Tumor Immunotargeting Using Innovative Radionuclides
Abstract
:1. Introduction
2. Principle of RIT
3. Radionuclides and Labeling Techniques for RIT
3.1. Radionuclides
| Radionuclide | T1/2 (hours) a | Main Emissions b | E Max (keV) | Range Max in Soft Tissue (mm) | Usual Labeling Method |
|---|---|---|---|---|---|
| Fluorine-18 | 1.83 | β+ | 633 | 3.1 | N-hydroxy-succinimide 18F-fluoro-benzotate, click chemistry, 18F-aluminum-NOTA |
| Gallium-68 | 1.13 | β+ | 1899 | 9.8 | Polyamino-carboxylic acids: DOTA, NOTA |
| Copper-64 | 12.7 | β+ | 653 | 3.2 | Many different chelating agents |
| β− | 579 | 2.8 | |||
| Yttrium-86 | 14.7 | β+ | 1220–2242 | 11 | Polyamino-carboxylic acids: DOTA |
| Bromine-76 | 16.2 | β+ | 1893 and 3382 | 19 | Direct bromination, bromine-labeled activated esters |
| Zirconium-89 | 78 | β+ | 902 | 4.6 | Desferroxamine |
| Iodine-124 | 100 | β+ | 1535 and 2138 | 7.9 and 10.9 | Direct labeling (tyrosine) |
| Scandium-44 | 3.97 | β+ | 1473 | 7.6 | Polyamino-carboxylic acids: DOTA |
| Iodine-131 | 193 | β− | 610 | 2.9 | Direct labeling (tyrosine) |
| γ | 362 | ||||
| Yttrium-90 | 64 | β− | 2250 | 11 | Polyamino-carboxylic acids: DOTA |
| Rhenium-188 | 17 | β− | 2120 | 10 | Direct labeling or N2S2 or N3S complexes (chemistry analogous to that of technetium) |
| γ | 155 | ||||
| Lutetium-177 | 162 | β− | 498 | 2.0 | Polyamino-carboxylic acids: DOTA |
| γ | 208 | ||||
| Copper-67 | 62 | β− | 392–577 | 1.8 | Many different chelating agents |
| γ | 184 | ||||
| Bismuth-212 | 1.01 | α | 6051 and 6090 | 0.07 | Polyamino-carboxylic acids: CHX-DTPA, DOTA |
| γ | 727 | ||||
| Bismuth-213 | 0.76 | α | 8,400 | 0.1 | Polyamino-carboxylic acids: CHX-DTPA, DOTA |
| γ | 440 | ||||
| Astatine-211 | 7.2 | α | 5870 and 7450 | 0.055–0.080 | Stannylated synthons: SAB, SAPS |
| X | 77–92 | ||||
| Actinium-225 | 240 | α | + alpha emitting daughters | * | Polyamino-carboxylic acids: DOTA |
| Thorium-227 | 449 | α | + alpha emitting daughters | * | Polyamino-carboxylic acids: DOTA |
| γ |
3.2. Labeling Techniques
4. RIT Efficacy Using Innovative β− Emitters
4.1. 177Lu-J591 Anti-PSMA in Metastatic Prostate Cancer (PCa)
4.2. Pretargeted 177Lu-Peptide in CEA-Positive Tumor
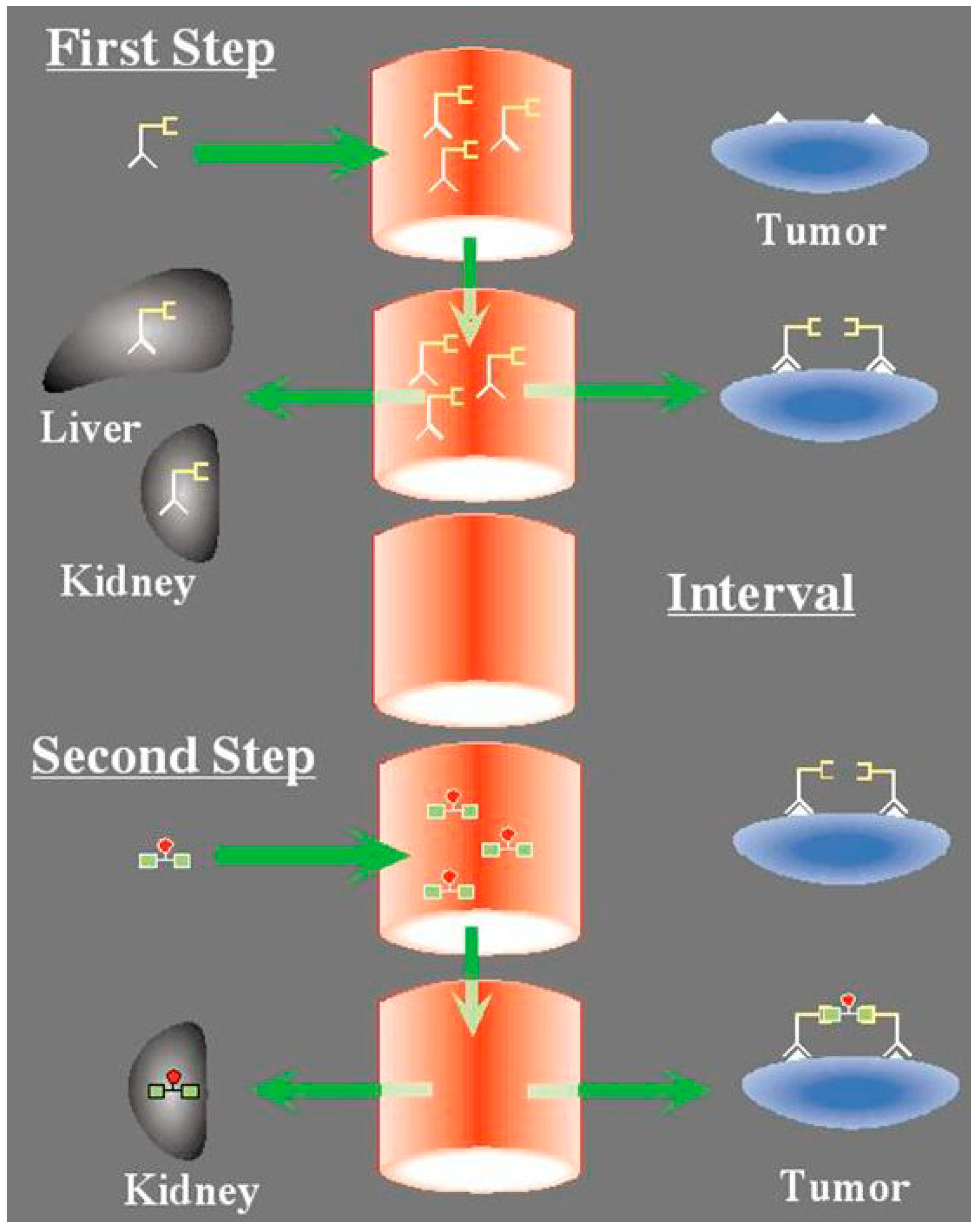

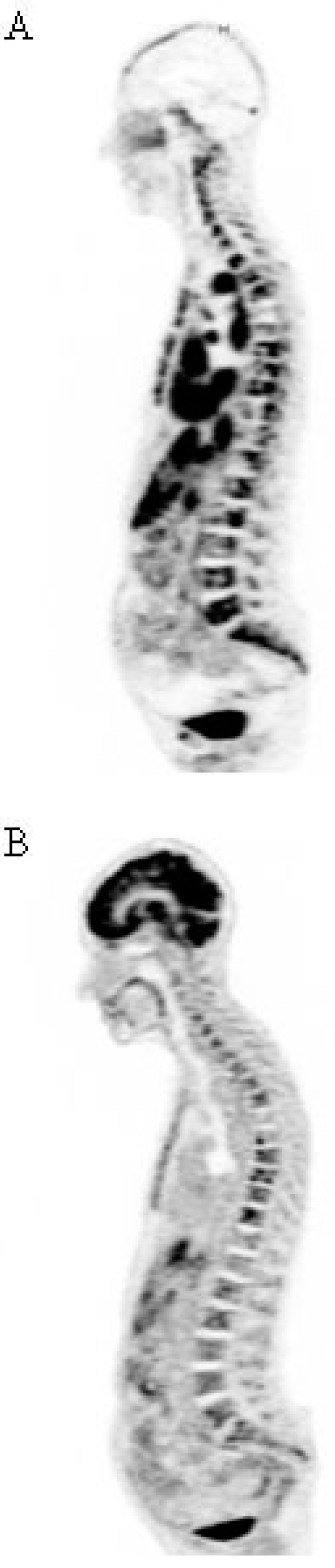
4.3. Interest of 67Cu for RIT
4.4. Other Radionuclides
5. RIT with Alpha-Emitting Radionuclides
6. Immuno-PET for Tumor Imaging and Theranostic Approaches
6.1. Interest of Immuno-PET and Choice of Radionuclides
6.2. 68Ga-Peptide for Pretargeted Immuno-PET in CEA Positive Tumors
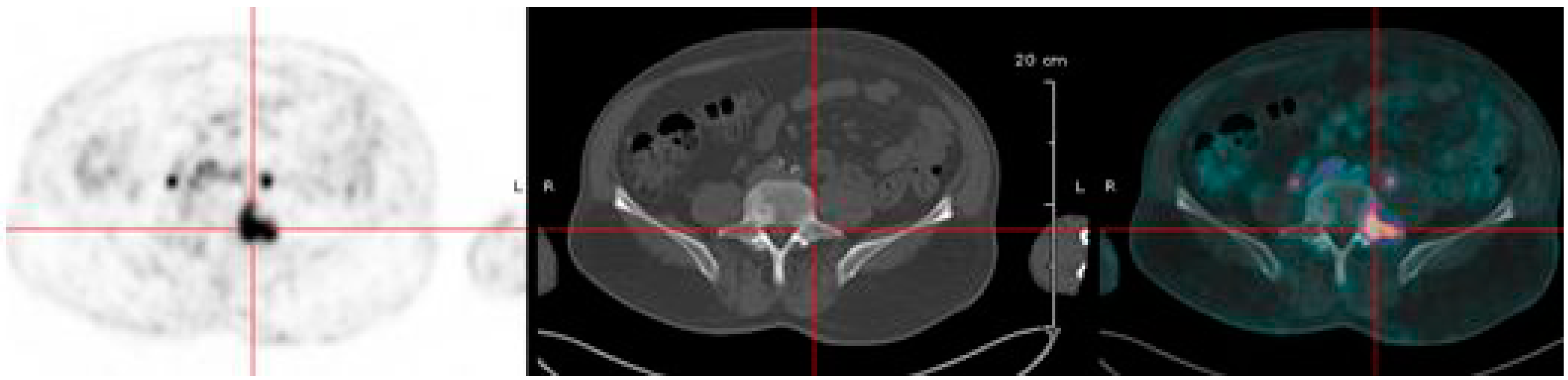
6.3. Immuno-PET of Carbonic Anhydrase IX for Renal Masses Exploration
6.4. Companion Anti-HER2 PET in Breast Cancer (BC)
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldenberg, D.M.; de Land, F.; Kim, E.; Bennett, S.; Primus, F.J.; van Nagell, J.R.; Estes, N.; de Simone, P.; Rayburn, P. Use of Radiolabeled Antibodies to Carcinoembryonic Antigen for the Detection and Localization of Diverse Cancers by External Photoscanning. N. Engl. J. Med. 1978, 298, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, H.; Steplewski, Z.; Herlyn, D.; Herlyn, M. Study of Antibodies against Human Melanoma Produced by Somatic Cell Hybrids. Proc. Natl. Acad. Sci. USA 1978, 75, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Herlyn, M.; Steplewski, Z.; Herlyn, D.; Koprowski, H. Colorectal Carcinoma-Specific Antigen: Detection by Means of Monoclonal Antibodies. Proc. Natl. Acad. Sci. USA 1979, 76, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Khaw, B.A.; Cooney, J.; Edgington, T.; Strauss, H.W. Differences in Experimental Tumor Localization of Dual-Labeled Monoclonal Antibody. J. Nucl. Med. 1986, 27, 1293–1299. [Google Scholar] [PubMed]
