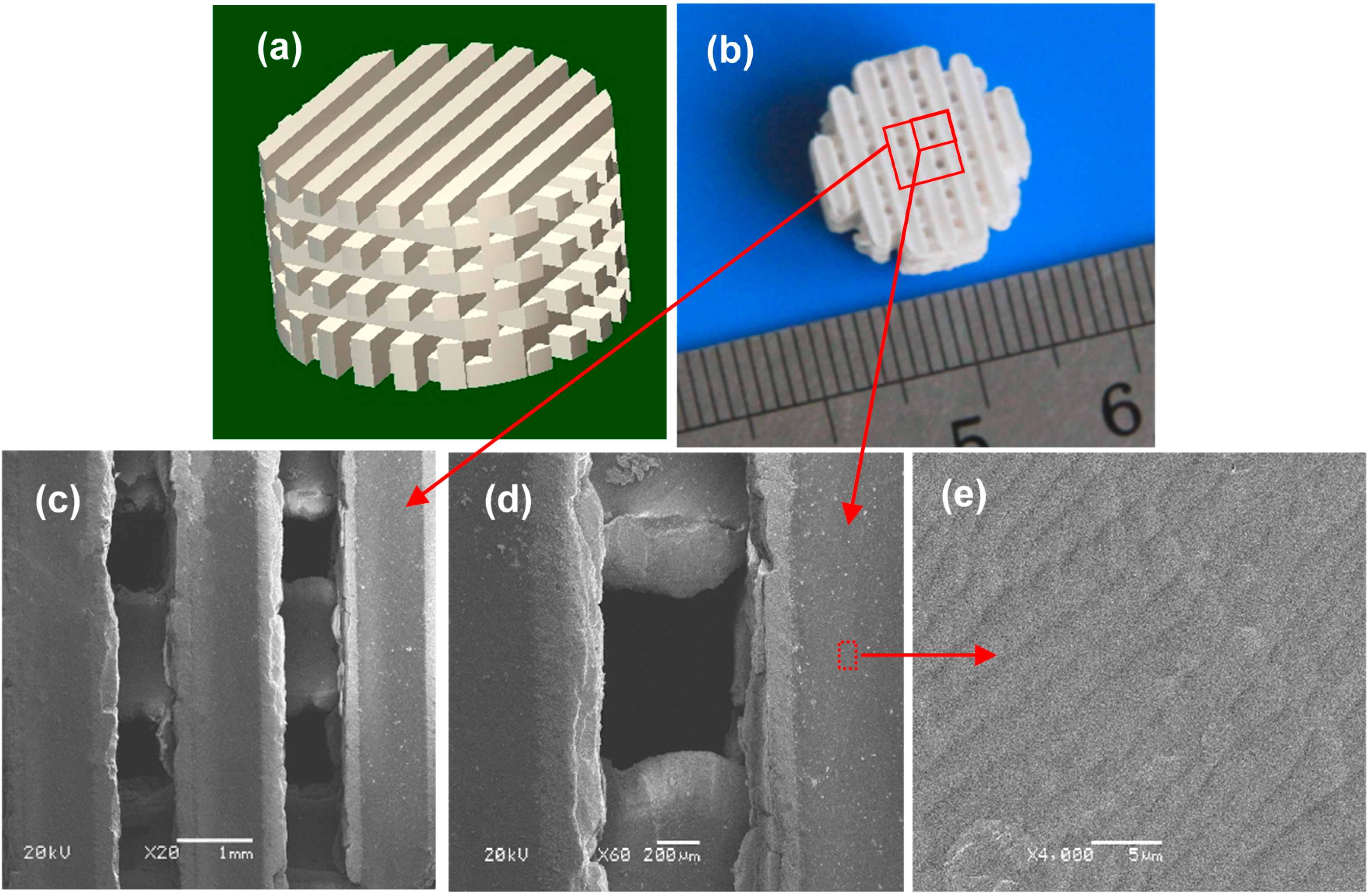
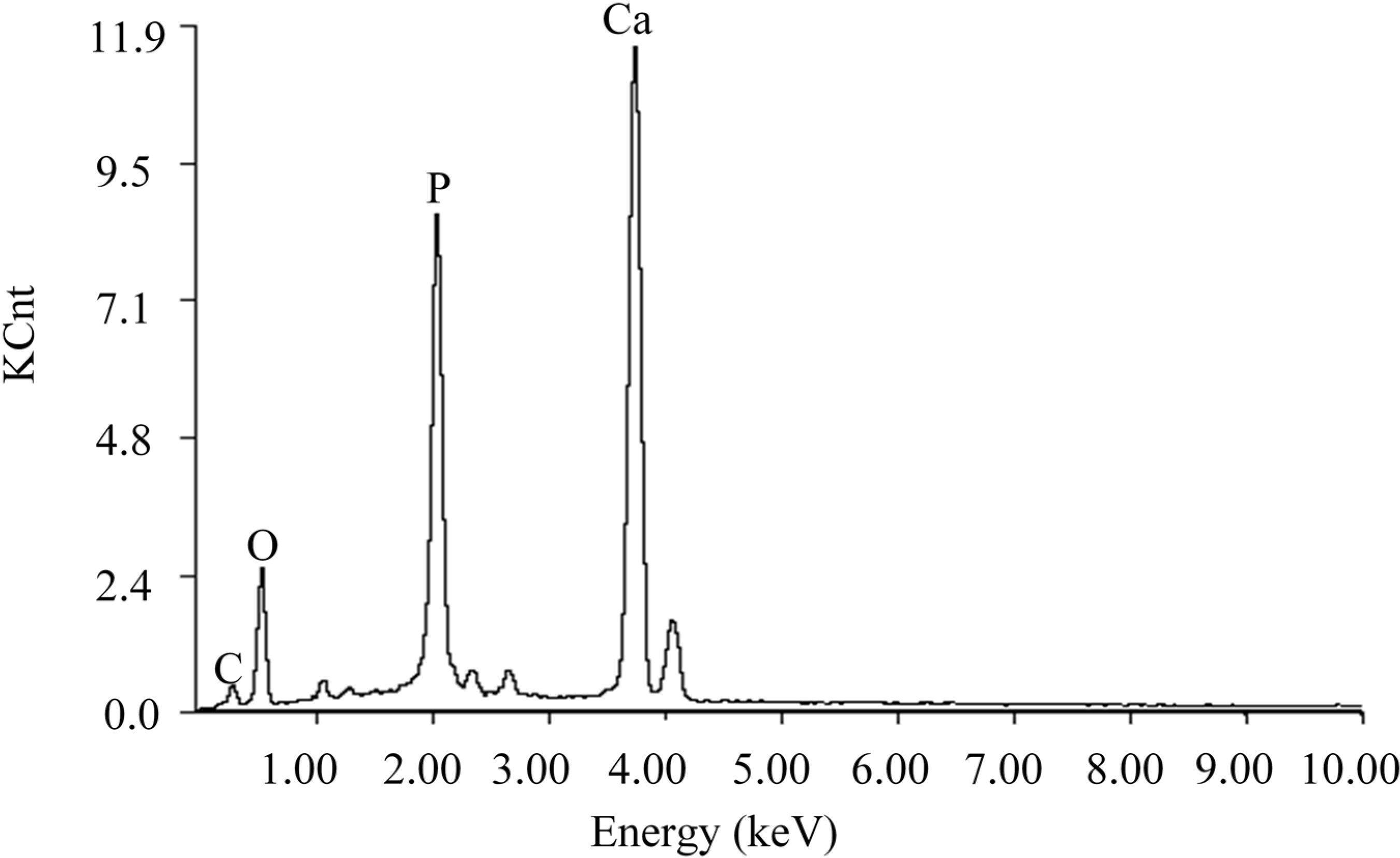
2.1. Characterization of the Scaffolds
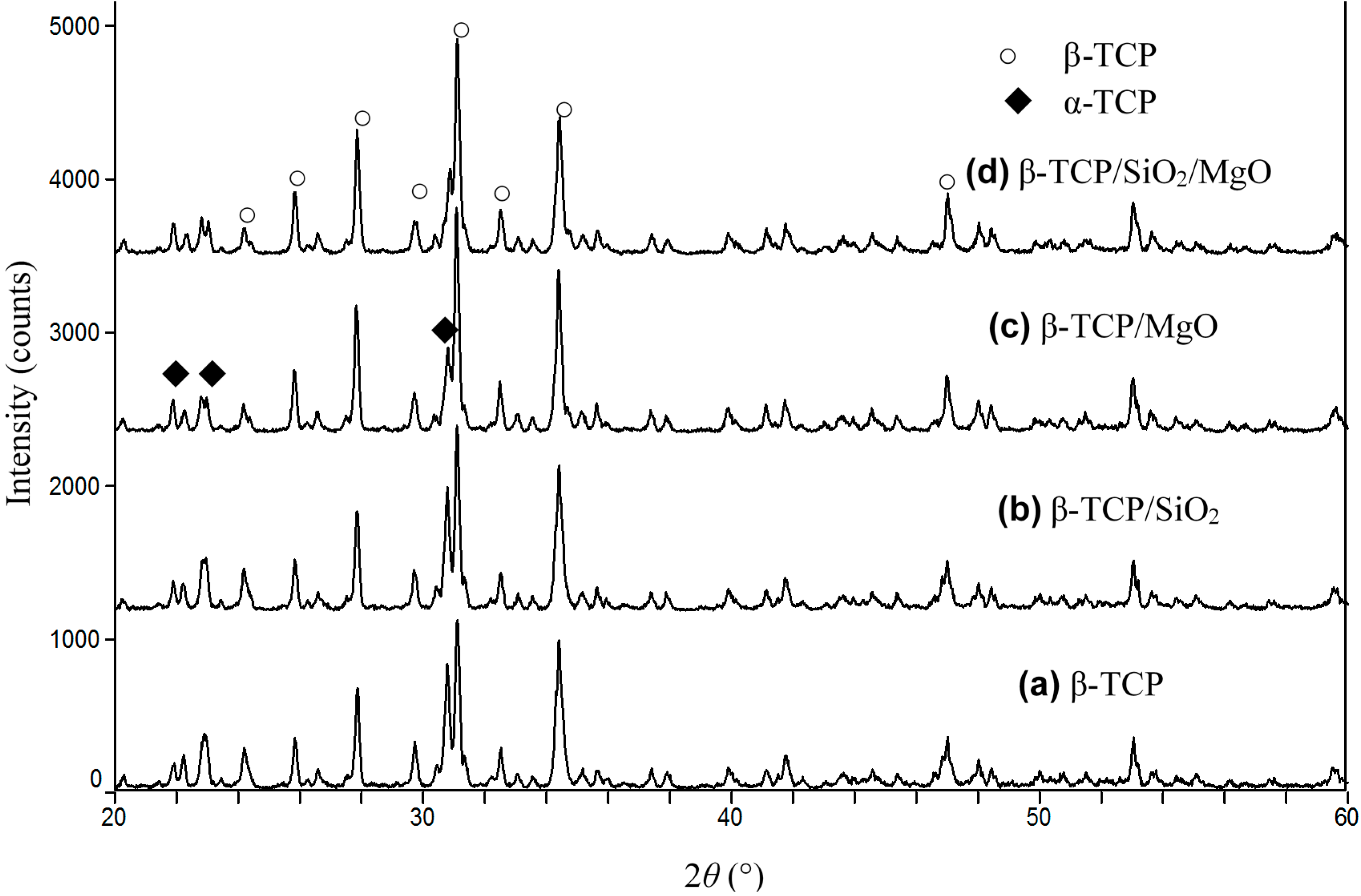
2.2. Phase Identification
| Compositions | β-TCP | β-TCP/SiO2 | β-TCP/MgO | β-TCP/SiO2/MgO |
|---|---|---|---|---|
| Weight percent of β-TCP (%) | 85.5 | 87.0 | 93.6 | 93.3 |
| Relative density (%) | 90.21 ± 1.53 | 91.35 ± 1.46 | 93.28 ± 1.08 | 95.53 ± 0.94 |
2.3. Mechanical Properties

2.4. Mineralization in Simulated Body Fluid (SBF)
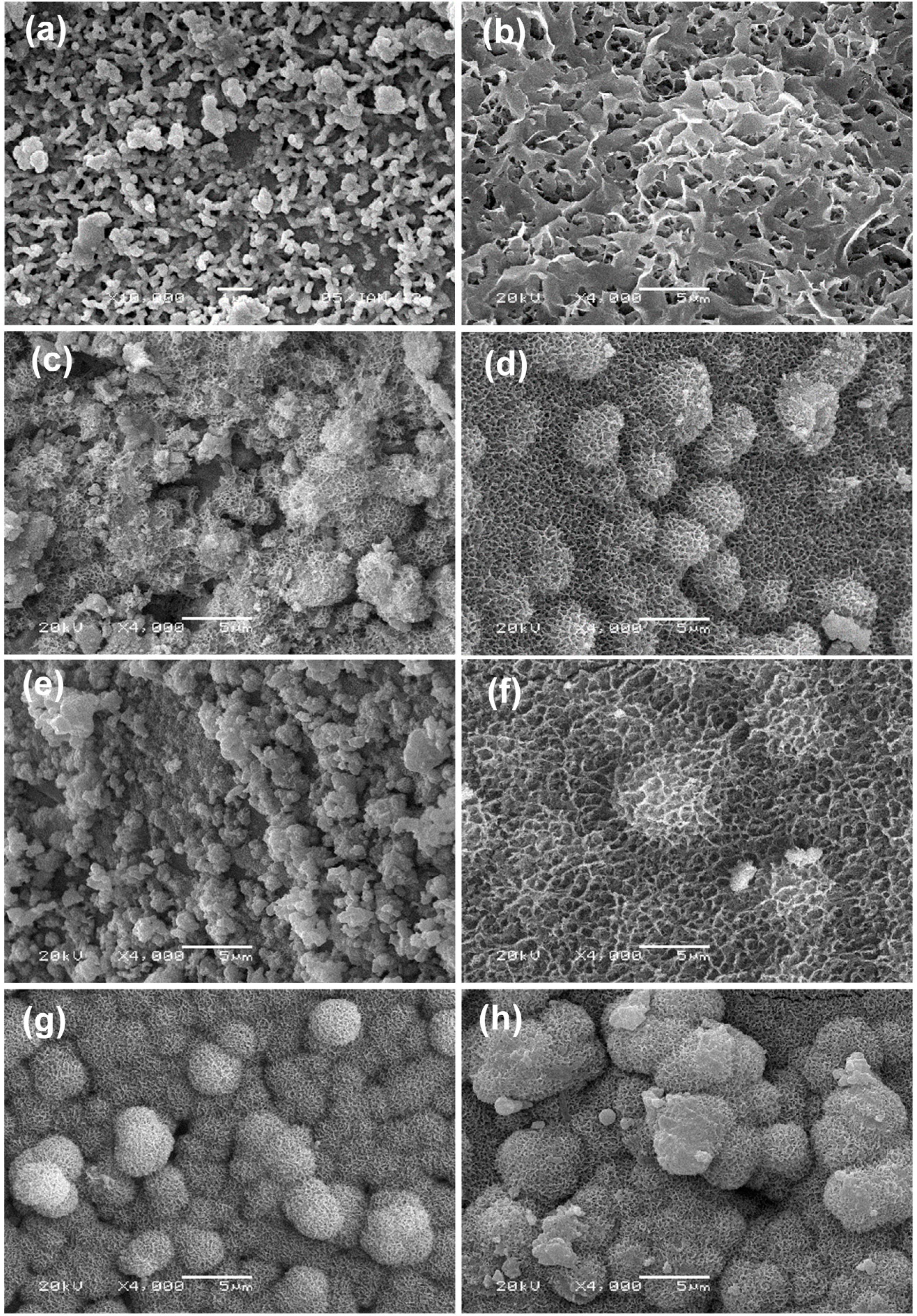
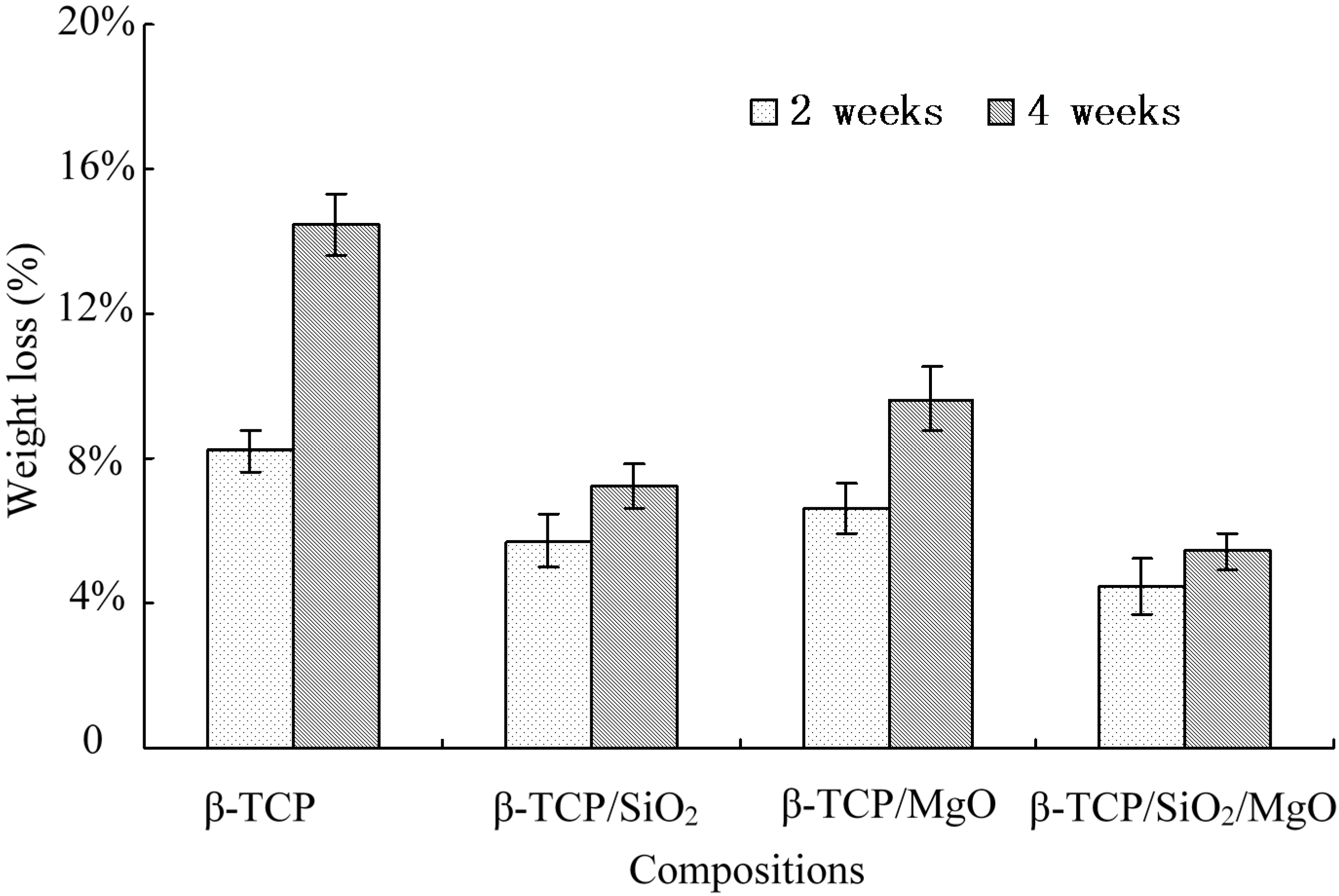
2.5. Weight Loss
2.6. Cell Attachment and Proliferation

3. Experimental Section
3.1. Materials
3.2. Scaffold Fabrication and Characterization
3.3. X-ray Diffraction (XRD)
3.4. The Degradability and Bioactivity
3.5. In Vitro Cell Culture
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Tricoteaux, A.; Rguiti, E.; Chicot, D.; Boilet, L.; Descamps, M.; Leriche, A.; Lesage, J. Influence of porosity on the mechanical properties of microporous β-TCP bioceramics by usual and instrumented Vickers microindentation. J. Eur. Ceram. Soc. 2011, 31, 1361–1369. [Google Scholar] [CrossRef]
