Thinopyrum ponticum Chromatin-Integrated Wheat Genome Shows Salt-Tolerance at Germination Stage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Transfer of Th. ponticum Genetic Material with Salt Tolerance to Wheat by Wide Hybridization

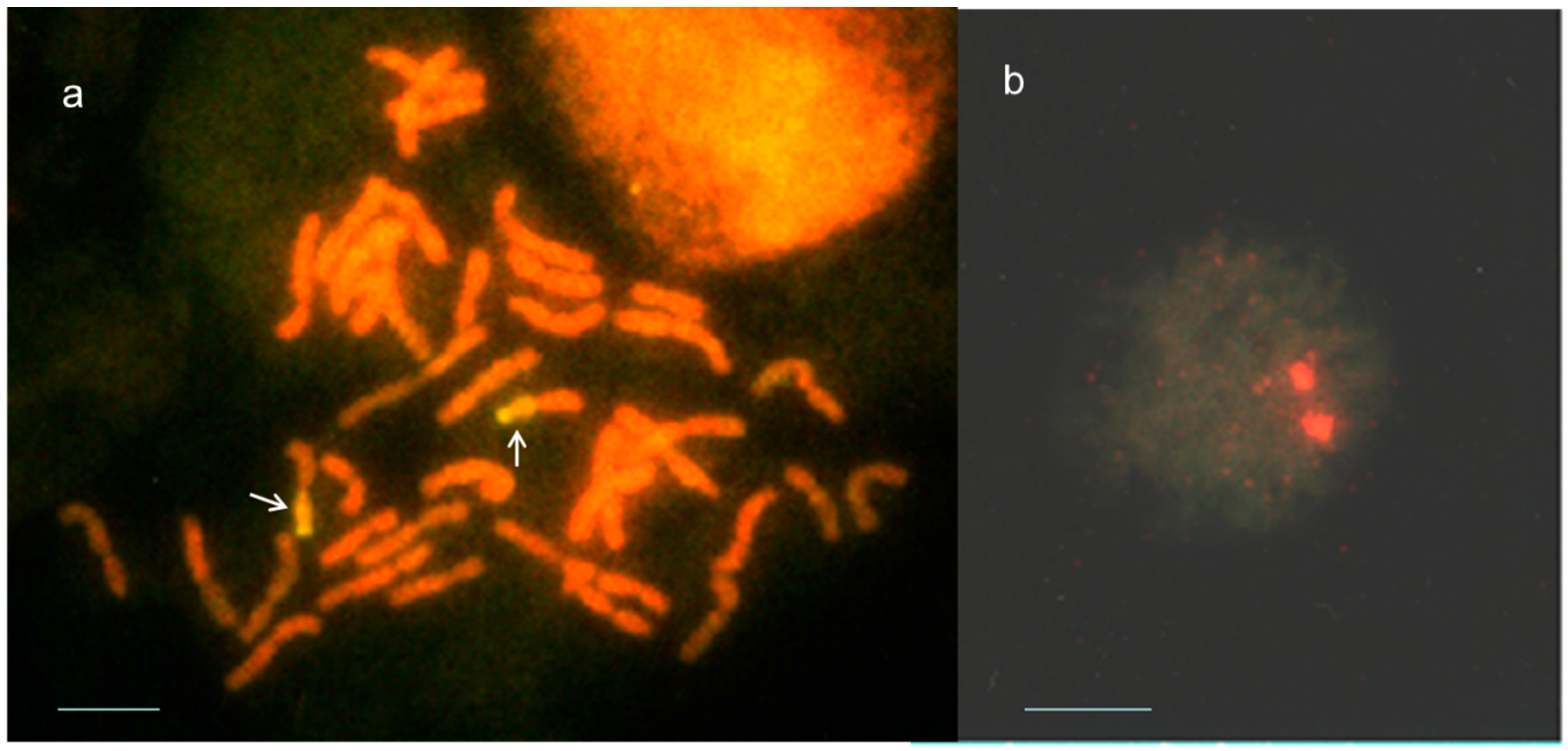
2.2. Identification of Th. ponticum Chromatin in Salt-Tolerant Recombinant Wheat
3. Experimental Section
3.1. Embryo Culture
3.2. Salinity Test
3.3. Fluorescence Genomic in Situ Hybridization
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinization of land and water resources. In Human Causes, Extent, Management and Case Studies; University of New South Wales: Sydney, Australia, 1995; p. 562. [Google Scholar]
- Mujeeb-Kazi, A.; Diaz de Leon, J.L. Conventional and alien genetic diversity for salt tolerant wheats: Focus on current status and new germplasm development. In Prospects for Saline Agriculture; Ahmad, R., Malik, K.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 69–82. [Google Scholar]
- Shalaby, E.E.; Epstein, E.; Qualset, C.O. Variation in salt tolerance among some wheat and triticale genotypes. J. Agron. Crop Sci. 1993, 171, 298–304. [Google Scholar] [CrossRef]
- Colmer, T.D.; Munns, R.; Flowers, T.J. Improving salt tolerance of wheat and barley: Future prospects. Aust. J. Exp. Agric. 2005, 45, 1425–1443. [Google Scholar] [CrossRef]
- Wang, R.R.C.; Li, X.M.; Hu, Z.M.; Zhang, J.Y.; Larson, S.R.; Zhang, X.Y.; Grieve, C.M.; Shannon, M.C. Development of salinity-tolerant wheat recombinant lines from a wheat disomic addition line carrying a Thinopyrum junceum chromosome. Int. J. Plant Sci. 2003, 164, 25–33. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- McGuire, G.E.; Dvořák, J. High salt tolerance potential in wheatgrasses. Crop Sci. 1981, 21, 702–705. [Google Scholar] [CrossRef]
- Dewey, D.R. Salt tolerance of twenty-five strains of Agropyron. Agron. J. 1960, 52, 631–635. [Google Scholar] [CrossRef]
- Akhtar, J.; Gorham, J.; Qureshi, R.H. Combined effect of salinity and hypoxia in wheat (Triticum aestivum L) and wheat-Thinopyrum amphiploids. Plant Soil 1994, 166, 47–54. [Google Scholar] [CrossRef]
- King, I.P.; Law, C.N.; Cant, K.A.; Orford, S.E.; Reader, S.M.; Miller, T.E. Tritipyrum, a potential new salt-tolerant cereal. Plant Breed. 1997, 116, 127–132. [Google Scholar] [CrossRef]
- Brasileiro-Vidal, A.C.; Cuadrado, A.; Brammer, S.P.; Benko-Iseppon, A.M.; Guerra, M. Molecular cytogenetic characterization of parental genomes in the partial amphidiploid Triticum aestivum × Thinopyrum ponticum. Genet. Mol. Biol. 2005, 28, 308–313. [Google Scholar] [CrossRef]
