IgG and IgA with Potential Microbial-Binding Activity Are Expressed by Normal Human Skin Epidermal Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immunoglobulin G (IgG) and Immunoglobulin A (IgA) Staining in Normal Human Epidermis
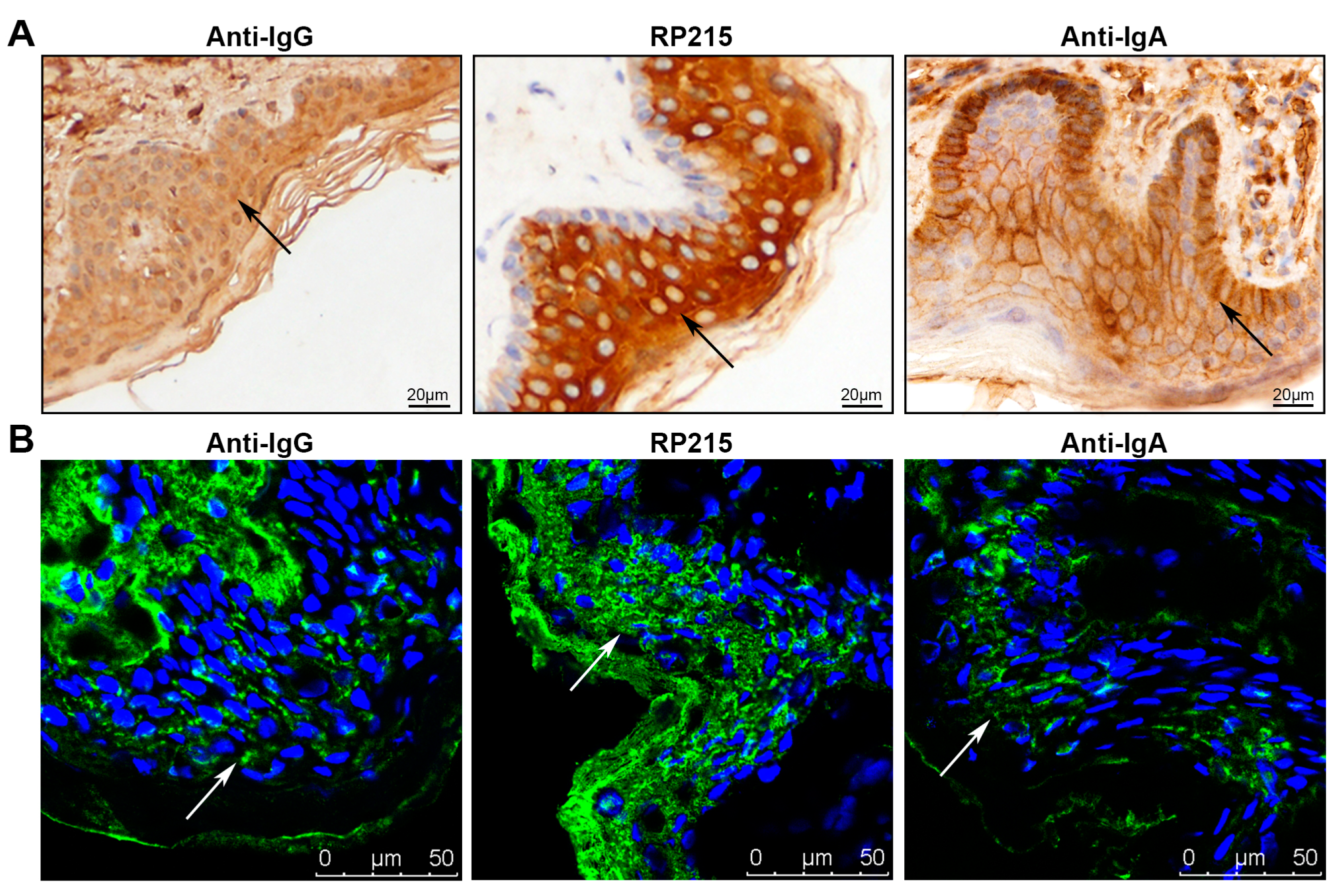
2.2. IgG and IgA in Epidermal Cells Confirmed by Western Blotting
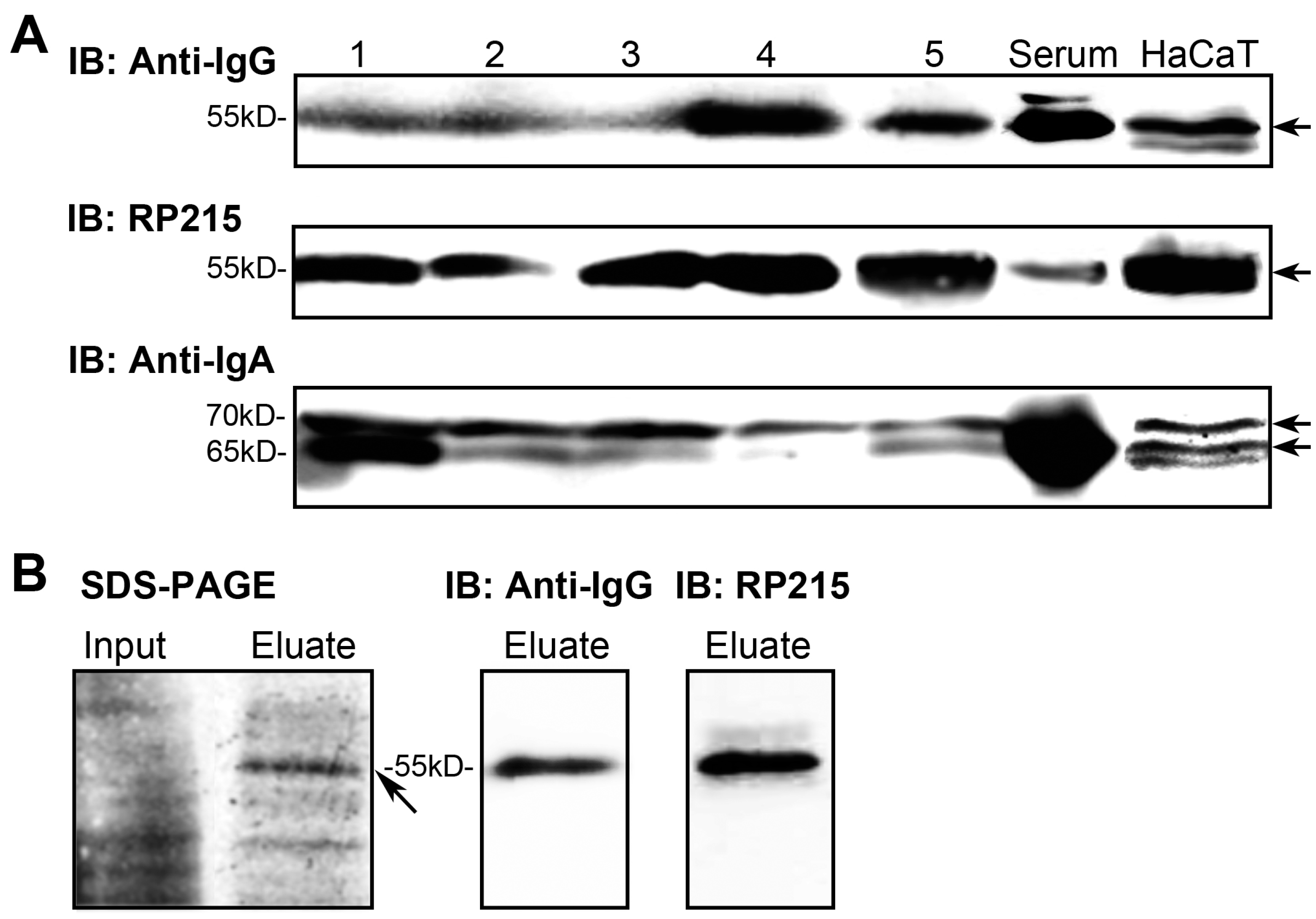
2.3. Rearranged IgG and IgA Heavy Chains Are Transcribed in Epidermal Cells
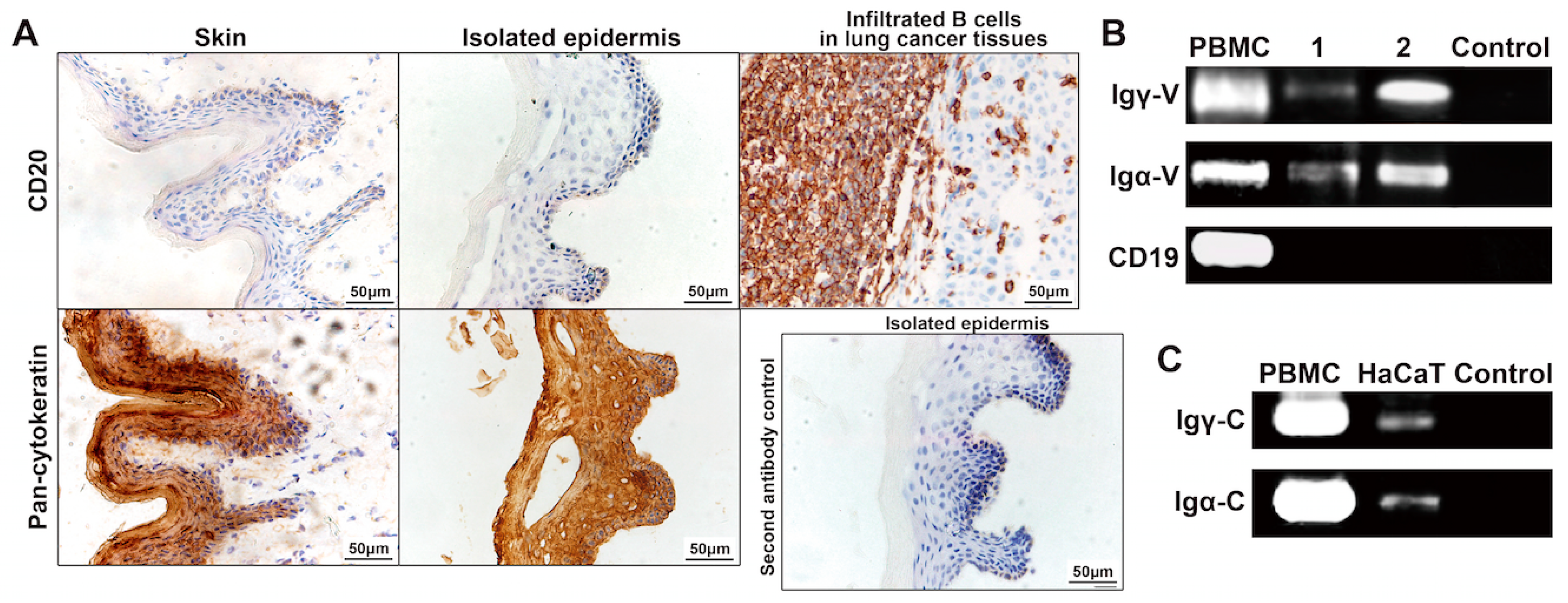
| Case | Igγ | Igα | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone | No. of Clones | VHDJH Recombination | V Segment Identity (%) with Germlines | Clone | No. of Clones | VHDJH Recombination | V Segment Identity (%) with Germlines | ||||||||
| Case 1 | 1 | 1 | VH1-2*02/D5-18*01/JH6*03 | 98.0 | 1 | 1 | VH7-4-1*02/D6-13*01/JH6*02 | 84.0 | |||||||
| 2 | 1 | VH1-2*02/D5-18*01/JH6*03 | 98.8 | 2 | 1 | VH7-4-1*02/D6-13*01/JH6*02 | 84.3 | ||||||||
| 3 | 1 | VH1-2*02/D5-18*01/JH6*03 | 98.4 | 3 | 1 | VH5-10-1*01/D2-2*01/JH3*03 | 79.9 | ||||||||
| 4 | 1 | VH1-2*02/D5-18*01/JH6*03 | 98.4 | 4 | 1 | VH5-10-1*01/D2-2*01/JH3*03 | 81.3 | ||||||||
| Case 2 | 1–6 | 6 | VH2-5*02/D2-21*02/JH4*02 | 96.3 | 1–2 | 2 | VH1-3*01/D4-17*01/JH1*01 | 77.4 | |||||||
| 7 | 1 | VH2-5*02/D2-21*02/JH4*02 | 96.3 | 3 | 1 | VH1-3*01/D4-17*01/JH1*01 | 78.8 | ||||||||
| 8 | 1 | VH2-5*02/D2-21*02/JH4*02 | 95.9 | 4 | 1 | VH1-3*01/D4-17*01/JH1*01 | 79.2 | ||||||||
| 9 | 1 | VH2-5*02/D2-21*02/JH4*02 | 95.9 | 5 | 1 | VH1-3*01/D4-17*01/JH4*02 | 79.2 | ||||||||
| 6 | 1 | VH1-3*01/D4-17*01/JH4*02 | 78.8 | ||||||||||||
| PBMC (Control) | 1 | 1 | VH1-69*13/D2-21*01/JH4*02 | 80.6 | 1 | 1 | VH1-46*01/D2-21*02/JH4*02 | 78.1 | |||||||
| 2 | 1 | VH1-8*01/D6-13*01/JH4*02 | 83.0 | 2 | 1 | VH1-2*02/D2-21*02/JH4*02 | 81.9 | ||||||||
| 3 | 1 | VH1-46*01/D1-7*01/JH4*02 | 90.5 | 3 | 1 | VH1-3*01/D1-7*01/JH4*02 | 81.6 | ||||||||
