Major Peptides from Amaranth (Amaranthus cruentus) Protein Inhibit HMG-CoA Reductase Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
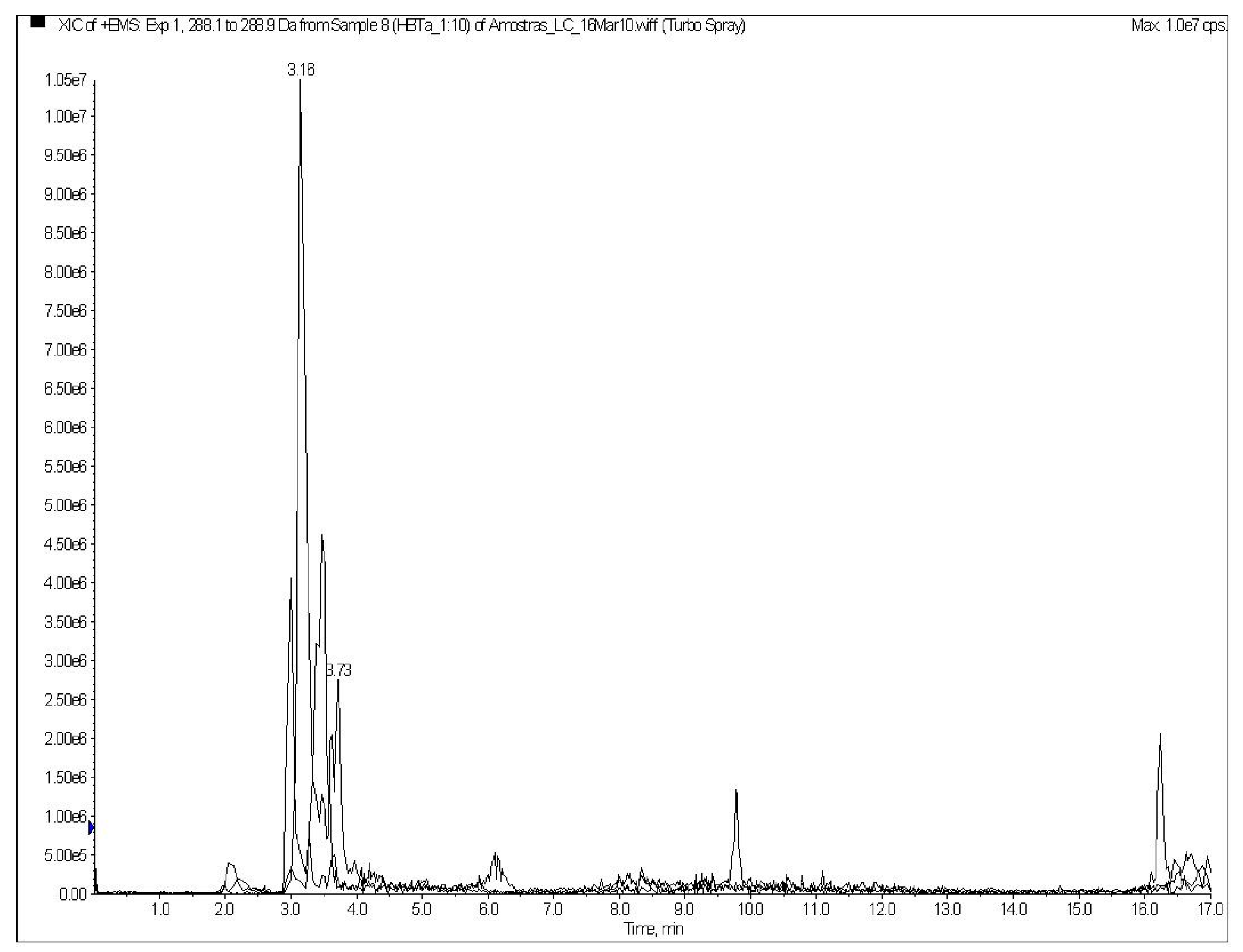
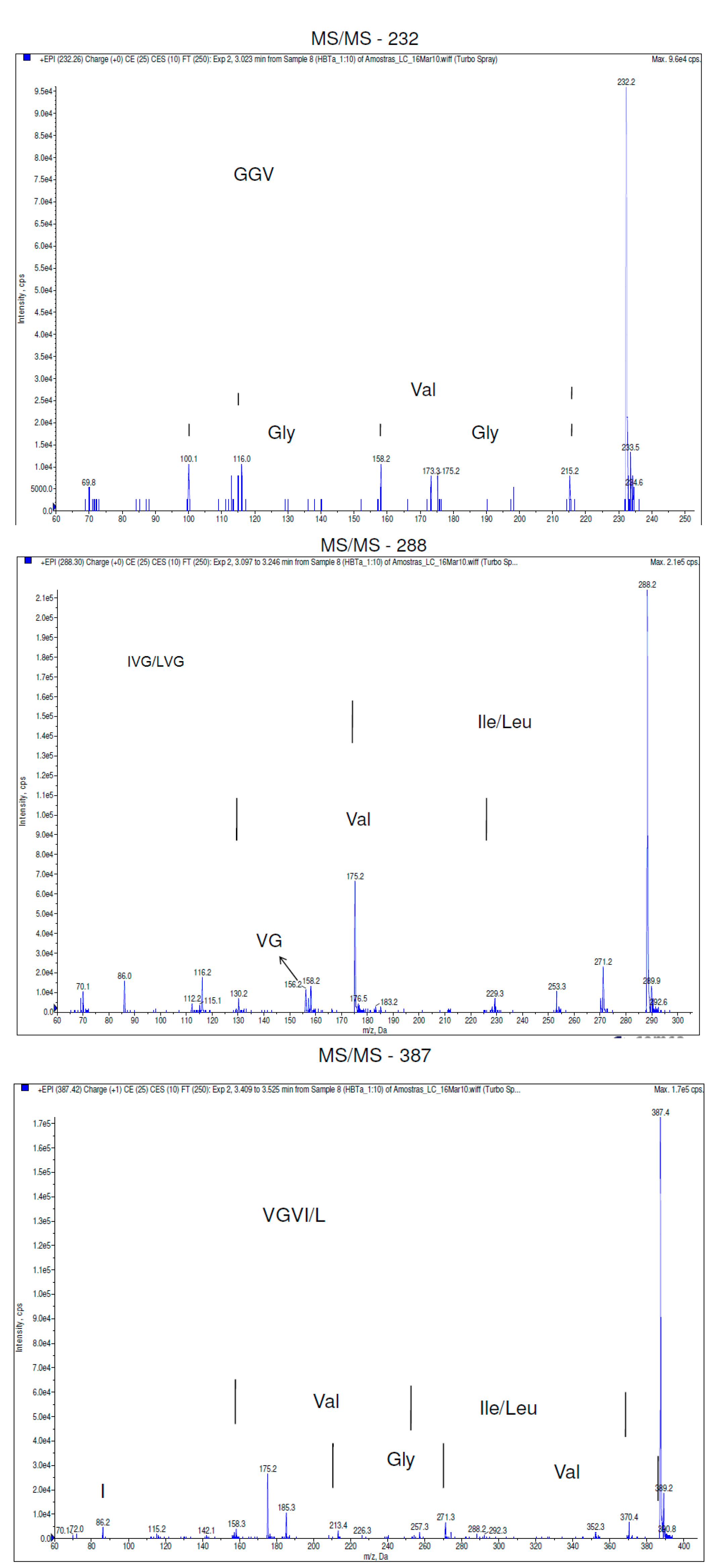
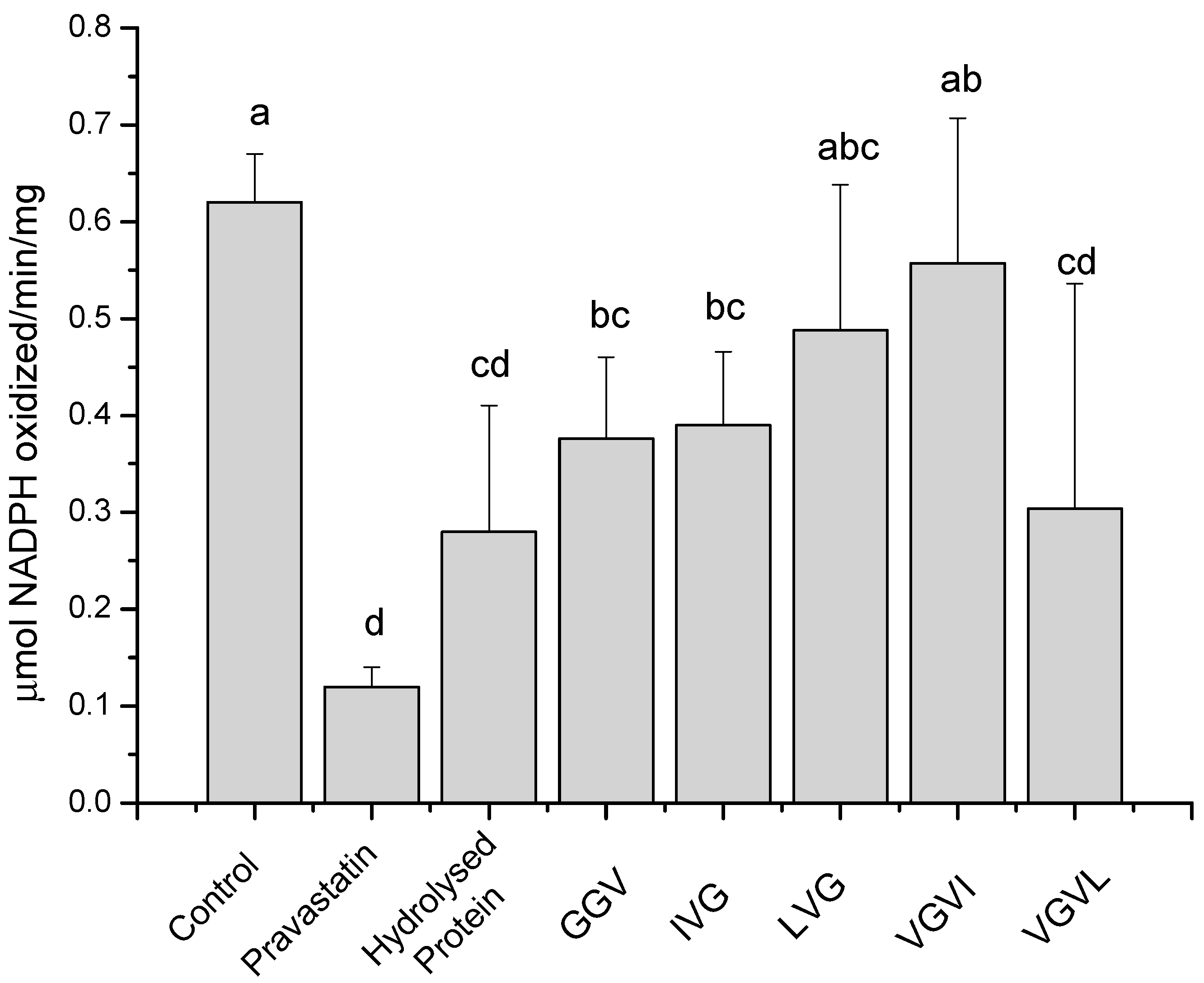
2.2. Discussion
3. Experimental Section
3.1. Material and Reagents
3.2. Sample Preparation
3.2.1. Protein Isolate Preparation
3.2.2. Protein in vitro Digestion
3.3. Analyses
3.3.1. Estimation of Soluble Nitrogen
3.3.2. Peptides Profile
3.3.3. LC-ESI MS/MS Analyses
3.3.4. HMG-CoA Reductase in vitro Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paredes-López, O. Amaranth: Biology, Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- National Academy of Science. Amaranth: Modern Prospects for an Ancient Crop; National Academy Press: Washington, DC, USA, 1984. [Google Scholar]
- Ebert, A.W. Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability 2014, 6, 319–335. [Google Scholar]
- Mendonça, S.; Saldiva, P.H.; Cruz, R.J.; Arêas, J.A.G. Amaranth protein presents cholesterol lowering effect. Food Chem. 2009, 116, 738–742. [Google Scholar]