- Wang, C.; Xue, Y.; Lin, K.; Lu, J.; Chang, J.; Sun, J. The enhancement of bone regeneration by a combination of osteoconductivity and osteostimulation using β-CaSiO3/β-Ca3(PO4)2 composite bioceramics. Acta Biomater. 2012, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.A.; Kalita, S.J. Influence of oxide-based sintering additives on densification and mechanical behavior of tricalcium phosphate (TCP). J. Mater. Sci. Mater. Med. 2007, 18, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Bodhak, S.; Bandyopadhyay, A.; Bose, S. Effect of electrical polarization and composition of biphasic calcium phosphates on early stage osteoblast interactions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Kasuga, T. SiO2-CaO-P2O5 sol-gel-derived glass coating on porous β-tricalcium phosphate ceramics. J. Ceram. Soc. Jpn. 2009, 117, 1120–1125. [Google Scholar] [CrossRef]
- Yu, X.; Wei, M. Cellular performance comparison of biomimetic calcium phosphate coating and alkaline-treated titanium surface. BioMed Res. Int. 2013, 2013, 832790. [Google Scholar] [PubMed]
- Zhang, M.; Wu, C.; Lin, K.; Fan, W.; Chen, L.; Xiao, Y.; Chang, J. Biological responses of human bone marrow mesenchymal stem cells to Sr-M-Si (M = Zn, Mg) silicate bioceramics. J. Biomed. Mater. Res. Part A 2012, 100, 2979–2990. [Google Scholar] [CrossRef]
- Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. 3D printed tricalcium phosphate bone tissue engineering scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci. 2013, 1, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, X.; Ding, C. Preparation and in vitro evaluation of nanostructured TiO2/TCP composite coating by plasma electrolytic oxidation. J. Alloys Compd. 2010, 498, 172–178. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, V.V.; Tirri, T.; Malin, M.; Seppälä, J.V.; Närhi, T.O. Enhanced osteogenicity of bioactive composites with biomimetic treatment. BioMed Res. Int. 2014, 2014, 207676. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Perez-Amodio, S.; Barrère-de Groot, F.Y.; Everts, V.; van Blitterswijk, C.A.; Habibovic, P. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials 2010, 31, 2976–2989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Kim, Y.; Riu, D.-H.; Jung, S.; Lee, Y.; Chung, S.; Kim, Y. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 2003, 24, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Bandyopadhyay, A.; Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 2012, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Petersen, J.; Fielding, G.; Banerjee, S.; Bose, S. ZnO, SiO2, and SrO doping in resorbable tricalcium phosphates: Influence on strength degradation, mechanical properties, and in vitro bone–cell material interactions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-S.; Hong, K.S.; Lee, J.-K.; Kim, D.J.; Lee, J.H.; Chang, B.-S.; Lee, D.-H.; Lee, C.-K.; Chung, S.-S. Magnesia-doped HA/β-TCP ceramics and evaluation of their biocompatibility. Biomaterials 2004, 25, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Yeong, W.; Sudarmadji, N.; Yu, H.; Chua, C.; Leong, K.; Venkatraman, S.; Boey, Y.; Tan, L. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Eosoly, S.; Brabazon, D.; Lohfeld, S.; Looney, L. Selective laser sintering of hydroxyapatite/poly-ε-caprolactone scaffolds. Acta Biomater. 2010, 6, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Lim, W.-K.; Huang, X.; Chen, Y. Preparation and characterization of interpenetrating phased TCP/HA/PLGA composites. Mater. Lett. 2005, 59, 4000–4005. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Banerjee, S.S.; Davies, N.M.; Bandyopadhyay, A. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped β-TCP. Bone 2011, 48, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J.; Beaupré, G.S.; Keller, T.S.; Carter, D.R. The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone 2001, 29, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Impens, S.; Schelstraete, R.; Mullens, S.; Thijs, I.; Luyten, J.; Schrooten, J. In vitro dissolution behavior of custom made CaP scaffolds for bone tissue engineering. Key Eng. Mater. 2008, 361, 7–10. [Google Scholar] [CrossRef]
- Wei, X.; Ugurlu, O.; Ankit, A.; Acar, H.Y.; Akinc, M. Dissolution behavior of Si, Zn-codoped tricalcium phosphates. Mater. Sci. Eng. C 2009, 29, 126–135. [Google Scholar] [CrossRef]
- Li, X.; Yasuda, H.; Umakoshi, Y. Bioactive ceramic composites sintered from hydroxyapatite and silica at 1200 °C: Preparation, microstructures and in vitro bone-like layer growth. J. Mater. Sci. Mater. Med. 2006, 17, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Zhou, Y.; Peng, S. Structure and properties of nano-hydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology 2011, 22, 285703. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Wei, P.; Feng, P.; Xiao, T.; Shuai, C.; Peng, S. Nano SiO2 and MgO Improve the Properties of Porous β-TCP Scaffolds via Advanced Manufacturing Technology. Int. J. Mol. Sci. 2015, 16, 6818-6830. https://doi.org/10.3390/ijms16046818
Gao C, Wei P, Feng P, Xiao T, Shuai C, Peng S. Nano SiO2 and MgO Improve the Properties of Porous β-TCP Scaffolds via Advanced Manufacturing Technology. International Journal of Molecular Sciences. 2015; 16(4):6818-6830. https://doi.org/10.3390/ijms16046818
Chicago/Turabian StyleGao, Chengde, Pingpin Wei, Pei Feng, Tao Xiao, Cijun Shuai, and Shuping Peng. 2015. "Nano SiO2 and MgO Improve the Properties of Porous β-TCP Scaffolds via Advanced Manufacturing Technology" International Journal of Molecular Sciences 16, no. 4: 6818-6830. https://doi.org/10.3390/ijms16046818
APA StyleGao, C., Wei, P., Feng, P., Xiao, T., Shuai, C., & Peng, S. (2015). Nano SiO2 and MgO Improve the Properties of Porous β-TCP Scaffolds via Advanced Manufacturing Technology. International Journal of Molecular Sciences, 16(4), 6818-6830. https://doi.org/10.3390/ijms16046818