- Gill, B.S.; Friebe, B.; Endo, T.R. Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 1991, 34, 830–839. [Google Scholar] [CrossRef]
- Tomita, M.; Shinohara, K.; Morimoto, M. Revolver is a new class of transposon-like gene composing the Triticeae genome. DNA Res. 2008, 15, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, R.V. Wheat × Thinopyrum hybrids. In Biotechnology in Agriculture and Forestry; Springer Berlin Heidelberg: New York, NY, USA, 1990; pp. 167–217. [Google Scholar]
- Fedak, G. Molecular aids for integration of alien chromatin through wide crosses. Genome 1999, 42, 584–591. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xia, G.M.; Quan, T.Y.; Xiang, F.N.; Yan, J.; Chen, H.M. Introgression of salt-tolerance from somatic hybrids between common wheat and Thinopyrum ponticum. Plant Sci. 2004, 167, 773–779. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, F.N.; Xia, G.M. Agropyron elongatum chromatin localization on the wheat chromosomes in an introgression line. Planta 2005, 221, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Peng, Z.Y.; Li, C.L.; Li, F.; Liu, C.; Xia, G.M. Proteomic analysis on a high salt tolerance introgression strain of Triticum aestivum/Thinopyrum ponticum. Proteomics 2008, 8, 1470–1489. [Google Scholar] [CrossRef] [PubMed]
- Mullan, D.J.; Mirzaghaderi, G.; Walker, E.; Colmer, T.D.; Francki, M.G. Development of wheat-Lophopyrum elongatum recombinant lines for enhanced sodium “exclusion” during salinity stress. Theor. Appl. Genet. 2009, 119, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Mott, I.W.; Wang, R.R.C. Comparative transcriptome analysis of salt-tolerant wheat germplasm lines using wheat genome arrays. Plant Sci. 2007, 173, 327–339. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.-Y.; Tomita, M. Thinopyrum ponticum Chromatin-Integrated Wheat Genome Shows Salt-Tolerance at Germination Stage. Int. J. Mol. Sci. 2015, 16, 4512-4517. https://doi.org/10.3390/ijms16034512
Yuan W-Y, Tomita M. Thinopyrum ponticum Chromatin-Integrated Wheat Genome Shows Salt-Tolerance at Germination Stage. International Journal of Molecular Sciences. 2015; 16(3):4512-4517. https://doi.org/10.3390/ijms16034512
Chicago/Turabian StyleYuan, Wen-Ye, and Motonori Tomita. 2015. "Thinopyrum ponticum Chromatin-Integrated Wheat Genome Shows Salt-Tolerance at Germination Stage" International Journal of Molecular Sciences 16, no. 3: 4512-4517. https://doi.org/10.3390/ijms16034512
APA StyleYuan, W.-Y., & Tomita, M. (2015). Thinopyrum ponticum Chromatin-Integrated Wheat Genome Shows Salt-Tolerance at Germination Stage. International Journal of Molecular Sciences, 16(3), 4512-4517. https://doi.org/10.3390/ijms16034512





