Faldaprevir for the Treatment of Hepatitis C
Abstract
:1. Introduction
2. HCV Genome and Its Coding Proteins
3. HCV NS3/4A Protease Inhibitors
4. Faldaprevir

4.1. Faldaprevir with Pegylated Interferon and Ribavirin for HCV Genotype 1-Infected Patients
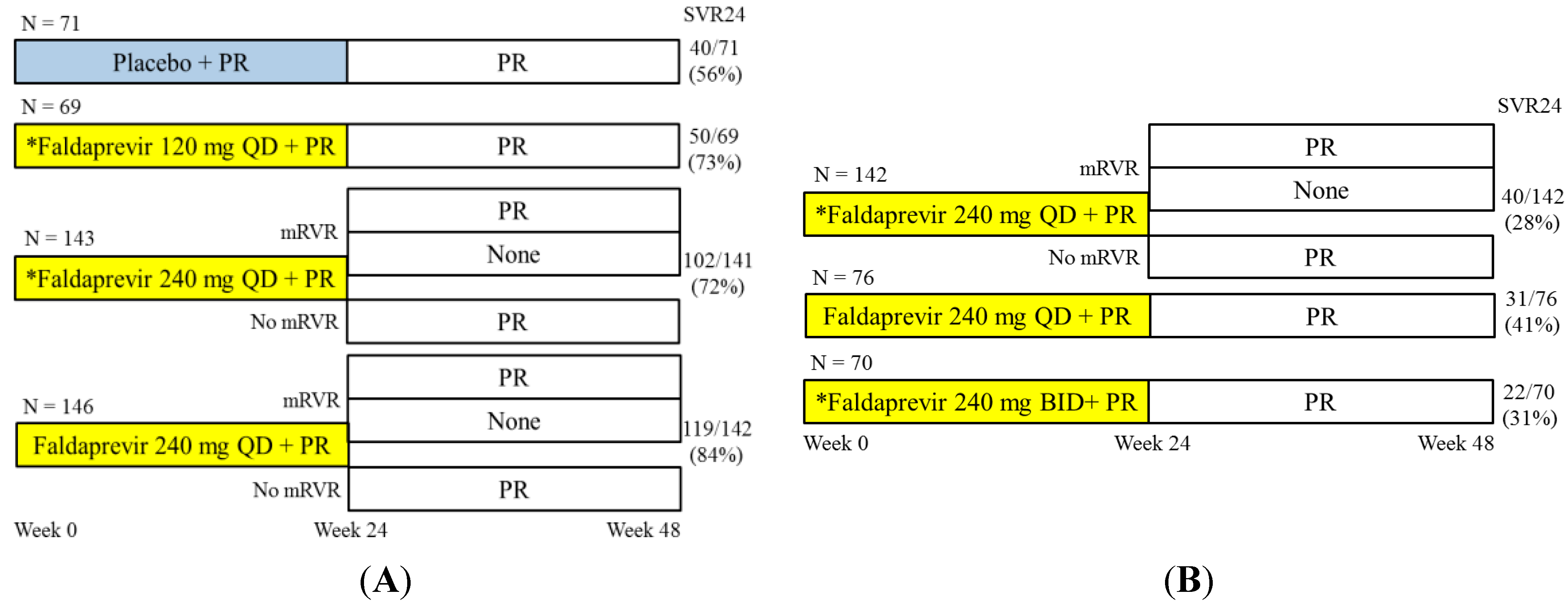
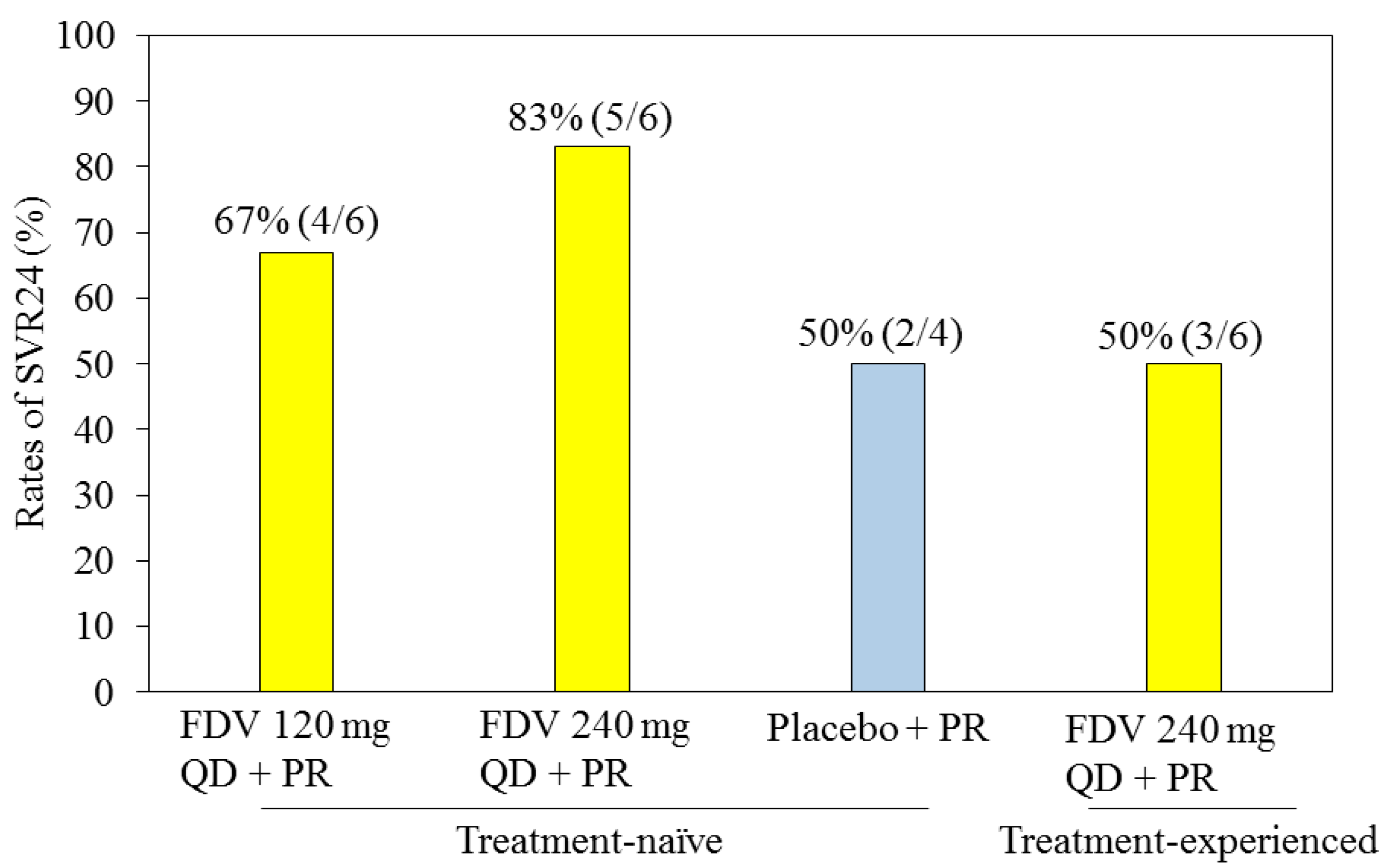
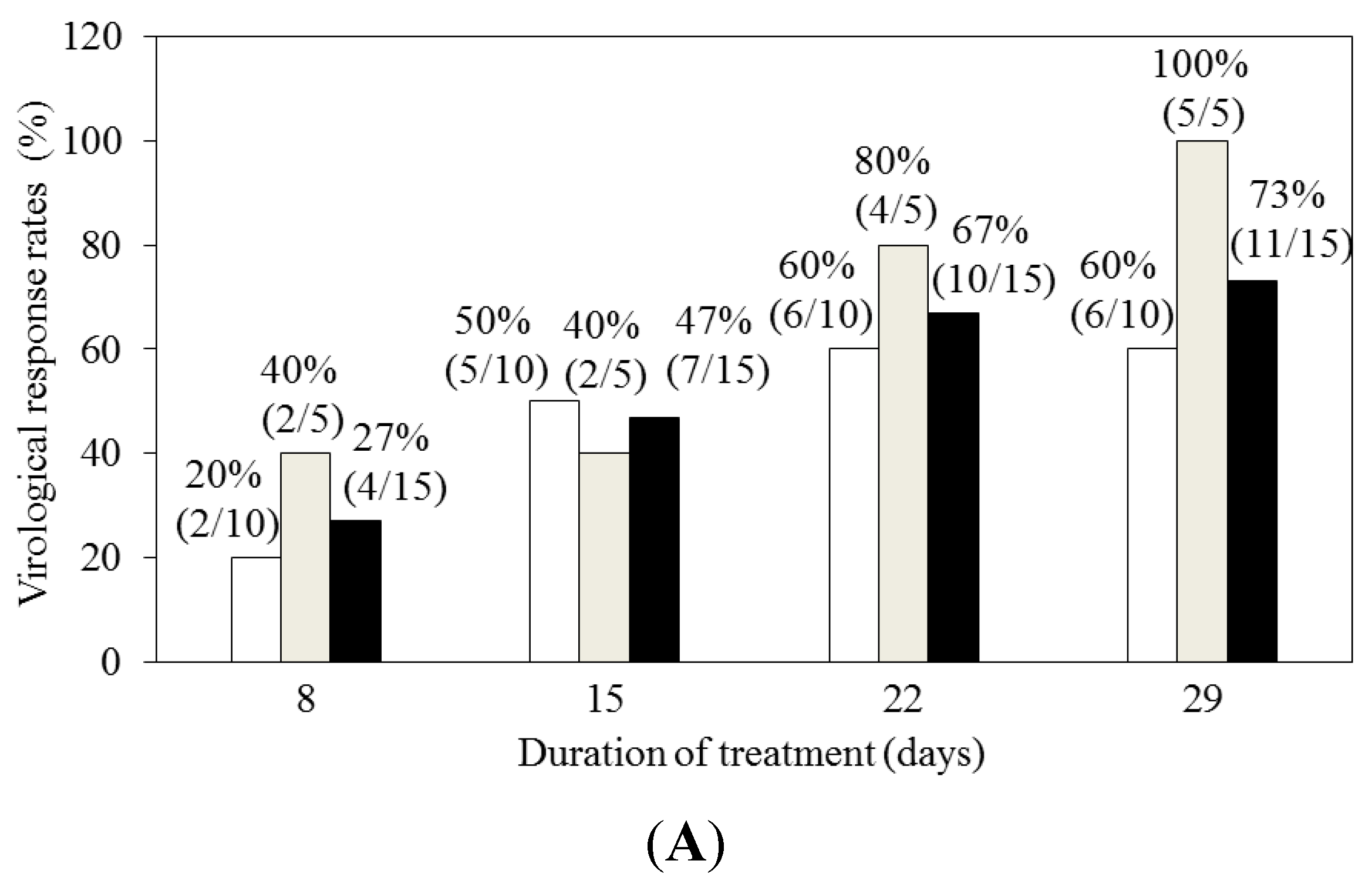
4.2. Faldaprevir and Deleobuvir (BI 207127) with or without Ribavirin for HCV Genotype 1-Infected Patients
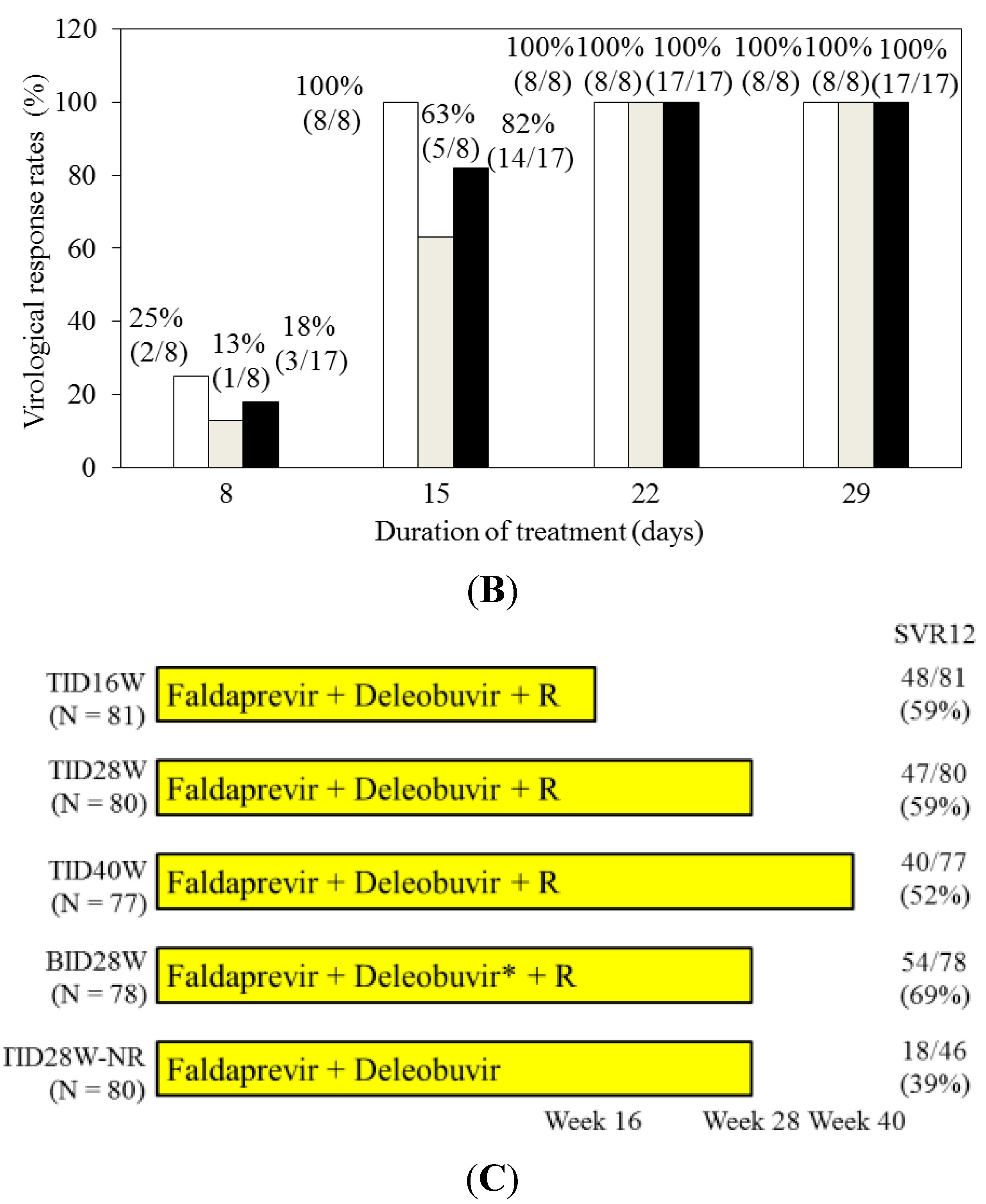
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Ghany, M.G.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2009, 49, 1335–1374. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R. The burden of hepatitis C in United States. Hepatology 2002, 36, S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Imazeki, F.; Yokosuka, O. New antiviral therapies for chronic hepatitis C. Hepatol. Int. 2010, 4, 548–561. [Google Scholar] [CrossRef] [PubMed]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J. Hepatol. 2014, 60, 392–420. [Google Scholar]
- Kanda, T.; Yokosuka, O.; Omata, M. Treatment of hepatitis C virus infection in the future. Clin. Transl. Med. 2013, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- McHutchison, J.G.; Everson, G.T.; Gordon, S.C.; Jacobson, I.M.; Sulkowski, M.; Kauffman, R.; McNair, L.; Alam, J.; Muir, A.J. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N. Engl. J. Med. 2009, 360, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Hezode, C.; Forestier, N.; Dusheiko, G.; Ferenci, P.; Pol, S.; Goeser, T.; Bronowicki, J.P.; Bourliere, M.; Gharakhanian, S.; Bengtsson, L.; et al. PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N. Engl. J. Med. 2009, 2, 1839–1850. [Google Scholar] [CrossRef]
- McHutchison, J.G.; Manns, M.P.; Muir, A.J.; Terrault, N.A.; Jacobson, I.M.; Afdhal, N.H.; Heathcote, E.J.; Zeuzem, S.; Reesink, H.W.; Garg, J.; et al. PROVE3 Study Team. Telaprevir for previously treated chronic HCV infection. N. Engl. J. Med. 2010, 2, 1292–1303. [Google Scholar] [CrossRef]