| 4 | 1 | VH4-59*03/D6-6*01/JH6*02 | 94.8 | 4 | 1 | VH4-59*08/D2-8*02/JH4*02 | 82.5 | ||||||||
| 5 | 1 | VH3-11*04/D5-12*01/JH5*02 | 85.7 | 5 | 1 | VH1-18*01/D3-3*01/JH4*02 | 81.9 | ||||||||
| 6 | 1 | VH4-34*01/D3-10*01/JH6*02 | 92.9 | ||||||||||||
| 7 | 1 | VH3-23*01/D6-13*01/JH4*02 | 91.7 | ||||||||||||
| 8 | 1 | VH3-74*03/D1-26*01/JH5*02 | 91.8 | ||||||||||||
2.4. Epidermis-Derived IgG and IgA Recognized Foreign Pathogens

2.5. Discussion
3. Experimental Section
3.1. Tissue Samples, Bacterial Strains and Cell Line
3.2. Cell Culture
3.3. Immunohistochemistry
3.4. Immunofluorescence Staining and Confocal Microscopy Analysis
3.5. Isolation of Epidermis
3.6. Isolation of Mononuclear Cells from Peripheral Blood
3.7. Protein Extraction and Purification
3.8. SDS-PAGE and Western Blotting
3.9. RT-PCR
3.10. Sequence Analysis
3.11. Microbes Stimulation and Enzyme-Linked Immunosorbent Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shishido, S.N.; Varahan, S.; Yuan, K.; Li, X.; Fleming, S.D. Humoral innate immune response and disease. Clin. Immunol. 2012, 144, 142–158. [Google Scholar]
- Li, M.; Feng, D.Y.; Ren, W.; Zheng, L.; Zheng, H.; Tang, M.; Cao, Y. Expression of immunoglobulin κ light chain constant region in abnormal human cervical epithelial cells. Int. J. Biochem. Cell Biol. 2004, 36, 2250–2257. [Google Scholar]
- Geng, L.Y.; Shi, Z.Z.; Dong, Q.; Cai, X.H.; Zhang, Y.M.; Cao, W.; Peng, J.P.; Fang, Y.M.; Zheng, L.; Zheng, S. Expression of SNC73, a transcript of the immunoglobulin α-1 gene, in human epithelial carcinomas. World J. Gastroenterol. 2007, 13, 2305–2311. [Google Scholar]
- Zhang, S.; Mao, Y.; Huang, J.; Ma, T.; Zhang, L.; Zhu, X.; Zheng, J.; Wu, L.; Yin, C.C.; Qiu, X. Immunoglobulin gene locus events in epithelial cells of lactating mouse mammary glands. Cell. Mol. Life Sci. 2010, 67, 985–994. [Google Scholar]