- Queiroz, Y.S.; Soares, R.A.M.; Capriles, V.D.; Torres, E.A.F.S.; Arêas, J.A.G. Efeito do processamento na atividade antioxidante do grão de amaranto (Amaranthus cruentus L. BRS-Alegria) (in Portuguese). Arch. Latinoam. Nutr. 2009, 59, 419–424. [Google Scholar]
- Jayaprakasm, B.; Zhang, Y.; Nair, M.G. Tumor cell proliferation and cyclooxygenase enzymes inhibitory compounds in Amaranthus tricolor. J. Agric. Food Chem. 2004, 52, 6939–6943. [Google Scholar]
- Maldonado-Cervantes, E.; Jeong, H.J.; León-Galván, F.; Barrera-Pacheco, A.; de León-Rodríguez, A.; de Mejia, E.G.; de Lumen, B.O.; de la Rosa, A.P.B. Amaranth lunasin-like peptide internalizes into the cell nucleus and inhibits chemical carcinogen-induced transformation of NIH-3T3 cells. Peptides 2010, 31, 1635–1642. [Google Scholar]
- Maier, S.M.; Turner, N.D.; Lupton, J.R. Serum lipids in hypercholesterolemic men and women consuming oat bran and amaranth products. Cereal Chem. 2000, 77, 297–302. [Google Scholar]