- Jacobson, I.M.; McHutchison, J.G.; Dusheiko, G.; di Bisceglie, A.M.; Reddy, K.R.; Bzowej, N.H.; Marcellin, P.; Muir, A.J.; Flisiak, R.; George, J.; et al. Telaprevir for previously untreated chronic HCV infection. N. Engl. J. Med. 2011, 2, 2405–2416. [Google Scholar] [CrossRef]
- Zeuzem, S.; Andreone, P.; Pol, S.; Lawitz, E.; Diago, M.; Roberts, S.; Focaccia, R.; Younossi, Z.; Foster, G.R.; Horban, A.; et al. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 2011, 2, 2417–2428. [Google Scholar] [CrossRef]
- Sherman, K.E.; Flamm, S.L.; Afdhal, N.H.; Nelson, D.R.; Sulkowski, M.S.; Everson, G.T.; Fried, M.W.; Adler, M.; Reesink, H.W.; Martin, M.; et al. Response-guided teraprevir combination treatment for hepatitis C virus infection. N. Engl. J. Med. 2011, 2, 1014–1024. [Google Scholar] [CrossRef]
- Poordad, F.; McCone, J., Jr.; Bacon, B.R.; Bruno, S.; Manns, M.P.; Sulkowski, M.S.; Jacobson, I.M.; Reddy, K.R.; Goodman, Z.D.; Boparai, N.; et al. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011, 2, 1195–1206. [Google Scholar] [CrossRef]
- Bacon, B.R.; Gordon, S.C.; Lawitz, E.; Marcellin, P.; Vierling, J.M.; Zeuzem, S.; Poordad, F.; Goodman, Z.D.; Sings, H.L.; Boparai, N.; et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011, 2, 1207–1217. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm376449.htm (accessed on 1 March 2014).
- U.S. Food and Drug Administration. Available online: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377920.htm (accessed on 1 March 2014).