- Zhang, L.; Hu, S.; Korteweg, C.; Chen, Z.; Qiu, Y.; Su, M.; Gu, J. Expression of immunoglobulin G in esophageal squamous cell carcinomas and its association with tumor grade and Ki67. Hum. Pathol. 2012, 43, 423–434. [Google Scholar]
- Hu, F.; Zhang, L.; Zheng, J.; Zhao, L.; Huang, J.; Shao, W.; Liao, Q.; Ma, T.; Geng, L.; Yin, C.C.; et al. Spontaneous production of immunoglobulin m in human epithelial cancer cells. PLoS One 2012, 7, e51423. [Google Scholar]
- Niu, N.; Zhang, J.; Huang, T.; Sun, Y.; Chen, Z.; Yi, W.; Korteweg, C.; Wang, J.; Gu, J. IgG expression in human colorectal cancer and its relationship to cancer cell behaviors. PLoS One 2012, 7, e47362. [Google Scholar]
- Huang, J.; Zhang, L.; Ma, T.; Zhang, P.; Qiu, X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse testis and epididymis. J. Histochem. Cytochem. 2009, 57, 339–349. [Google Scholar]
- Zhao, Y.; Liu, Y.; Chen, Z.; Korteweg, C.; Gu, J. Immunoglobulin g (IgG) expression in human umbilical cord endothelial cells. J. Histochem. Cytochem. 2011, 59, 474–488. [Google Scholar]
- Kang, B.Y.; Hu, C.; Prayaga, S.; Khaidakov, M.; Sawamura, T.; Seung, K.B.; Mehta, J.L. Lox-1 dependent over-expression of immunoglobulins in cardiomyocytes in response to angiotensin II. Biochem. Biophys. Res. Commun. 2009, 379, 395–399. [Google Scholar]
- Huang, J.; Sun, X.; Mao, Y.; Zhu, X.; Zhang, P.; Zhang, L.; Du, J.; Qiu, X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int. J. Biochem. Cell Biol. 2008, 40, 1604–1615. [Google Scholar]
- Niu, N.; Zhang, J.; Guo, Y.; Zhao, Y.; Korteweg, C.; Gu, J. Expression and distribution of immunoglobulin G and its receptors in the human nervous system. Int. J. Biochem. Cell Biol. 2011, 43, 556–563. [Google Scholar]
- Chen, Z.; Li, J.; Xiao, Y.; Zhang, J.; Zhao, Y.; Liu, Y.; Ma, C.; Qiu, Y.; Luo, J.; Huang, G.; et al. Immunoglobulin G locus events in soft tissue sarcoma cell lines. PLoS One 2011, 6, e21276. [Google Scholar]