- Bruneau, N.; Lombardo, D.; Bendayan, M. Participation of GRP94-related protein in secretion of pancreatic bile salt-dependent lipase in its internalization by the intestinal epithelium. J. Cell Sci. 1998, 111, 2665–2679. [Google Scholar]
- Gardner, M.G. Intestinal assimilation of intact peptides and proteins from the diet—A neglected field. Biol. Rev. Camb. Philos. Soc. 1984, 59, 289–331. [Google Scholar]
- Segura-Campos, M.; Chel-Guerrero, L.; Ancona, D.B.; Hernandez-Escalante, V.M. Bioavailability of bioactive peptides. Food Rev. Int. 2011, 27, 213–226. [Google Scholar]
- Hinsberger, A.; Sandhu, B.K. Digestion and absorption. Curr. Paediatr. 2004, 14, 605–611. [Google Scholar]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Saari, N. Production of defatted palm kernel cake protein hydrolysate as a valuable source of natural antioxidants. Int. J. Mol. Sci. 2012, 13, 8097–8111. [Google Scholar]
- Silva-Sánchez, C.; Barba de La Rosa, A.P.; León-Galván, M.F.; de Lumen, B.O.; León Rodriguez, A.; de Mejía, E.G. Bioactive peptides in amaranth (Amaranthus hypochondriacus) Seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar]
- Tovar-Pérez, E.G.; Guerrero-Legarreta, I.; Farrés-González, A.; Soriano-Santos, J. Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem. 2009, 116, 437–444. [Google Scholar]
- Barba de la Rosa, A.P.; Barba Montoya, A.; Martínez-Cuevas, P.; Hernández-Ledesma, B.; León-Galván, M.F.; de León-Rodríguez, A.; González, C. Tryptic amaranth glutelin digests induce endothelial nitric oxide production through inhibition of ACE: Antihypertensive role of amaranth peptides. Nitric Oxide 2010, 23, 106–111. [Google Scholar]
- Luna-Suárez, S.; Medina-Godoy, S.; Cruz-Hernández, A.; Paredes-López, O. Modification of the amaranth 11S globulin storage protein to produce an inhibitory peptide of the angiotensin I converting enzyme, and its expression in Escherichia coli. J. Biotechnol. 2010, 148, 240–247. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar]
- BIOPEP Database. Available online: http://www.uwm.edu.pl/biochemia/ (accessed on 15 July 2014).