- Wu, S.; Kanda, T.; Nakamoto, S.; Imazeki, F.; Yokosuka, O. Hepatitis C virus protease inhibitor-resistance mutations: Our experience and review. World J. Gastroenterol. 2013, 19, 8940–8948. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, R. Molecular virology of the hepatitis C virus. J. Hepatol. 1999, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Atoom, A.M.; Taylor, N.G.; Russell, R.S. The elusive function of the hepatitis C virus p7 protein. Virology 2014, 462, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ray, R. Progress toward development of a hepatitis C vaccine with broad shoulders. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Kanda, T.; Nakamoto, S.; Nakamura, M.; Jiang, X.; Miyamura, T.; Wu, S.; Yokosuka, O. Treatment with direct-acting antiviral agents for chronic hepatitis C virus infection. J. Clin. Transl. Hepatol. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- White, P.W.; Llinàs-Brunet, M.; Amad, M.; Bethell, R.C.; Bolger, G.; Cordingley, M.G.; Duan, J.; Garneau, M.; Lagacé, L.; Thibeault, D.; et al. Preclinical characterization of BI 201335, a C-terminal carboxylic acid inhibitor of the hepatitis C virus NS3-NS4A protease. Antimicrob. Agents Chemother. 2010, 54, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Lamarre, D.; Anderson, P.C.; Bailey, M.; Beaulieu, P.; Bolger, G.; Bonneau, P.; Bös, M.; Cameron, D.R.; Cartier, M.; Cordingley, M.G.; et al. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 2003, 426, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Vanwolleghem, T.; Meuleman, P.; Libbrecht, L.; Roskams, T.; de Vos, R.; Leroux-Roels, G. Ultra-rapid cardiotoxicity of hepatitis C virus protease inhibitor BILN 2061 in the urokinase-type plasminogen activator mouse. Gastroenterology 2007, 133, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Bourliere, M.; Benhamou, Y.; Pol, S.; Bonacini, M.; Trepo, C.; Wright, D.; Berg, T.; Calleja, J.L.; White, P.W.; et al. Potency, safety, and pharmacokinetics of the NS3/4A protease inhibitor BI201335 in patients with chronic HCV genotype-1 infection. J. Hepatol. 2011, 54, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S.; Asselah, T.; Lalezari, J.; Ferenci, P.; Fainboim, H.; Leggett, B.; Bessone, F.; Mauss, S.; Heo, J.; Datsenko, Y.; et al. Faldaprevir combined with pegylated interferon α-2a and ribavirin in treatment-naive patients with chronic genotype 1 HCV: SILEN-C1 Trial. Hepatology 2013, 57, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S.; Bourliere, M.; Bronowicki, J.P.; Asselah, T.; Pawlotsky, J.M.; Shafran, S.D.; Pol, S.; Mauss, S.; Larrey, D.; Datsenko, Y.; et al. Faldaprevir combined with peginterferon α-2a and ribavirin in chronic hepatitis C virus genotype-1 patients with prior nonresponse: SILEN-C2 Trial. Hepatology 2013, 57, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Sakai, Y.; Kuboki, M.; Tsunematsu, S.; Urano, Y.; Sakamoto, W.; Tsuda, Y.; Steinmann, G.; Omata, M. Safety and efficacy of faldaprevir with pegylated interferon α-2a and ribavirin in Japanese patients with chronic genotype-1 hepatitis C infection. Liver Int. 2013, 34, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Asselah, T.; Angus, P.; Zarski, J.P.; Larrey, D.; Müllhaupt, B.; Gane, E.; Schuchmann, M.; Lohse, A.; Pol, S.; et al. Efficacy of the protease inhibitor BI 20135, polymerase inhibitor BI 207127, and ribavirin in patients with chronic HCV infection. Gastroenterology 2011, 141, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Soriano, V.; Asselah, T.; Bronowicki, J.P.; Lohse, A.W.; Müllhaupt, B.; Schuchmann, M.; Bourlière, M.; Buti, M.; Roberts, S.K.; et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N. Engl. J. Med. 2013, 369, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Lagace, L.; White, P.W.; Bousquet, C.; Dansereau, N.; Dô, F.; Llinas-Brunet, M.; Marquis, M.; Massariol, M.J.; Maurice, R.; Spickler, C.; et al. In vitro resistance profile of the hepatitis C virus NS3 protease inhibitor BI 201335. Antimicrob. Agents Chemother. 