- Barbet, J.; Peltier, P.; Bardet, S.; Vuillez, J.P.; Bachelot, I.; Denet, S.; Olivier, P.; Leccia, F.; Corcuff, B.; Huglo, D.; et al. Radioimmunodetection of Medullary Thyroid Carcinoma Using Indium-111 Bivalent Hapten and Anti-CEA X Anti-DTPA-Indium Bispecific Antibody. J. Nucl. Med. 1998, 39, 1172–1178. [Google Scholar]
- Teillaud, J.-L. Engineering of Monoclonal Antibodies and Antibody-Based Fusion Proteins: Successes and Challenges. Expert Opin. Biol. Ther. 2005, 5, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Press, O.W.; Leonard, J.P.; Coiffier, B.; Levy, R.; Timmerman, J. Immunotherapy of Non-Hodgkin’s Lymphomas. Hematol. Am. Soc. Hematol. Educ. Program 2001, 221–240. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar]
- Kaminski, M.S.; Estes, J.; Zasadny, K.R.; Francis, I.R.; Ross, C.W.; Tuck, M.; Regan, D.; Fisher, S.; Gutierrez, J.; Kroll, S.; et al. Radioimmunotherapy with Iodine (131)I Tositumomab for Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma: Updated Results and Long-Term Follow-up of the University of Michigan Experience. Blood 2000, 96, 1259–1266. [Google Scholar]
- Press, O.W.; Eary, J.F.; Gooley, T.; Gopal, A.K.; Liu, S.; Rajendran, J.G.; Maloney, D.G.; Petersdorf, S.; Bush, S.A.; Durack, L.D.; et al. A Phase I/II Trial of Iodine-131-Tositumomab (anti-CD20), Etoposide, Cyclophosphamide, and Autologous Stem Cell Transplantation for Relapsed B-Cell Lymphomas. Blood 2000, 96, 2934–2942. [Google Scholar]
- Kraeber-Bodéré, F.; Bodet-Milin, C.; Rousseau, C.; Eugène, T.; Pallardy, A.; Frampas, E.; Carlier, T.; Ferrer, L.; Gaschet, J.; Davodeau, F.; et al. Radioimmunoconjugates for the Treatment of Cancer. Semin. Oncol. 2014, 41, 613–622. [Google Scholar]
- Boerman, O.C.; Oyen, W.J.G. Immuno-PET of Cancer: A Revival of Antibody Imaging. J. Nucl. Med. 2011, 52, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, G.A.M.S.; Poot, A.J.; Vugts, D.J. PET Imaging with Radiolabeled Antibodies and Tyrosine Kinase Inhibitors: Immuno-PET and TKI-PET. Tumour Biol. 2012, 33, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Barbet, J.; Bardiès, M.; Bourgeois, M.; Chatal, J.-F.; Chérel, M.; Davodeau, F.; Faivre-Chauvet, A.; Gestin, J.-F.; Kraeber-Bodéré, F. Radiolabeled Antibodies for Cancer Imaging and Therapy. Methods Mol. Biol. 2012, 907, 681–697. [Google Scholar]
- Pouget, J.-P.; Navarro-Teulon, I.; Bardiès, M.; Chouin, N.; Cartron, G.; Pèlegrin, A.; Azria, D. Clinical Radioimmunotherapy—The Role of Radiobiology. Nat. Rev. Clin. Oncol. 2011, 8, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing Radiation Inhibition of Distant Untreated Tumors (abscopal Effect) Is Immune Mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Bodet-Milin, C.; Ferrer, L.; Pallardy, A.; Eugène, T.; Rauscher, A.; Faivre-Chauvet, A.; Barbet, J.; Kraeber-Bodéré, F. Radioimmunotherapy of B-Cell Non-Hodgkin’s Lymphoma. Front. Oncol. 2013, 3, 177. [Google Scholar] [CrossRef]
- Liersch, T.; Meller, J.; Kulle, B.; Behr, T.M.; Markus, P.; Langer, C.; Ghadimi, B.M.; Wegener, W.A.; Kovacs, J.; Horak, I.D.; et al. Phase II Trial of Carcinoembryonic Antigen Radioimmunotherapy with 131I-Labetuzumab after Salvage Resection of Colorectal Metastases in the Liver: Five-Year Safety and Efficacy Results. J. Clin. Oncol. 2005, 23, 6763–6770. [Google Scholar]
- Tagawa, S.T.; Beltran, H.; Vallabhajosula, S.; Goldsmith, S.J.; Osborne, J.; Matulich, D.; Petrillo, K.; Parmar, S.; Nanus, D.M.; Bander, N.H. Anti-Prostate-Specific Membrane Antigen-Based Radioimmunotherapy for Prostate Cancer. Cancer 2010, 116, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Sharkey, R.M.; Paganelli, G.; Barbet, J.; Chatal, J.-F. Antibody Pretargeting Advances Cancer Radioimmunodetection and Radioimmunotherapy. J. Clin. Oncol. 2006, 24, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Chatal, J.-F.; Campion, L.; Kraeber-Bodéré, F.; Bardet, S.; Vuillez, J.-P.; Charbonnel, B.; Rohmer, V.; Chang, C.-H.; Sharkey, R.M.; Goldenberg, D.M.; et al. Survival Improvement in Patients with Medullary Thyroid Carcinoma Who Undergo Pretargeted Anti-Carcinoembryonic-Antigen Radioimmunotherapy: A Collaborative Study with the French Endocrine Tumor Group. J. Clin. Oncol. 2006, 24, 1705–1711. [Google Scholar]