- Pepbank Database. Available online: http://pepbank.mgh.harvard.edu (accessed on 15 July 2014).
- Oleszek, W.; Junkuszew, M.; Stochmal, A. Determination and toxicity of saponins from Amaranthus cruentus seeds. J. Agric. Food Chem. 1999, 47, 3685–3687. [Google Scholar]
- Kwon, D.Y.; Oh, S.H.; Lee, J.S.; Yang, H.J.; Lee, S.H.; Lee, J.H. Amino acid substitution of hypocholesterolemic peptide originated from glycinin hydrolyzate. Food Sci. Biotechnol. 2002, 11, 55–61. [Google Scholar]
- Takenaka, Y.; Nakamura, F.; Yamamoto, T.; Yoshikawa, M. Enterostatin (VPDPR) and its peptide fragment DPR reduce serum cholesterol levels after oral administration in mice. Biosci. Biotechnol. Biochem. 2003, 67, 1620–1622. [Google Scholar]
- Pak, V.V.; Koo, M.; Kwon, D.Y.; Yun, L. Design of a highly potent inhibitory peptide acting as a competitive inhibitor of HMG-CoA reductase. Amino Acids 2012, 43, 2015–2025. [Google Scholar]
- Vaughan, C.J.; Gotto, A.M.; Basson, P.C.T. The evolving role of statins in the management of atherosclerosis. Am. Coll. Cardiol. 2000, 35, 1–10. [Google Scholar]
- Arêas, J.A.G. Lipid–protein interactions in offal protein isolates: Effect of several solvents on lipid extraction. J. Food Sci. 1985, 50, 1392–1398. [Google Scholar]
- Gueguen, J.; Chevalier, M.; Barbot, J.; Schaeffer, F. Dissociation and aggregation of pea legumin induced by pH and ionic-strenght. J. Sci. Food Agric. 1988, 44, 167–182. [Google Scholar]
- Association of Official Analytical Chemists International (AOAC). Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Qiao, Y.; Gumpertz, M.; van Kempen, T. Stability of pepsin (EC 3.4.23.1) during in vitro protein digestibility assay. J. Food Biochem. 2002, 26, 355–375. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, R.A.M.; Mendonça, S.; De Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major Peptides from Amaranth (Amaranthus cruentus) Protein Inhibit HMG-CoA Reductase Activity. Int. J. Mol. Sci. 2015, 16, 4150-4160. https://doi.org/10.3390/ijms16024150
Soares RAM, Mendonça S, De Castro LÍA, Menezes ACCCC, Arêas JAG. Major Peptides from Amaranth (Amaranthus cruentus) Protein Inhibit HMG-CoA Reductase Activity. International Journal of Molecular Sciences. 2015; 16(2):4150-4160. https://doi.org/10.3390/ijms16024150
Chicago/Turabian StyleSoares, Rosana Aparecida Manólio, Simone Mendonça, Luíla Ívini Andrade De Castro, Amanda Caroline Cardoso Corrêa Carlos Menezes, and José Alfredo Gomes Arêas. 2015. "Major Peptides from Amaranth (Amaranthus cruentus) Protein Inhibit HMG-CoA Reductase Activity" International Journal of Molecular Sciences 16, no. 2: 4150-4160. https://doi.org/10.3390/ijms16024150
APA StyleSoares, R. A. M., Mendonça, S., De Castro, L. Í. A., Menezes, A. C. C. C. C., & Arêas, J. A. G. (2015). Major Peptides from Amaranth (Amaranthus cruentus) Protein Inhibit HMG-CoA Reductase Activity. International Journal of Molecular Sciences, 16(2), 4150-4160. https://doi.org/10.3390/ijms16024150



