CXCL13 Promotes the Effect of Bone Marrow Mesenchymal Stem Cells (MSCs) on Tendon-Bone Healing in Rats and in C3HIOT1/2 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
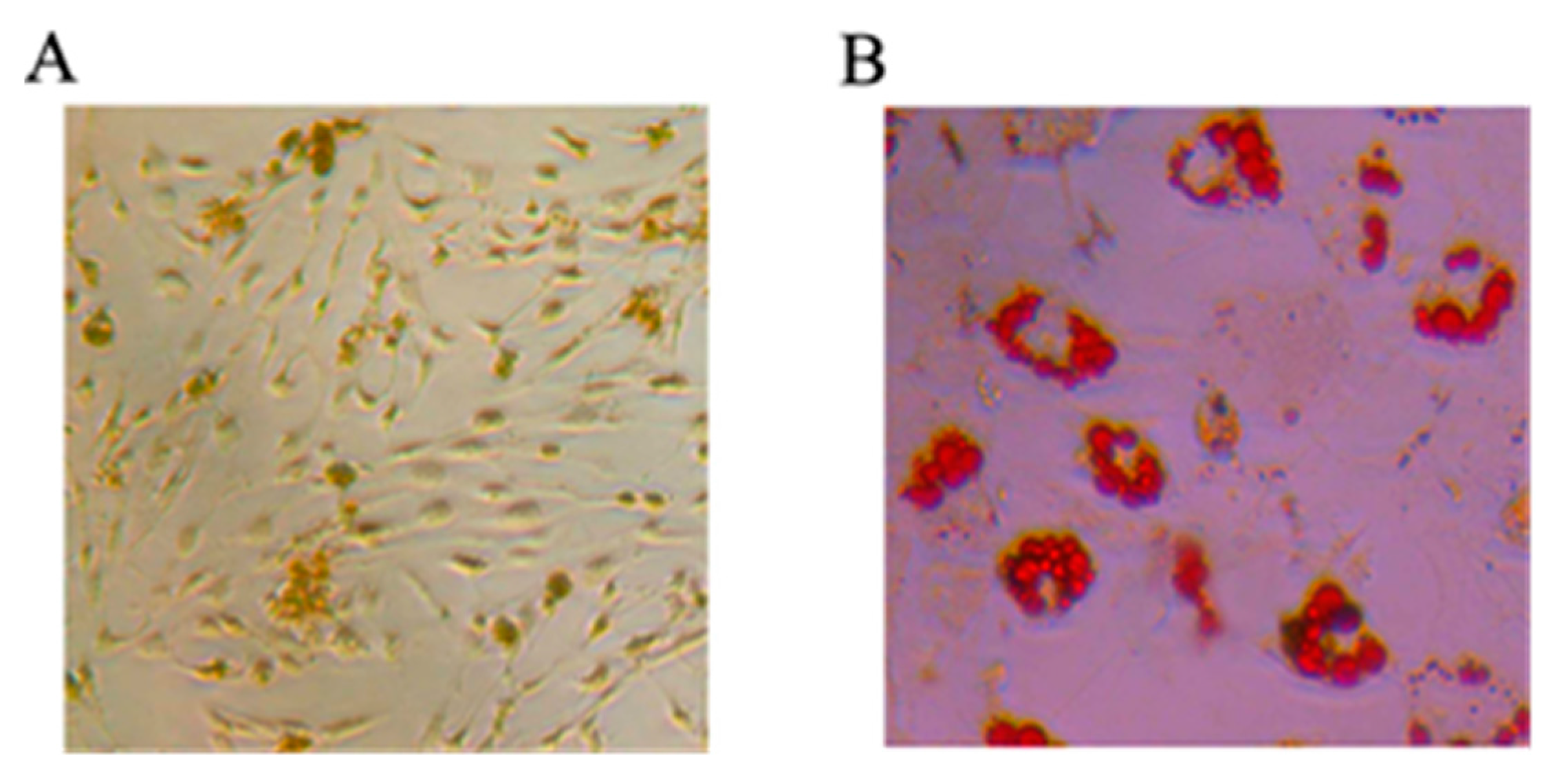
2.1.1. Mesenchymal Stem Cells Differentiating into Osteoblasts and Adipocytes
2.1.2. Biomechanical Assessment
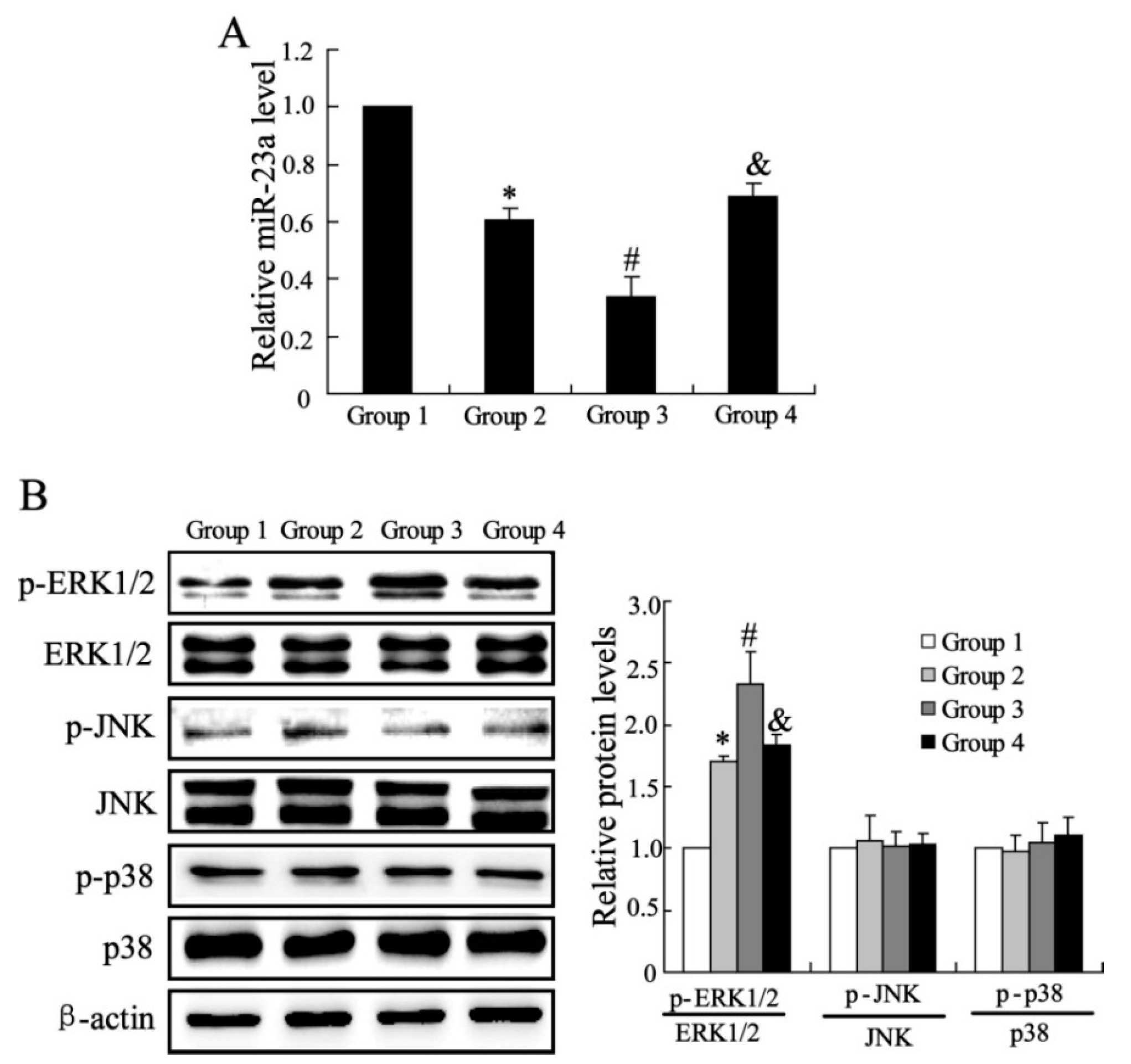
2.1.3. The Effects of CXCL13 on miR-23a and MAPK Molecules Expression in Vivo

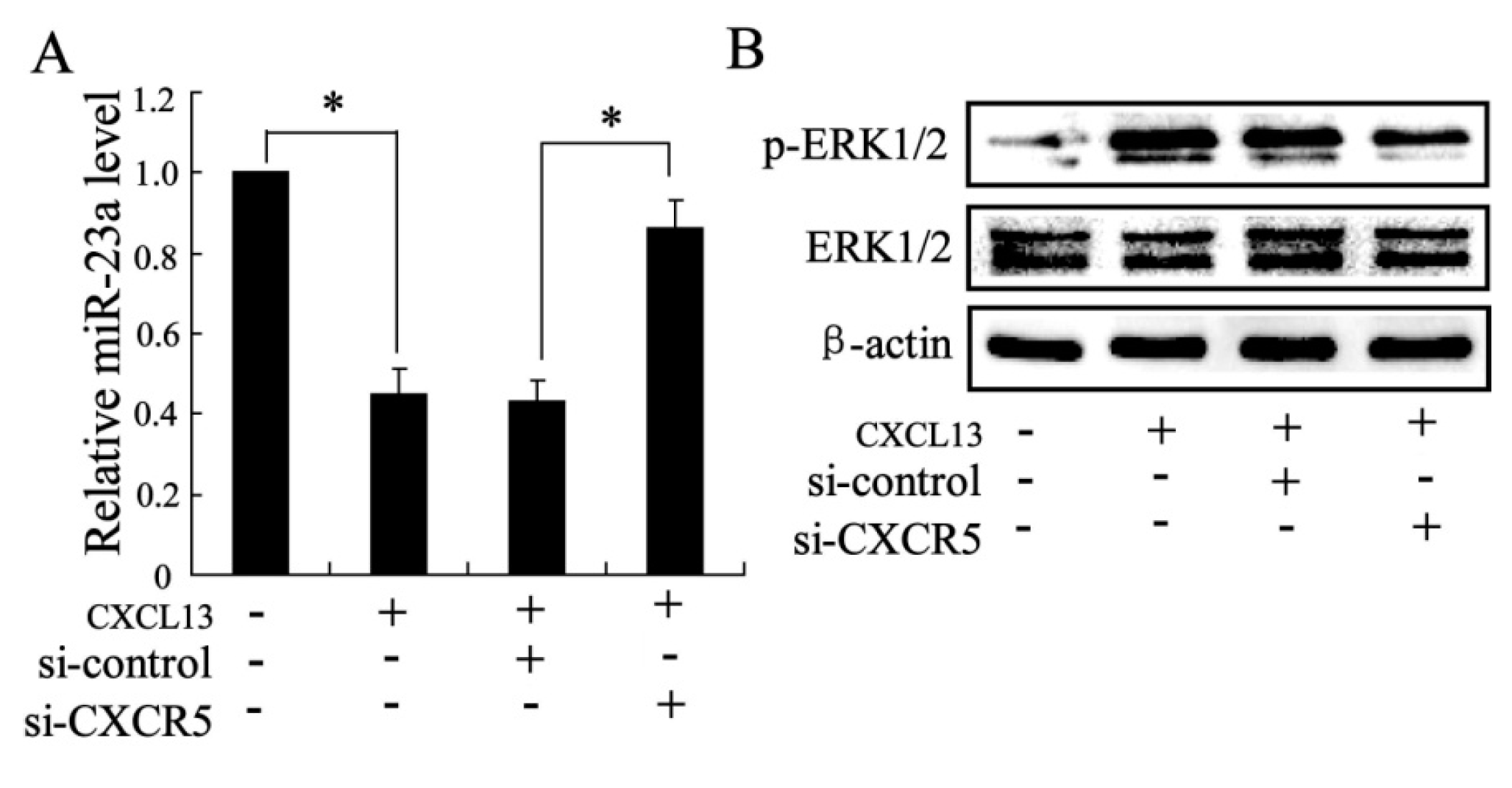
2.1.4. The Effects of CXCL13 on miR-23a and MAPK Molecules Expression in Vitro
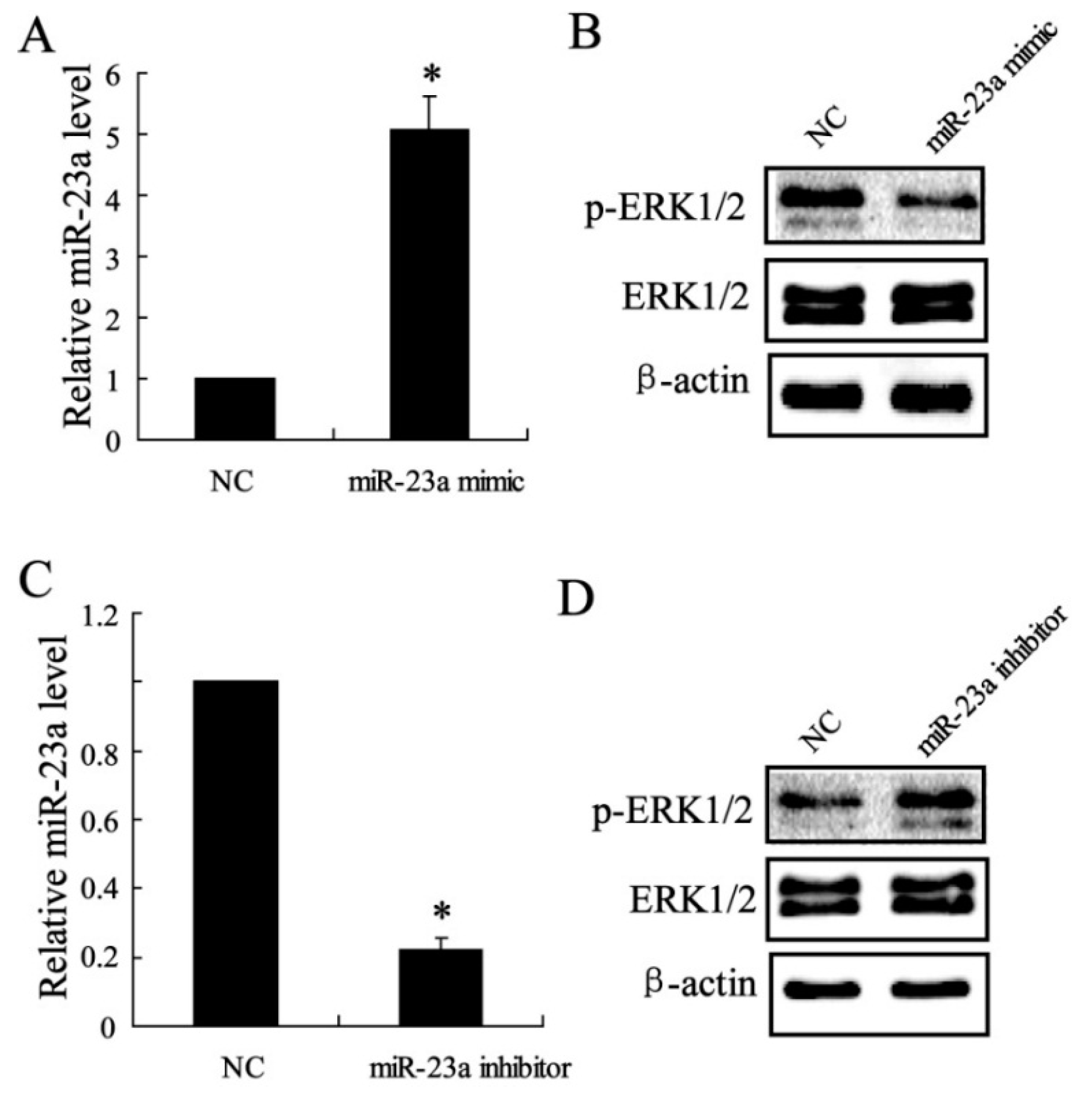
2.1.5. The Effect of miR-23a on ERK1/2 Expression in Vitro
2.2. Discussion
3. Experimental Section
3.1. Isolation and Culture of MSCs
3.2. Tendon-Bone Healing Model of Rat
3.3. Biomechanical Testing
3.4. Cell Culture
3.5. Real-Time PCR
3.6. Western Blotting
3.7. Overexpression and Down-Regulation of miR-23a
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Li, M.; Ikehara, S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013, 2013. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar]