- Kraeber-Bodéré, F.; Rousseau, C.; Bodet-Milin, C.; Ferrer, L.; Faivre-Chauvet, A.; Campion, L.; Vuillez, J.-P.; Devillers, A.; Chang, C.-H.; Goldenberg, D.M.; et al. Targeting, Toxicity, and Efficacy of 2-Step, Pretargeted Radioimmunotherapy Using a Chimeric Bispecific Antibody and 131I-Labeled Bivalent Hapten in a Phase I Optimization Clinical Trial. J. Nucl. Med. 2006, 47, 247–255. [Google Scholar]
- Salaun, P.-Y.; Campion, L.; Bournaud, C.; Faivre-Chauvet, A.; Vuillez, J.-P.; Taieb, D.; Ansquer, C.; Rousseau, C.; Borson-Chazot, F.; Bardet, S.; et al. Phase II Trial of Anticarcinoembryonic Antigen Pretargeted Radioimmunotherapy in Progressive Metastatic Medullary Thyroid Carcinoma: Biomarker Response and Survival Improvement. J. Nucl. Med. 2012, 53, 1185–1192. [Google Scholar]
- Schoffelen, R.; Boerman, O.C.; Goldenberg, D.M.; Sharkey, R.M.; van Herpen, C.M.L.; Franssen, G.M.; McBride, W.J.; Chang, C.-H.; Rossi, E.A.; van der Graaf, W.T.A.; et al. Development of an Imaging-Guided CEA-Pretargeted Radionuclide Treatment of Advanced Colorectal Cancer: First Clinical Results. Br. J. Cancer 2013, 109, 934–942. [Google Scholar]
- Ocean, A.J.; Pennington, K.L.; Guarino, M.J.; Sheikh, A.; Bekaii-Saab, T.; Serafini, A.N.; Lee, D.; Sung, M.W.; Gulec, S.A.; Goldsmith, S.J.; et al. Fractionated Radioimmunotherapy with (90) Y-Clivatuzumab Tetraxetan and Low-Dose Gemcitabine Is Active in Advanced Pancreatic Cancer: A Phase 1 Trial. Cancer 2012, 118, 5497–5506. [Google Scholar]
- Chatal, J.-F.; Davodeau, F.; Cherel, M.; Barbet, J. Different Ways to Improve the Clinical Effectiveness of Radioimmunotherapy in Solid Tumors. J. Cancer Res. Ther. 2009, 5, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, J.A.; Bardiès, M.; Wheldon, T.E. Relationships between Tumor Size and Curability for Uniformly Targeted Therapy with Beta-Emitting Radionuclides. J. Nucl. Med. 1995, 36, 1902–1909. [Google Scholar]
- Morschhauser, F.; Radford, J.; van Hoof, A.; Vitolo, U.; Soubeyran, P.; Tilly, H.; Huijgens, P.C.; Kolstad, A.; D’Amore, F.; Gonzalez Diaz, M.; et al. Phase III Trial of Consolidation Therapy with Yttrium-90-Ibritumomab Tiuxetan Compared with No Additional Therapy after First Remission in Advanced Follicular Lymphoma. J. Clin. Oncol. 2008, 26, 5156–5164. [Google Scholar]
- Morschhauser, F.; Radford, J.; van Hoof, A.; Botto, B.; Rohatiner, A.Z.S.; Salles, G.; Soubeyran, P.; Tilly, H.; Bischof-Delaloye, A.; van Putten, W.L.J.; et al. 90Yttrium-Ibritumomab Tiuxetan Consolidation of First Remission in Advanced-Stage Follicular Non-Hodgkin Lymphoma: Updated Results after a Median Follow-up of 7.3 Years from the International, Randomized, Phase III First-LineIndolent Trial. J. Clin. Oncol. 2013, 31, 1977–1983. [Google Scholar]
- Boudousq, V.; Ricaud, S.; Garambois, V.; Bascoul-Mollevi, C.; Boutaleb, S.; Busson, M.; Quenet, F.; Colombo, P.-E.; Bardiès, M.; Kotzki, P.-O.; et al. Brief Intraperitoneal Radioimmunotherapy of Small Peritoneal Carcinomatosis Using High Activities of Noninternalizing 125I-Labeled Monoclonal Antibodies. J. Nucl. Med. 2010, 51, 1748–1755. [Google Scholar]
