Biochemical Characterization of An Arginine-Specific Alkaline Trypsin from Bacillus licheniformis
Abstract
:1. Introduction
2. Results and Discussion
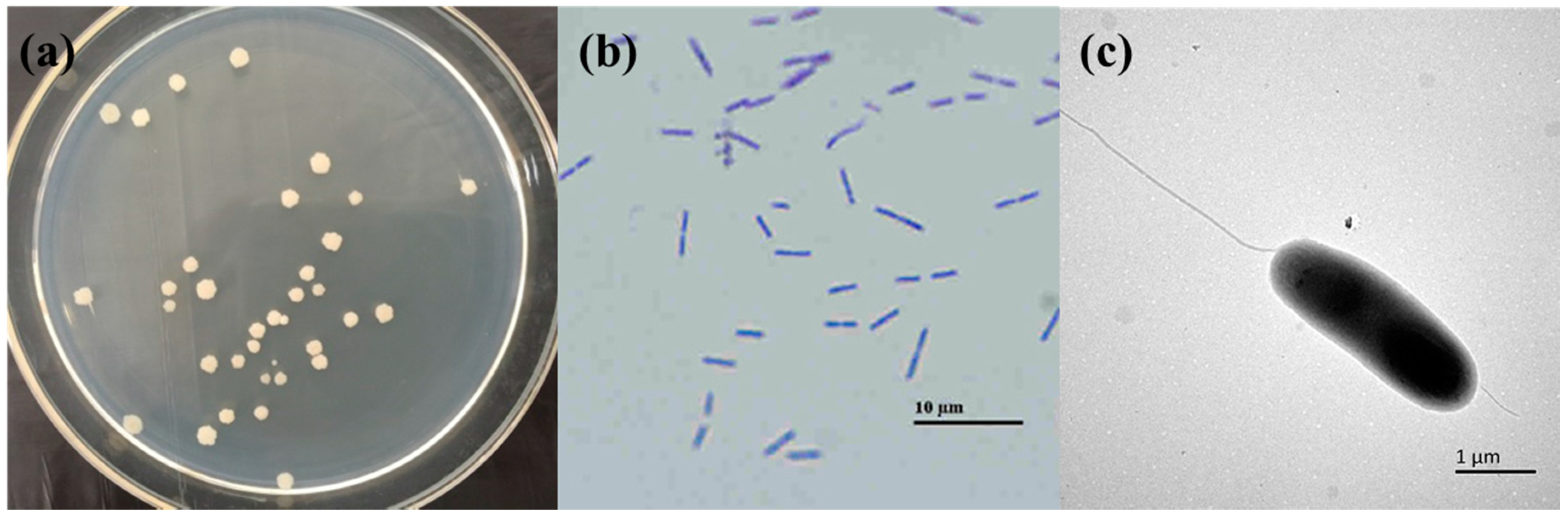
2.1. Isolation of Trypsin-Producing Strains
| Strain Number | Transparent Circle Diameter/mm | Diameter Ratio | Protease Activity (U/mL) | Trypsin Activity (U/mL) |
|---|---|---|---|---|
| ZM4 | 30 | 3.46 | 38 | 8 |
| ZM5 | 32 | 5.65 | 73 | 7.5 |
| CD2 | 30.33 | 5.69 | 67 | 4.5 |
| CD7 | 29.67 | 9.89 | 91 | 6.5 |
| DMN1 | 31.33 | 4.09 | 23 | 4 |
| DMN3 | 28.67 | 8.6 | 13 | 10 |
| DMN6 | 15.55 | 1.35 | 14 | 20.5 |
| WS6 | 31.33 | 3.36 | 76 | 5.5 |
2.2. Strain Identification
| Characteristics | DMN6 | Bacillus licheniformis a | Characteristics | DMN6 | Bacillus licheniformis |
|---|---|---|---|---|---|
| Oxidase | − | + b | 7% NaCl growth | + | + |
| Anaerobic growth | + | + | Methyl red | + | + |
| Voges-Proskauer reaction | + | + | Hippurate hydrolyzation | − | − |
| Glucose acid production | − | + | 5 °C growth | − | − |
| Glucose gas production | − | W c/− | 40 °C growth | + | + |
| Citrate utilization | + | + | 44 °C growth | + | + |
| Gelatin hydrolyzation | + | + | 50 °C growth | + | + |
| Amylolysis | + | + | 55 °C growth | − | + |
| Indole production | − | − |

2.3. Optimization of Fermentation Conditions


2.4. Enzyme Purification

| Purification Step | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Yield (%) | Purification |
|---|---|---|---|---|---|
| Culture filtrate | 73.1 | 3000 | 41.04 | 100 | 1 |
| DEAE | 4.05 | 1200 | 296.3 | 5.54 | 7.2 |
| Superdex G75 | 2.10 | 735.0 | 350.0 | 2.87 | 8.5 |
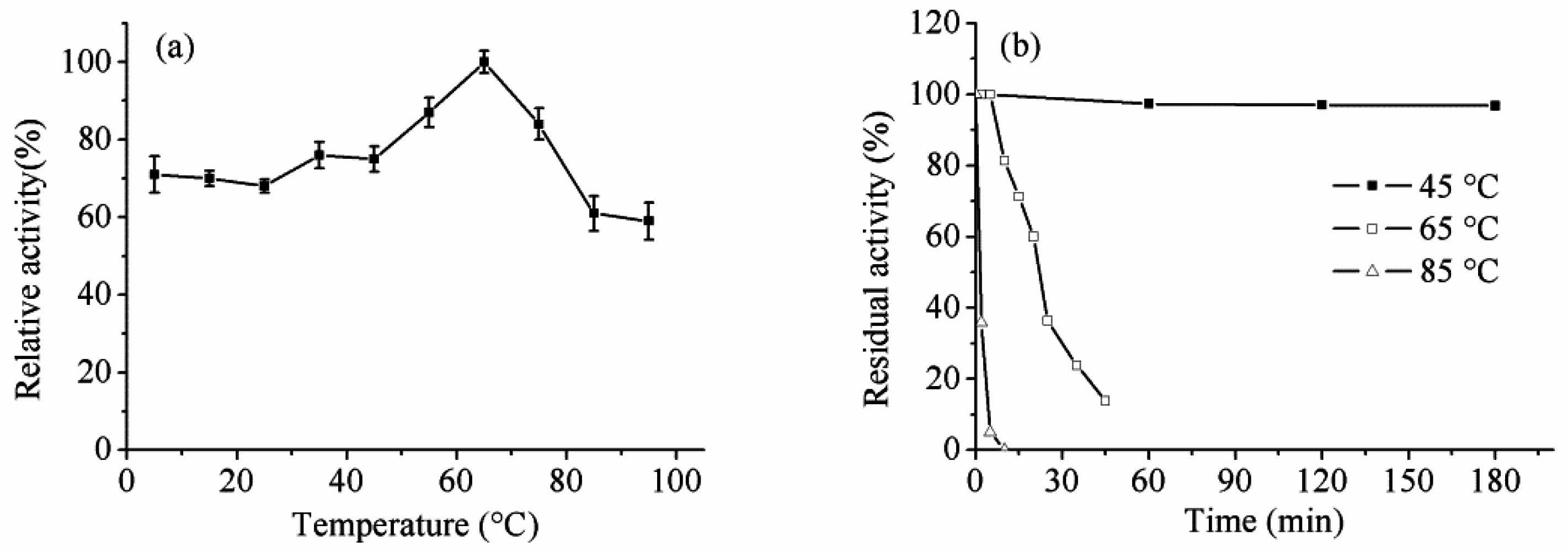
2.5. Effect of Temperature and pH on Enzyme Activity and Stability

2.6. Effect of Metal Ions, Inhibitors and Surfactants on Trypsin Activity
| Metal Ions | Concentration (mM) | Relative Activity (%) | Concentration (mM) | Relative Activity (%) |
|---|---|---|---|---|
| Control | 0 | 100 | – | – |
| K+ | 1 | 106.67 ± 1.36 | 5 | 44.44 ± 2.59 |
| Zn2+ | 1 | ND | 5 | ND |
| Mg2+ | 1 | 65.00 ± 6.24 | 5 | 23.02 ± 1.50 |
| Na+ | 1 | 95.00 ± 4.08 | 5 | 30.95 ± 3.68 |
| Fe3+ | 1 | ND | 5 | ND |
| Ba2+ | 1 | 182.46 ± 2.96 | 5 | 60.32 ± 1.30 |
| Al3+ | 1 | ND | 5 | ND |
| Co2+ | 1 | 34.49 ± 2.61 | 5 | 21.43 ± 3.57 |
| Ca2+ | 1 | 67.14 ± 3.59 | 5 | 38.10 ± 0.99 |
| Sr2+ | 1 | 59.24 ± 0.00 | 5 | 36.51 ± 1.98 |
| Mn2+ | 1 | 69.64 ± 7.99 | 5 | 46.83 ± 3.89 |
| Ag+ | 1 | ND | 5 | ND |
| Surfactants & Inhibitors | Concentration | Relative Activity (%) |
|---|---|---|