2012, 56, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Sabo, J.P.; Kort, J.; Ballow, C.; Kashuba, A.D.; Haschke, M.; Battegay, M.; Girlich, B.; Ting, N.; Lang, B.; Zhang, W.; et al. Interactions of the hepatitis C virus protease inhibitor faldaprevir with cytochrome P450 enzymes: In vitro and in vivo correlation. J. Clin. Pharmacol. 2015. [Google Scholar] [CrossRef]
- Ghany, M.G.; Nelson, D.R.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Kanda, T.; Yu, M.L.; Yokosuka, O.; Lim, S.G.; Jafri, W.; Tateishi, R.; Hamid, S.S.; Chuang, W.L.; Chutaputti, A.; et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol. Int. 2012, 6, 409–435. [Google Scholar] [CrossRef]
- Kanda, T.; Nakamoto, S.; Wu, S.; Yokosuka, O. Role of IL28B genotype in older hepatitis C virus-infected patients. World J. Immunol. 2013, 3, 54–61. [Google Scholar] [CrossRef]
- Ferenci, P.; Asselah, T.; Foster, G.R.; Zeuzem, S.; Sarrazin, C.; Moreno, C.; Ouzan, D.; Maevskaya, M.; Calinas, F.; Morano, L.E.; et al. STARTVerso1: A randomized trial of faldaprevir plus pegylated interferon/ribavirin for chronic HCV genotype-1 infection. J. Hepatol. 2015. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Kanda, T.; Matsumura, H.; Moriyama, M.; Yokosuka, O.; Omata, M. HCV NS5A resistance-associated variants in a group of real-world Japanese patients chronically infected with HCV genotype 1b. Hepatol. Int. 2015, in press. [Google Scholar]
- McCown, M.F.; Rajyaguru, S.; le Pogam, S.; Ali, S.; Jiang, W.R.; Kang, H.; Symons, J.; Cammack, N.; Najera, I. The hepatitis C virus replicon presents a higher barrier to resistance to nucleoside analogs than to nonnucleoside polymerase or protease inhibitors. Antimicrob. Agents Chemother. 2008, 52, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Omata, M. Antiviral therapy for “difficult-to-treat” hepatitis C virus-infected patients. Chin. Med. J. 2013, 126, 4568–4574. [Google Scholar] [PubMed]
- Zeuzem, S.; Dufour, J.F.; Buti, M.; Soriano, V.; Buynak, R.J.; Mantry, P.; Taunk, J.; Stern, J.O.; Vinisko, R.; Gallivan, J.P.; et al. Interferon-free treatment of chronic hepatitis C with faldaprevir, deleobuvir and ribavirin: SOUND-C3, a Phase 2b study. Liver Int. 2015, 35, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Soriano, V.; Asselah, T.; Gane, E.J.; Bronowicki, J.P.; Angus, P.; Lohse, A.W.; Stickel, F.; Müllhaupt, B.; Roberts, S.; et al. Efficacy and safety of faldaprevir, deleobuvir, and ribavirin in treatment-naive patients with chronic hepatitis C virus infection and advanced liver fibrosis or cirrhosis. Antimicrob. Agents Chemother. 2015, 59, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Yokosuka, O.; Omata, M. Faldaprevir for the Treatment of Hepatitis C. Int. J. Mol. Sci. 2015, 16, 4985-4996. https://doi.org/10.3390/ijms16034985
Kanda T, Yokosuka O, Omata M. Faldaprevir for the Treatment of Hepatitis C. International Journal of Molecular Sciences. 2015; 16(3):4985-4996. https://doi.org/10.3390/ijms16034985
Chicago/Turabian StyleKanda, Tatsuo, Osamu Yokosuka, and Masao Omata. 2015. "Faldaprevir for the Treatment of Hepatitis C" International Journal of Molecular Sciences 16, no. 3: 4985-4996. https://doi.org/10.3390/ijms16034985