- Santoro, L.; Boutaleb, S.; Garambois, V.; Bascoul-Mollevi, C.; Boudousq, V.; Kotzki, P.-O.; Pèlegrin, M.; Navarro-Teulon, I.; Pèlegrin, A.; Pouget, J.-P. Noninternalizing Monoclonal Antibodies Are Suitable Candidates for 125I Radioimmunotherapy of Small-Volume Peritoneal Carcinomatosis. J. Nucl. Med. 2009, 50, 2033–2041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (abridged): Radiobiology and Dosimetry of Alpha-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar]
- Medvedev, D.G.; Mausner, L.F.; Meinken, G.E.; Kurczak, S.O.; Schnakenberg, H.; Dodge, C.J.; Korach, E.M.; Srivastava, S.C. Development of a Large Scale Production of 67Cu from 68Zn at the High Energy Proton Accelerator: Closing the 68Zn Cycle. Appl. Radiat. Isot. 2012, 70, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Chérel, M.; Davodeau, F.; Kraeber-Bodéré, F.; Chatal, J.F. Current Status and Perspectives in Alpha Radioimmunotherapy. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 322–329. [Google Scholar]
- Fraker, P.J.; Speck, J.C., Jr. Protein and Cell Membrane Iodinations with a Sparingly Soluble Chloroamide, 1,3,4,6-Tetrachloro-3a,6a-Diphrenylglycoluril. Biochem. Biophys. Res. Commun. 1978, 80, 849–857. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Jestin, E.; Olafsen, T.; Wu, A.M.; Zalutsky, M.R. Evaluation of an Anti-p185HER2 (scFv-CH2-CH3)2 Fragment Following Radioiodination Using Two Different Residualizing Labels: SGMIB and IB-Mal-d-GEEEK. Nucl. Med. Biol. 2009, 36, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Brechbiel, M.W. Bifunctional Chelates for Metal Nuclides. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 166–173. [Google Scholar] [PubMed]
- O’Donnell, R.T.; DeNardo, S.J.; Miers, L.A.; Lamborn, K.R.; Kukis, D.L.; DeNardo, G.L.; Meyers, F.J. Combined Modality Radioimmunotherapy for Human Prostate Cancer Xenografts with Taxanes and 90yttrium-DOTA-Peptide-ChL6. Prostate 2002, 50, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Richman, C.M.; Denardo, S.J.; O’Donnell, R.T.; Yuan, A.; Shen, S.; Goldstein, D.S.; Tuscano, J.M.; Wun, T.; Chew, H.K.; Lara, P.N.; et al. High-Dose Radioimmunotherapy Combined with Fixed, Low-Dose Paclitaxel in Metastatic Prostate and Breast Cancer by Using a MUC-1 Monoclonal Antibody, m170, Linked to Indium-111/yttrium-90 via a Cathepsin Cleavable Linker with Cyclosporine to Prevent Human Anti-Mouse Antibody. Clin. Cancer Res. 2005, 11, 5920–5927. [Google Scholar]
- Meredith, R.F.; Bueschen, A.J.; Khazaeli, M.B.; Plott, W.E.; Grizzle, W.E.; Wheeler, R.H.; Schlom, J.; Russell, C.D.; Liu, T.; LoBuglio, A.F. Treatment of Metastatic Prostate Carcinoma with Radiolabeled Antibody CC49. J. Nucl. Med. 1994, 35, 1017–1022. [Google Scholar] [PubMed]
- Sokoloff, R.L.; Norton, K.C.; Gasior, C.L.; Marker, K.M.; Grauer, L.S. A Dual-Monoclonal Sandwich Assay for Prostate-Specific Membrane Antigen: Levels in Tissues, Seminal Fluid and Urine. Prostate 2000, 43, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.L., Jr.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of Prostate-Specific Membrane Antigen in Normal, Benign, and Malignant Prostate Tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Israeli, R.S.; Powell, C.T.; Corr, J.G.; Fair, W.R.; Heston, W.D. Expression of the Prostate-Specific Membrane Antigen. Cancer Res. 1994, 54, 1807–1811. [Google Scholar] [PubMed]
- Israeli, R.S.; Powell, C.T.; Fair, W.R.; Heston, W.D. Molecular Cloning of a Complementary DNA Encoding a Prostate-Specific Membrane Antigen. Cancer Res. 1993, 53, 227–230. [Google Scholar] [PubMed]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal Antibodies to a New Antigenic Marker in Epithelial Prostatic Cells and Serum of Prostatic Cancer Patients. Anticancer Res. 1987, 7, 927–935. [Google Scholar] [PubMed]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate Specific Membrane Antigen Expression in Prostatic Intraepithelial Neoplasia and Adenocarcinoma: A Study of 184 Cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Rajasekaran, A.K.; Moy, P.; Xia, Y.; Kim, S.; Navarro, V.; Rahmati, R.; Bander, N.H. Constitutive and Antibody-Induced Internalization of Prostate-Specific Membrane Antigen. Cancer Res. 1998, 58, 4055–4060. [Google Scholar] [PubMed]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal Antibodies to the Extracellular Domain of Prostate-Specific Membrane Antigen Also React with Tumor Vascular Endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar] [PubMed]