- Rui, Y.F.; Lui, P.P.; Lee, Y.W.; Chan, K.M. Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. Int. Orthop. 2012, 36, 1099–1107. [Google Scholar]
- Backesjo, C.M.; Li, Y.; Lindgren, U.; Haldosen, L.A. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J. Bone Miner. Res. 2006, 21, 993–1002. [Google Scholar]
- Zhou, S.B.; Wang, J.; Chiang, C.A.; Sheng, L.L.; Li, Q.F. Mechanical stretch upregulates SDF-1α in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells 2013, 31, 2703–2713. [Google Scholar]
- Singh, S.; Singh, R.; Sharma, P.K.; Singh, U.P.; Rai, S.N.; Chung, L.W.; Cooper, C.R.; Novakovic, K.R.; Grizzle, W.E.; Lillard, J.W., Jr. Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Lett. 2009, 283, 29–35. [Google Scholar]
- Judson, R.L.; Babiarz, J.E.; Venere, M.; Blelloch, R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat. Biotechnol. 2009, 27, 459–461. [Google Scholar]
- Gruber, A.J.; Grandy, W.A.; Balwierz, P.J.; Dimitrova, Y.A.; Pachkov, M.; Ciaudo, C.; van Nimwegen, E.; Zavolan, M. Embryonic stem cell-specific microRNAs contribute to pluripotency by inhibiting regulators of multiple differentiation pathways. Nucleic Acids Res. 2014, 42, 9313–9326. [Google Scholar]