| Control | 0 | 100 |
| DMSO | 1% | 89.71 ± 1.20 |
| Triton100 | 1% | 86.27 ± 1.39 |
| Tween80 | 1% | 93.63 ± 0.69 |
| SDS | 1% | 39.77 ± 0.83 |
| PMSF | 5 mM | 38.73 ± 0.69 |
| DTT | 5 mM | 110.29 ± 1.2 |
| EDTA | 5 mM | 136.27 ± 1.39 |
| Benzamidine | 5 mM | 47.92 ± 1.28 |
| Aprotinin | 5 mM | 40.79 ± 1.04 |
2.7. Kinetic Parameters of Enzyme
2.8. Cloning of Trypsin Gene from B. licheniformis
3. Materials and Methods
3.1. Materials
3.2. Strain Screening
3.3. Taxonomic Identification
3.4. Culture Optimization
3.5. Enzyme Purification
3.6. Effect of Temperature, pH and Stability on Trypsin Activity
3.7. Effect of Metals, Inhibitors and Surfactants on Trypsin Activity
3.8. Kinetic Parameter Determination
3.9. Cloning of B. licheniformis Trypsin Gene
3.10. Enzyme Assay
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Walsh, K. Trypsinogens and trypsins of various species. Methods Enzymol. 1970, 19, 41–63. [Google Scholar]
- Aguirre, C.; Condado-Morales, I.; Olguin, L.F.; Costas, M. Isothermal titration calorimetry determination of individual rate constants of trypsin catalytic activity. Anal. Biochem. 2015, 479, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Craik, C.S.; Largman, C.; Fletcher, T.; Roczniak, S.; Barr, P.J.; Fletterick, R.; Rutter, W.J. Redesigning trypsin: Alteration of substrate specificity. Science 1985, 228, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, A.; Buck, F.; Bier, M.; Nord, F. On the mechanism of enzyme action: LXXII. comparative studies on trypsins of various origins. Arch. Biochem. Biophys. 1961, 92, 532–540. [Google Scholar] [CrossRef]
- Mahmoud, M.I.; Malone, W.T.; Cordle, C.T. Enzymatic hydrolysis of casein: Effect of degree of hydrolysis on antigenicity and physical properties. J. Food Sci. 1992, 57, 1223–1229. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Paul, R.G.; Attenburrow, G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: Animal species and collagen extraction method. J. Biomed. Mater. Res. A 2008, 86, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Adler-Nissen, J. Enzymic hydrolysis of proteins for increased solubility. J. Agric. Food Chem. 1976, 24, 1090–1093. [Google Scholar] [CrossRef]
- Zambare, V.; Nilegaonkar, S.; Kanekar, P. Application of protease from Bacillus cereus MCM B-326 as a bating agent in leather processing. IIOAB J. 2010, 1, 18–21. [Google Scholar]