- Bander, N.H.; Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J. Phase I Trial of 177lutetium-Labeled J591, a Monoclonal Antibody to Prostate-Specific Membrane Antigen, in Patients with Androgen-Independent Prostate Cancer. J. Clin. Oncol. 2005, 23, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II Study of Lutetium-177-Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar]
- Rossi, E.A.; Goldenberg, D.M.; Cardillo, T.M.; McBride, W.J.; Sharkey, R.M.; Chang, C.-H. Stably Tethered Multifunctional Structures of Defined Composition Made by the Dock and Lock Method for Use in Cancer Targeting. Proc. Natl. Acad. Sci. USA 2006, 103, 6841–6846. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.M.; McBride, W.J.; Karacay, H.; Chang, K.; Griffiths, G.L.; Hansen, H.J.; Goldenberg, D.M. A Universal Pretargeting System for Cancer Detection and Therapy Using Bispecific Antibody. Cancer Res. 2003, 63, 354–363. [Google Scholar] [PubMed]
- McBride, W.J.; Zanzonico, P.; Sharkey, R.M.; Norén, C.; Karacay, H.; Rossi, E.A.; Losman, M.J.; Brard, P.-Y.; Chang, C.-H.; Larson, S.M.; et al. Bispecific Antibody Pretargeting PET (immunoPET) with an 124I-Labeled Hapten-Peptide. J. Nucl. Med. 2006, 47, 1678–1688. [Google Scholar]
- McBride, W.J.; Sharkey, R.M.; Karacay, H.; D’Souza, C.A.; Rossi, E.A.; Laverman, P.; Chang, C.-H.; Boerman, O.C.; Goldenberg, D.M. A Novel Method of 18F Radiolabeling for PET. J. Nucl. Med. 2009, 50, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; van der Graaf, W.T.A.; Franssen, G.; Sharkey, R.M.; Goldenberg, D.M.; McBride, W.J.; Rossi, E.A.; Eek, A.; Oyen, W.J.G.; Boerman, O.C. Pretargeted 177Lu Radioimmunotherapy of Carcinoembryonic Antigen-Expressing Human Colonic Tumors in Mice. J. Nucl. Med. 2010, 51, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; van der Weg, W.; Visser, E.P.; Goldenberg, D.M.; Sharkey, R.M.; McBride, W.J.; Chang, C.-H.; Rossi, E.A.; van der Graaf, W.T.A.; Oyen, W.J.G.; et al. Predictive Patient-Specific Dosimetry and Individualized Dosing of Pretargeted Radioimmunotherapy in Patients with Advanced Colorectal Cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1593–1602. [Google Scholar]
- Frampas, E.; Maurel, C.; Remaud-Le Saëc, P.; Mauxion, T.; Faivre-Chauvet, A.; Davodeau, F.; Goldenberg, D.M.; Bardiès, M.; Barbet, J. Pretargeted Radioimmunotherapy of Colorectal Cancer Metastases: Models and Pharmacokinetics Predict Influence of the Physical and Radiochemical Properties of the Radionuclide. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, S.J.; DeNardo, G.L.; Kukis, D.L.; Shen, S.; Kroger, L.A.; DeNardo, D.A.; Goldstein, D.S.; Mirick, G.R.; Salako, Q.; Mausner, L.F.; et al. 67Cu-2IT-BAT-Lym-1 Pharmacokinetics, Radiation Dosimetry, Toxicity and Tumor Regression in Patients with Lymphoma. J. Nucl. Med. 1999, 40, 302–310. [Google Scholar]
- DeNardo, G.L.; DeNardo, S.J.; O’Donnell, R.T.; Kroger, L.A.; Kukis, D.L.; Meares, C.F.; Goldstein, D.S.; Shen, S. Are Radiometal-Labeled Antibodies Better than Iodine-131-Labeled Antibodies: Comparative Pharmacokinetics and Dosimetry of Copper-67-, Iodine-131-, and Yttrium-90-Labeled Lym-1 Antibody in Patients with Non-Hodgkin’s Lymphoma. Clin. Lymphoma 2000, 1, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, A.B.; Delaloye, B.; Buchegger, F.; Vogel, C.A.; Gillet, M.; Mach, J.P.; Smith, A.; Schubiger, P.A. Comparison of Copper-67- and Iodine-125-Labeled Anti-CEA Monoclonal Antibody Biodistribution in Patients with Colorectal Tumors. J. Nucl. Med. 1997, 38, 847–853. [Google Scholar] [PubMed]