- Niu, N.; Zhang, J.; Sun, Y.; Wang, S.; Sun, Y.; Korteweg, C.; Gao, W.; Gu, J. Expression and distribution of immunoglobulin G and its receptors in an immune privileged site: The eye. Cell. Mol. Life Sci. 2011, 68, 2481–2492. [Google Scholar]
- Hu, D.; Zheng, H.; Liu, H.; Li, M.; Ren, W.; Liao, W.; Duan, Z.; Li, L.; Cao, Y. Immunoglobulin expression and its biological significance in cancer cells. Cell. Mol. Immunol. 2008, 5, 319–324. [Google Scholar]
- Zheng, H.; Li, M.; Ren, W.; Zeng, L.; Liu, H.D.; Hu, D.; Deng, X.; Tang, M.; Shi, Y.; Gong, J.; et al. Expression and secretion of immunoglobulin α heavy chain with diverse VDJ recombinations by human epithelial cancer cells. Mol. Immunol. 2007, 44, 2221–2227. [Google Scholar]
- Duan, Z.; Zheng, H.; Xu, S.; Jiang, Y.; Liu, H.; Li, M.; Hu, D.; Li, W.; Bode, A.M.; Dong, Z.; et al. Activation of the Ig Iα1 promoter by the transcription factor Ets-1 triggers Ig Iα1–Cα1 germline transcription in epithelial cancer cells. Cell. Mol. Immunol. 2014, 11, 197–205. [Google Scholar]
- Wang, J.; Lin, D.; Peng, H.; Huang, Y.; Huang, J.; Gu, J. Cancer-derived immunoglobulin G promotes tumor cell growth and proliferation through inducing production of reactive oxygen species. Cell Death Dis. 2013, 4, e945. [Google Scholar]
- Qiu, X.; Zhu, X.; Zhang, L.; Mao, Y.; Zhang, J.; Hao, P.; Li, G.; Lv, P.; Li, Z.; Sun, X.; et al. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003, 63, 6488–6495. [Google Scholar]
- Wen, Y.J.; Mancino, A.; Pashov, A.; Whitehead, T.; Stanley, J.; Kieber-Emmons, T. Antigen binding of human IgG Fabs mediate ERK-associated proliferation of human breast cancer cells. DNA Cell Biol. 2005, 24, 73–84. [Google Scholar]
- Liang, P.Y.; Li, H.Y.; Zhou, Z.Y.; Jin, Y.X.; Wang, S.X.; Peng, X.H.; Ou, S.J. Over-expression of immunoglobulin G prompts cell proliferation and inhibits cell apoptosis in human urothelial carcinoma. Tumour Biol. 2013, 34, 1783–1791. [Google Scholar]