- Zhang, H.; Huang, R.Y.C.; Jalili, P.R.; Irungu, J.W.; Nicol, G.R.; Ray, K.B.; Rohrs, H.W.; Gross, M.L. Improved mass spectrometric characterization of protein glycosylation reveals unusual glycosylation of maize-derived bovine trypsin. Anal. Chem. 2010, 82, 10095–10101. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.L.; Fonseca, S.G.; Ishigaki, S.; Nguyen, L.X.; Foss, E.; Bortell, R.; Rossini, A.A.; Urano, F. Regulation of insulin biosynthesis in pancreatic β cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell. Metab. 2006, 4, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Tubio, G.; Picó, G.A.; Nerli, B.B. Extraction of trypsin from bovine pancreas by applying polyethyleneglycol/sodium citrate aqueous two-phase systems. J. Chromatogr. B 2009, 877, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hedlin, P.; Taschuk, R.; Potter, A.; Griebel, P.; Napper, S. Detection and control of prion diseases in food animals. ISRN Vet. Sci. 2012, 2012, 24. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.; Blanch, H. Recombinant trypsin production in high cell density fed-batch cultures in Escherichia coli. Biotechnol. Bioeng. 1993, 41, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Hanquier, J.; Sorlet, Y.; Desplancq, D.; Baroche, L.; Ebtinger, M.; Lefevre, J.-F.; Pattus, F.; Hershberger, C.L.; Vertès, A.A. A single mutation in the activation site of bovine trypsinogen enhances its accumulation in the fermentation broth of the yeast Pichia pastoris. Appl. Environ. Microbiol. 2003, 69, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, N.E.; Robson, G.D.; Trinci, A.P.; Wiebe, M.G. Trypsin-like protease (TLP) production in Fusarium oxysporum and Fusarium venenatum and use of the TLP promoter for recombinant protein (glucoamylase) production. Enzyme Microb. Technol. 2003, 33, 85–91. [Google Scholar] [CrossRef]
- Vukoti, K.M.; Kadiyala, C.S.R.; Miyagi, M. Streptomyces erythraeus trypsin inactivates α1-antitrypsin. FEBS Lett. 2011, 585, 3898–3902. [Google Scholar] [CrossRef] [PubMed]
- Dienes, D.; Börjesson, J.; Hägglund, P.; Tjerneld, F.; Lidén, G.; Réczey, K.; Stålbrand, H. Identification of a trypsin-like serine protease from Trichoderma reesei QM9414. Enzym. Microb. Technol. 2007, 40, 1087–1094. [Google Scholar] [CrossRef]
- Ling, Z.; Ma, T.; Li, J.; Du, G.; Kang, Z.; Chen, J. Functional expression of trypsin from Streptomyces griseus by Pichia pastoris. J. Ind. Microbiol. Biotechnol. 2012, 39, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Rooney, A.P.; Price, N.P.J.; Ehrhardt, C.; Swezey, J.L.; Bannan, J.D. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. Inaquosorum. subsp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, Z.; Xu, S.; Hu, Y.; Guo, C.; Li, F.; Li, J.; Liu, J.; Wang, H. Peptidomic analysis of antimicrobial peptides in skin secretions of Amolops mantzorum. Zool. Sci. 2014, 31, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sriket, P.; Sriket, C. A trypsin from royal red prawn (Haliporoides sibogae) and its possible application for collagen hydrolysis. Sep. Sci. Technol. 2015, 50, 1073–1082. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell. Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, A.; Traeger, G.; Hansen, U.; Weis, M.A.; Ravindran, S.; Wirthlin, L.; Eyre, D.R.; Fernandes, R.J. Molecular properties and fibril ultrastructure of types II and XI collagens in cartilage of mice expressing exclusively the α1(IIA) collagen isoform. Matrix Biol. 2014, 34, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.-K.; Chan, E.Y.-M.; Fong, W.-P. Identification of an “alcohol dehydrogenase-activating” protease in grass carp hepatopancreas as a chymotrypsin. IUBMB Life 1997, 43, 1231–1239. [Google Scholar] [CrossRef]
- BLAST Assembled Genomes. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 December 2015).
- Schwert, G.W.; Takenaka, Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim. Biophys. Acta. 1955, 16, 570–575. [Google Scholar] [CrossRef]
- Cui, H.; Wang, L. Screening of a newly marine bacteria producing alkaline protease from Qinhuangdao sea area and its characterization of alkaline protease. J. Chem. Pharm Res. 2014, 6, 1236–1242. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.-S.; Li, W.; Zhang, D.-D.; Xie, M.-F.; Yang, B.; Zhang, R.-X.; Li, H.; Lu, Z.-M.; Xu, Z.-H.; Shi, J.-S. Biochemical Characterization of An Arginine-Specific Alkaline Trypsin from Bacillus licheniformis. Int. J. Mol. Sci. 2015, 16, 30061-30074. https://doi.org/10.3390/ijms161226200
Gong J-S, Li W, Zhang D-D, Xie M-F, Yang B, Zhang R-X, Li H, Lu Z-M, Xu Z-H, Shi J-S. Biochemical Characterization of An Arginine-Specific Alkaline Trypsin from Bacillus licheniformis. International Journal of Molecular Sciences. 2015; 16(12):30061-30074. https://doi.org/10.3390/ijms161226200
Chicago/Turabian StyleGong, Jin-Song, Wei Li, Dan-Dan Zhang, Min-Feng Xie, Biao Yang, Rong-Xian Zhang, Heng Li, Zhen-Ming Lu, Zheng-Hong Xu, and Jin-Song Shi. 2015. "Biochemical Characterization of An Arginine-Specific Alkaline Trypsin from Bacillus licheniformis" International Journal of Molecular Sciences 16, no. 12: 30061-30074. https://doi.org/10.3390/ijms161226200