- Pietrelli, L.; Mausner, L.F.; Kolsky, K.L. Separation of Carrier-Free Sc-47 from Titanium Targets. J. Radioanal. Nucl. Chem. 1992, 157, 335–345. [Google Scholar] [CrossRef]
- Müller, C.; Bunka, M.; Reber, J.; Fischer, C.; Zhernosekov, K.; Türler, A.; Schibli, R. Promises of Cyclotron-Produced 44Sc as a Diagnostic Match for Trivalent Β--Emitters: In vitro and in vivo Study of a 44Sc-DOTA-Folate Conjugate. J. Nucl. Med. 2013, 54, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; van der Walt, N.T.; Türler, A.; Schibli, R. A Unique Matched Quadruplet of Terbium Radioisotopes for PET and SPECT and for Α- and Β- Radionuclide Therapy: An in vivo Proof-of-Concept Study with a New Receptor-Targeted Folate Derivative. J. Nucl. Med. 2012, 53, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, A.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted Alpha Particle Immunotherapy for Myeloid Leukemia. Blood 2002, 100, 1233–1239. [Google Scholar]
- Chérel, M.; Gouard, S.; Gaschet, J.; Saï-Maurel, C.; Bruchertseifer, F.; Morgenstern, A.; Bourgeois, M.; Gestin, J.-F.; Bodéré, F.K.; Barbet, J.; et al. 213Bi Radioimmunotherapy with an Anti-mCD138 Monoclonal Antibody in a Murine Model of Multiple Myeloma. J. Nucl. Med. 2013, 54, 1597–1604. [Google Scholar]
- Supiot, S.; Faivre-Chauvet, A.; Couturier, O.; Heymann, M.F.; Robillard, N.; Kraeber-Bodéré, F.; Morandeau, L.; Mahé, M.A.; Chérel, M. Comparison of the Biologic Effects of MA5 and B-B4 Monoclonal Antibody Labeled with Iodine-131 and Bismuth-213 on Multiple Myeloma. Cancer 2002, 94, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.R.; Ma, D.; Lai, L.T.; Simon, J.; Borchardt, P.; Frank, R.K.; Wu, K.; Pellegrini, V.; Curcio, M.J.; Miederer, M.; et al. Tumor Therapy with Targeted Atomic Nanogenerators. Science 2001, 294, 1537–1540. [Google Scholar]
- Miederer, M.; McDevitt, M.R.; Sgouros, G.; Kramer, K.; Cheung, N.-K.V.; Scheinberg, D.A. Pharmacokinetics, Dosimetry, and Toxicity of the Targetable Atomic Generator, 225Ac-HuM195, in Nonhuman Primates. J. Nucl. Med. 2004, 45, 129–137. [Google Scholar] [PubMed]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M. Dose Escalation and Dosimetry of First-in-Human Α Radioimmunotherapy with 212Pb-TCMC-Trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and Imaging of 212Pb-TCMC-Trastuzumab after Intraperitoneal Administration in Ovarian Cancer Patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Jaggi, J.S.; O’Donoghue, J.A.; Ruan, S.; McDevitt, M.; Larson, S.M.; Scheinberg, D.A.; Humm, J.L. Renal Uptake of Bismuth-213 and Its Contribution to Kidney Radiation Dose Following Administration of Actinium-225-Labeled Antibody. Phys. Med. Biol. 2011, 56, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Heyerdahl, H.; Abbas, N.; Sponheim, K.; Mollatt, C.; Bruland, Ø.; Dahle, J. Targeted Alpha Therapy with 227Th-Trastuzumab of Intraperitoneal Ovarian Cancer in Nude Mice. Curr. Radiopharm. 2013, 6, 106–116. [Google Scholar]
- Boudousq, V.; Bobyk, L.; Busson, M.; Garambois, V.; Jarlier, M.; Charalambatou, P.; Pèlegrin, A.; Paillas, S.; Chouin, N.; Quenet, F.; et al. Comparison between Internalizing Anti-HER2 mAbs and Non-Internalizing Anti-CEA mAbs in Alpha-Radioimmunotherapy of Small Volume Peritoneal Carcinomatosis Using 212Pb. PLoS One 2013, 8, e69613. [Google Scholar]
- Zalutsky, M.R.; Garg, P.K.; Friedman, H.S.; Bigner, D.D. Labeling Monoclonal Antibodies and F(ab’)2 Fragments with the Alpha-Particle-Emitting Nuclide Astatine-211: Preservation of Immunoreactivity and in vivo Localizing Capacity. Proc. Natl. Acad. Sci. USA 1989, 86, 7149–7153. [Google Scholar] [CrossRef] [PubMed]