- Mao, J.; Lv, Z.; Zhuang, Y. MicroRNA-23a is involved in tumor necrosis factor-α induced apoptosis in mesenchymal stem cells and myocardial infarction. Exp. Mol. Pathol. 2014, 97, 23–30. [Google Scholar]
- Nie, Y.; Han, B.M.; Liu, X.B.; Yang, J.J.; Wang, F.; Cong, X.F.; Chen, X. Identification of microRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int. J. Biol. Sci. 2011, 7, 762–768. [Google Scholar]
- Zhang, Y.; Xie, R.L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar]
- Carcamo-Orive, I.; Tejados, N.; Delgado, J.; Gaztelumendi, A.; Otaegui, D.; Lang, V.; Trigueros, C. ERK2 protein regulates the proliferation of human mesenchymal stem cells without affecting their mobilization and differentiation potential. Exp. Cell Res. 2008, 314, 1777–1788. [Google Scholar]
- Jaiswal, R.K.; Jaiswal, N.; Bruder, S.P.; Mbalaviele, G.; Marshak, D.R.; Pittenger, M.F. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 2000, 275, 9645–9652. [Google Scholar]
- Fu, L.; Tang, T.; Miao, Y.; Zhang, S.; Qu, Z.; Dai, K. Stimulation of osteogenic differentiation and inhibition of adipogenic differentiation in bone marrow stromal cells by alendronate via ERK and JNK activation. Bone 2008, 43, 40–47. [Google Scholar]