- Pan, B.; Zheng, S.; Liu, C.; Xu, Y. Suppression of IGHG1 gene expression by siRNA leads to growth inhibition and apoptosis induction in human prostate cancer cell. Mol. Biol. Rep. 2013, 40, 27–33. [Google Scholar]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. A peptide antibiotic from human skin. Nature 1997, 387, 861. [Google Scholar]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Novel formulations for antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 18040–18083. [Google Scholar]
- Ryu, S.; Song, P.I.; Seo, C.H.; Cheong, H.; Park, Y. Colonization and infection of the skin by s. Aureus: Immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 8753–8772. [Google Scholar]
- Coruh, G.; Mason, D.Y. Serum proteins in human squamous epithelium. Br. J. Dermatol. 1980, 102, 497–505. [Google Scholar]
- Gilliam, J.N.; Cheatum, D.E.; Hurd, E.R.; Stastny, P.; Ziff, M. Immunoglobulin in clinically uninvolved skin in systemic lupus erythematosus: Association with renal disease. J. Clin. Investig. 1974, 53, 1434–1440. [Google Scholar]
- Mshana, R.N.; Humber, D.P.; Belehu, A.; Harboe, M. Immunohistological studies of skin biopsies from patients with lepromatous leprosy. J. Clin. Immunol. 1983, 3, 22–29. [Google Scholar]
- Yaoita, H.; Briggaman, R.A.; Lawley, T.J.; Provost, T.T.; Katz, S.I. Epidermolysis bullosa acquisita: Ultrastructural and immunological studies. J. Investig. Dermatol. 1981, 76, 288–292. [Google Scholar]
- Blenkinsopp, W.K.; Clayton, R.J.; Haffenden, G.P. Immunoglobulin and complement in normal skin. J. Clin. Pathol. 1978, 31, 1143–1146. [Google Scholar]
- Zhu, X.; Li, C.; Sun, X.; Mao, Y.; Li, G.; Liu, X.; Zhang, Y.; Qiu, X. Immunoglobulin mRNA and protein expression in human oral epithelial tumor cells. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 232–238. [Google Scholar]