- Reist, C.J.; Foulon, C.F.; Alston, K.; Bigner, D.D.; Zalutsky, M.R. Astatine-211 Labeling of Internalizing Anti-EGFRvIII Monoclonal Antibody Using N-Succinimidyl 5-[211At]astato-3-Pyridinecarboxylate. Nucl. Med. Biol. 1999, 26, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.J.; Bäck, T.; Kenoyer, A.; Balkin, E.R.; Hamlin, D.K.; Wilbur, D.S.; Fisher, D.R.; Frayo, S.L.; Hylarides, M.D.; Green, D.J.; et al. Anti-CD45 Radioimmunotherapy Using 211At with Bone Marrow Transplantation Prolongs Survival in a Disseminated Murine Leukemia Model. Blood 2013, 121, 3759–3767. [Google Scholar]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical Experience with Alpha-Particle Emitting 211At: Treatment of Recurrent Brain Tumor Patients with 211At-Labeled Chimeric Antitenascin Monoclonal Antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Cederkrantz, E.; Bäck, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal Alpha-Particle Radioimmunotherapy of Ovarian Cancer Patients: Pharmacokinetics and Dosimetry of (211)At-MX35 F(ab’)2—A Phase I Study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar]
- Stute, S.; Benoit, D.; Martineau, A.; Rehfeld, N.S.; Buvat, I. A Method for Accurate Modelling of the Crystal Response Function at a Crystal Sub-Level Applied to PET Reconstruction. Phys. Med. Biol. 2011, 56, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Moses, W.W. Recent Advances and Future Advances in Time-of-Flight PET. Nucl. Instrum. Methods Phys. Res. A 2007, 580, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Lewellen, T.K. Recent Developments in PET Detector Technology. Phys. Med. Biol. 2008, 53, R287–R317. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.M.; Wu, A.M. Advances in Immuno-Positron Emission Tomography: Antibodies for Molecular Imaging in Oncology. J. Clin. Oncol. 2012, 30, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, G.A. M.S.; Visser, G.W.M.; Lub-de Hooge, M.N.; de Vries, E.G.; Perk, L.R. Immuno-PET: A Navigator in Monoclonal Antibody Development and Applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Heskamp, S.; van Laarhoven, H.W.M.; Molkenboer-Kuenen, J.D.M.; Franssen, G.M.; Versleijen-Jonkers, Y.M.H.; Oyen, W.J.G.; van der Graaf, W.T.A.; Boerman, O.C. ImmunoSPECT and immunoPET of IGF-1R Expression with the Radiolabeled Antibody R1507 in a Triple-Negative Breast Cancer Model. J. Nucl. Med. 2010, 51, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Prasad, V.; Müller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular Imaging of HER2-Expressing Malignant Tumors in Breast Cancer Patients Using Synthetic 111In- or 68Ga-Labeled Affibody Molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; Sharkey, R.M.; Goldenberg, D.M.; Franssen, G.; McBride, W.J.; Rossi, E.A.; Chang, C.-H.; Laverman, P.; Disselhorst, J.A.; Eek, A.; et al. Pretargeted Immuno-Positron Emission Tomography Imaging of Carcinoembryonic Antigen-Expressing Tumors with a Bispecific Antibody and a 68Ga- and 18F-Labeled Hapten Peptide in Mice with Human Tumor Xenografts. Mol. Cancer Ther. 2010, 9, 1019–1027. [Google Scholar]
- Börjesson, P.K.E.; Jauw, Y.W.S.; Boellaard, R.; de Bree, R.; Comans, E.F.I.; Roos, J.C.; Castelijns, J.A.; Vosjan, M.J.W.D.; Kummer, J.A.; Leemans, C.R.; et al. Performance of Immuno-Positron Emission Tomography with Zirconium-89-Labeled Chimeric Monoclonal Antibody U36 in the Detection of Lymph Node Metastases in Head and Neck Cancer Patients. Clin. Cancer Res. 2006, 12, 2133–2140. [Google Scholar]