- Chang, J.; Sonoyama, W.; Wang, Z.; Jin, Q.; Zhang, C.; Krebsbach, P.H.; Giannobile, W.; Shi, S.; Wang, C.Y. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J. Biol. Chem. 2007, 282, 30938–30948. [Google Scholar]
- Smith, R.K.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One 2013, 8, e75697. [Google Scholar]
- Takano, T.; Li, Y.J.; Kukita, A.; Yamaza, T.; Ayukawa, Y.; Moriyama, K.; Uehara, N.; Nomiyama, H.; Koyano, K.; Kukita, T. Mesenchymal stem cells markedly suppress inflammatory bone destruction in rats with adjuvant-induced arthritis. Lab. Investig. 2014, 94, 286–296. [Google Scholar]
- Phipps, M.C.; Xu, Y.; Bellis, S.L. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PLoS One 2012, 7, e40831. [Google Scholar]
- Shahnazari, M.; Chu, V.; Wronski, T.J.; Nissenson, R.A.; Halloran, B.P. CXCL12/CXCR4 signaling in the osteoblast regulates the mesenchymal stem cell and osteoclast lineage populations. FASEB J. 2013, 27, 3505–3513. [Google Scholar]
- Hwang, J.H.; Shim, S.S.; Seok, O.S.; Lee, H.Y.; Woo, S.K.; Kim, B.H.; Song, H.R.; Lee, J.K.; Park, Y.K. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J. Korean Med. Sci. 2009, 24, 547–554. [Google Scholar]
- Lisignoli, G.; Cristino, S.; Toneguzzi, S.; Grassi, F.; Piacentini, A.; Cavallo, C.; Facchini, A.; Mariani, E. IL1β and TNFα differently modulate CXCL13 chemokine in stromal cells and osteoblasts isolated from osteoarthritis patients: Evidence of changes associated to cell maturation. Exp. Gerontol. 2004, 39, 659–665. [Google Scholar]
- Donzelli, E.; Lucchini, C.; Ballarini, E.; Scuteri, A.; Carini, F.; Tredici, G.; Miloso, M. ERK1 and ERK2 are involved in recruitment and maturation of human mesenchymal stem cells induced to adipogenic differentiation. J. Mol. Cell Biol. 2011, 3, 123–131. [Google Scholar]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar]
- Liu, Q.; Cen, L.; Zhou, H.; Yin, S.; Liu, G.; Liu, W.; Cao, Y.; Cui, L. The role of the extracellular signal-related kinase signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng. Part A 2009, 15, 3487–3497. [Google Scholar]
- Salasznyk, R.M.; Klees, R.F.; Hughlock, M.K.; Plopper, G.E. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun. Adhes. 2004, 11, 137–153. [Google Scholar]