- Lee, G.; Laflamme, E.; Chien, C.H.; Ting, H.H. Molecular identity of a pan cancer marker, CA215. Cancer Biol. Ther. 2008, 7, 2007–2014. [Google Scholar]
- Lee, G. Cancer cell-expressed immunoglobulins: CA215 as a pan cancer marker and its diagnostic applications. Cancer Biomark. 2009, 5, 137–142. [Google Scholar]
- Schneider, T.E.; Barland, C.; Alex, A.M.; Mancianti, M.L.; Lu, Y.; Cleaver, J.E.; Lawrence, H.J.; Ghadially, R. Measuring stem cell frequency in epidermis: A quantitative in vivo functional assay for long-term repopulating cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11412–11417. [Google Scholar]
- Kanno, H.; Kubo, A.; Yoshizumi, T.; Mikami, T.; Maegawa, J. Isolation of multipotent nestin-expressing stem cells derived from the epidermis of elderly humans and TAT-VHL peptide-mediated neuronal differentiation of these cells. Int. J. Mol. Sci. 2013, 14, 9604–9617. [Google Scholar]
- Van Dongen, J.J.; Langerak, A.W.; Bruggemann, M.; Evans, P.A.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; Garcia-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar]
- Zheng, J.; Huang, J.; Mao, Y.; Liu, S.; Sun, X.; Zhu, X.; Ma, T.; Zhang, L.; Ji, J.; Zhang, Y.; et al. Immunoglobulin gene transcripts have distinct VHDJH recombination characteristics in human epithelial cancer cells. J. Biol. Chem. 2009, 284, 13610–13619. [Google Scholar]
- Tigalonowa, M.; Bjerke, J.R.; Matre, R. Fc gamma-receptors on langerhans’ cells and keratinocytes in suspension from normal skin characterized using soluble immune complexes and monoclonal antibodies. Acta Derm. Venereol. 1991, 71, 99–103. [Google Scholar]
- Huff, J.C. Epithelial polymeric immunoglobulin receptors. J. Investig. Dermatol. 1990, 94, 74S–78S. [Google Scholar]
- Glanville, J.; Zhai, W.; Berka, J.; Telman, D.; Huerta, G.; Mehta, G.R.; Ni, I.; Mei, L.; Sundar, P.D.; Day, G.M.; et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc. Natl. Acad. Sci. USA 2009, 106, 20216–20221. [Google Scholar]