- Perk, L.R.; Stigter-van Walsum, M.; Visser, G.W.M.; Kloet, R.W.; Vosjan, M.J.W.D.; Leemans, C.R.; Giaccone, G.; Albano, R.; Comoglio, P.M.; van Dongen, G.A.M.S. Quantitative PET Imaging of Met-Expressing Human Cancer Xenografts with 89Zr-Labelled Monoclonal Antibody DN30. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Divgi, C.R.; Uzzo, R.G.; Gatsonis, C.; Bartz, R.; Treutner, S.; Yu, J.Q.; Chen, D.; Carrasquillo, J.A.; Larson, S.; Bevan, P.; et al. Positron Emission Tomography/computed Tomography Identification of Clear Cell Renal Cell Carcinoma: Results from the REDECT Trial. J. Clin. Oncol. 2013, 31, 187–194. [Google Scholar]
- Divgi, C.R.; Pandit-Taskar, N.; Jungbluth, A.A.; Reuter, V.E.; Gönen, M.; Ruan, S.; Pierre, C.; Nagel, A.; Pryma, D.A.; Humm, J.; et al. Preoperative Characterisation of Clear-Cell Renal Carcinoma Using Iodine-124-Labelled Antibody Chimeric G250 (124I-cG250) and PET in Patients with Renal Masses: A Phase I Trial. Lancet Oncol. 2007, 8, 304–310. [Google Scholar]
- Pryma, D.A.; O’Donoghue, J.A.; Humm, J.L.; Jungbluth, A.A.; Old, L.J.; Larson, S.M.; Divgi, C.R. Correlation of in vivo and in vitro Measures of Carbonic Anhydrase IX Antigen Expression in Renal Masses Using Antibody 124I-cG250. J. Nucl. Med. 2011, 52, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-Trastuzumab PET Imaging in Patients with HER2-Positive Breast Cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar]
- Cheal, S.M.; Punzalan, B.; Doran, M.G.; Evans, M.J.; Osborne, J.R.; Lewis, J.S.; Zanzonico, P.; Larson, S.M. Pairwise Comparison of 89Zr- and 124I-Labeled cG250 Based on Positron Emission Tomography Imaging and Nonlinear Immunokinetic Modeling: In vivo Carbonic Anhydrase IX Receptor Binding and Internalization in Mouse Xenografts of Clear-Cell Renal Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Schwartz, L.H.; Larson, S.M. Imaging Surrogates of Tumor Response to Therapy: Anatomic and Functional Biomarkers. J. Nucl. Med. 2009, 50, 239–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoeben, B.A.W.; Kaanders, J.H.A.M.; Franssen, G.M.; Troost, E.G.C.; Rijken, P.F.J.W.; Oosterwijk, E.; van Dongen, G.A.M.S.; Oyen, W.J.G.; Boerman, O.C.; Bussink, J. PET of Hypoxia with 89Zr-Labeled cG250-F(ab’)2 in Head and Neck Tumors. J. Nucl. Med. 2010, 51, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-Trastuzumab and PET Imaging of HER2-Positive Lesions in Patients with Metastatic Breast Cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional Imaging of Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Using (64)Cu-DOTA-Trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraeber-Bodéré, F.; Rousseau, C.; Bodet-Milin, C.; Mathieu, C.; Guérard, F.; Frampas, E.; Carlier, T.; Chouin, N.; Haddad, F.; Chatal, J.-F.; et al. Tumor Immunotargeting Using Innovative Radionuclides. Int. J. Mol. Sci. 2015, 16, 3932-3954. https://doi.org/10.3390/ijms16023932
Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, Mathieu C, Guérard F, Frampas E, Carlier T, Chouin N, Haddad F, Chatal J-F, et al. Tumor Immunotargeting Using Innovative Radionuclides. International Journal of Molecular Sciences. 2015; 16(2):3932-3954. https://doi.org/10.3390/ijms16023932
Chicago/Turabian StyleKraeber-Bodéré, Françoise, Caroline Rousseau, Caroline Bodet-Milin, Cédric Mathieu, François Guérard, Eric Frampas, Thomas Carlier, Nicolas Chouin, Ferid Haddad, Jean-François Chatal, and et al. 2015. "Tumor Immunotargeting Using Innovative Radionuclides" International Journal of Molecular Sciences 16, no. 2: 3932-3954. https://doi.org/10.3390/ijms16023932
APA StyleKraeber-Bodéré, F., Rousseau, C., Bodet-Milin, C., Mathieu, C., Guérard, F., Frampas, E., Carlier, T., Chouin, N., Haddad, F., Chatal, J.-F., Faivre-Chauvet, A., Chérel, M., & Barbet, J. (2015). Tumor Immunotargeting Using Innovative Radionuclides. International Journal of Molecular Sciences, 16(2), 3932-3954. https://doi.org/10.3390/ijms16023932