- Zhao, S.; Peng, L.; Xie, G.; Li, D.; Zhao, J.; Ning, C. Effect of the interposition of calcium phosphate materials on tendon-bone healing during repair of chronic rotator cuff tear. Am. J. Sports Med. 2014, 42, 1920–1929. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Ji, X.-L.; Xiao, W.-A.; Wang, B.; Wang, F. CXCL13 Promotes the Effect of Bone Marrow Mesenchymal Stem Cells (MSCs) on Tendon-Bone Healing in Rats and in C3HIOT1/2 Cells. Int. J. Mol. Sci. 2015, 16, 3178-3187. https://doi.org/10.3390/ijms16023178
Tian F, Ji X-L, Xiao W-A, Wang B, Wang F. CXCL13 Promotes the Effect of Bone Marrow Mesenchymal Stem Cells (MSCs) on Tendon-Bone Healing in Rats and in C3HIOT1/2 Cells. International Journal of Molecular Sciences. 2015; 16(2):3178-3187. https://doi.org/10.3390/ijms16023178
Chicago/Turabian StyleTian, Feng, Xiang-Lu Ji, Wan-An Xiao, Bin Wang, and Fei Wang. 2015. "CXCL13 Promotes the Effect of Bone Marrow Mesenchymal Stem Cells (MSCs) on Tendon-Bone Healing in Rats and in C3HIOT1/2 Cells" International Journal of Molecular Sciences 16, no. 2: 3178-3187. https://doi.org/10.3390/ijms16023178