- Huang, J.; Sun, X.; Gong, X.; He, Z.; Chen, L.; Qiu, X.; Yin, C.C. Rearrangement and expression of the immunoglobulin µ-chain gene in human myeloid cells. Cell. Mol. Immunol. 2014, 11, 94–104. [Google Scholar]
- Qiu, X.; Sun, X.; He, Z.; Huang, J.; Hu, F.; Chen, L.; Lin, P.; You, M.J.; Medeiros, L.J.; Yin, C.C. Immunoglobulin γ heavy chain gene with somatic hypermutation is frequently expressed in acute myeloid leukemia. Leukemia 2013, 27, 92–99. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Ge, J.; Liao, Q.; Ma, J.; Liu, Y.; Huang, J.; Wang, C.; Xu, W.; Zheng, J.; Shao, W.; et al. IgG and IgA with Potential Microbial-Binding Activity Are Expressed by Normal Human Skin Epidermal Cells. Int. J. Mol. Sci. 2015, 16, 2574-2590. https://doi.org/10.3390/ijms16022574
Jiang D, Ge J, Liao Q, Ma J, Liu Y, Huang J, Wang C, Xu W, Zheng J, Shao W, et al. IgG and IgA with Potential Microbial-Binding Activity Are Expressed by Normal Human Skin Epidermal Cells. International Journal of Molecular Sciences. 2015; 16(2):2574-2590. https://doi.org/10.3390/ijms16022574
Chicago/Turabian StyleJiang, Dongyang, Jing Ge, Qinyuan Liao, Junfan Ma, Yang Liu, Jing Huang, Chong Wang, Weiyan Xu, Jie Zheng, Wenwei Shao, and et al. 2015. "IgG and IgA with Potential Microbial-Binding Activity Are Expressed by Normal Human Skin Epidermal Cells" International Journal of Molecular Sciences 16, no. 2: 2574-2590. https://doi.org/10.3390/ijms16022574
APA StyleJiang, D., Ge, J., Liao, Q., Ma, J., Liu, Y., Huang, J., Wang, C., Xu, W., Zheng, J., Shao, W., Lee, G., & Qiu, X. (2015). IgG and IgA with Potential Microbial-Binding Activity Are Expressed by Normal Human Skin Epidermal Cells. International Journal of Molecular Sciences, 16(2), 2574-2590. https://doi.org/10.3390/ijms16022574





