Theoretical Mechanistic and Kinetic Studies on Homogeneous Gas-Phase Formation of Polychlorinated Naphthalene from 2-Chlorophenol as Forerunner
Abstract
:1. Introduction
2. Results and Discussion
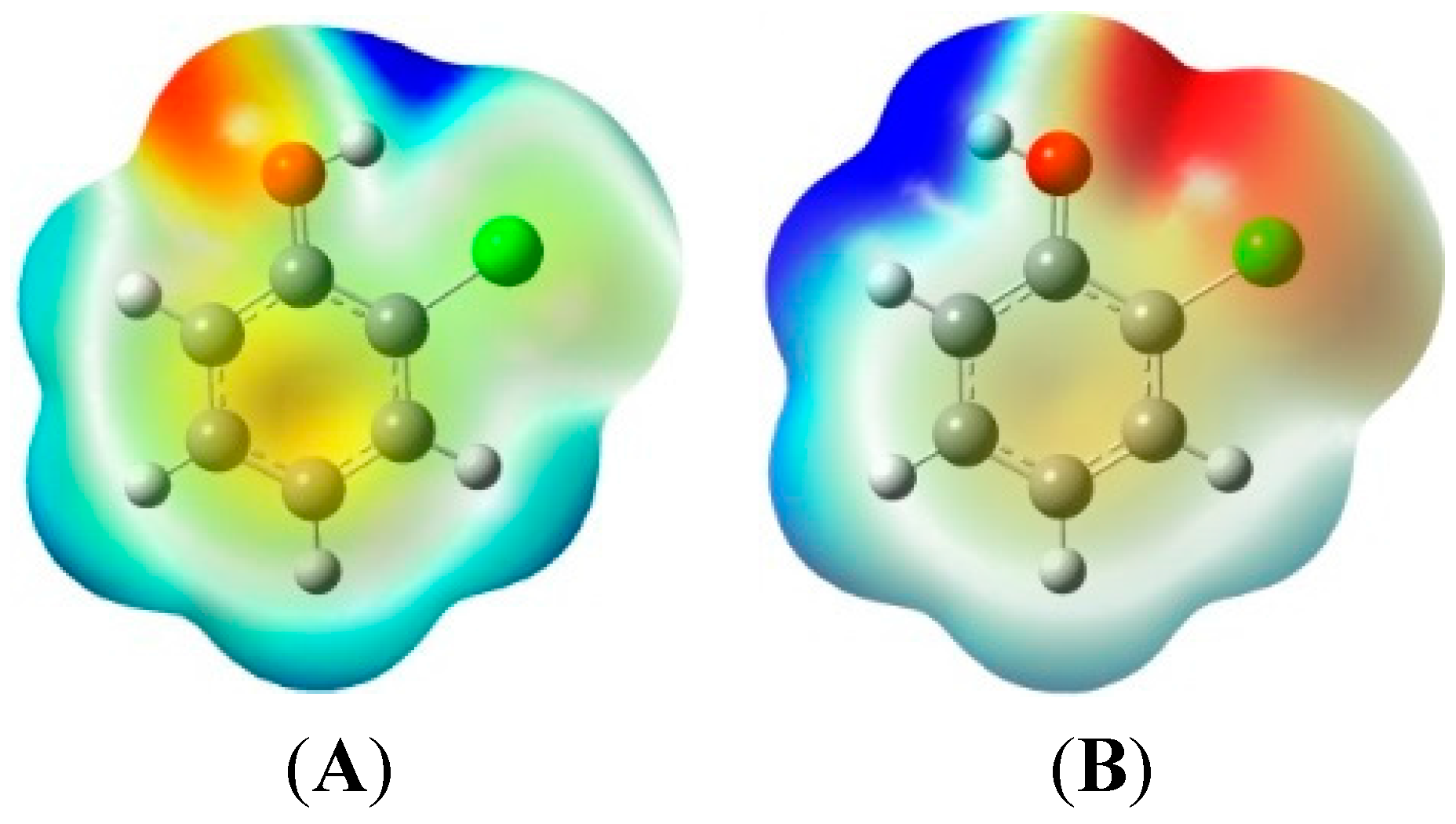
2.1. 2-Chlorophenoxy Radical Formation from 2-Chlorophenols
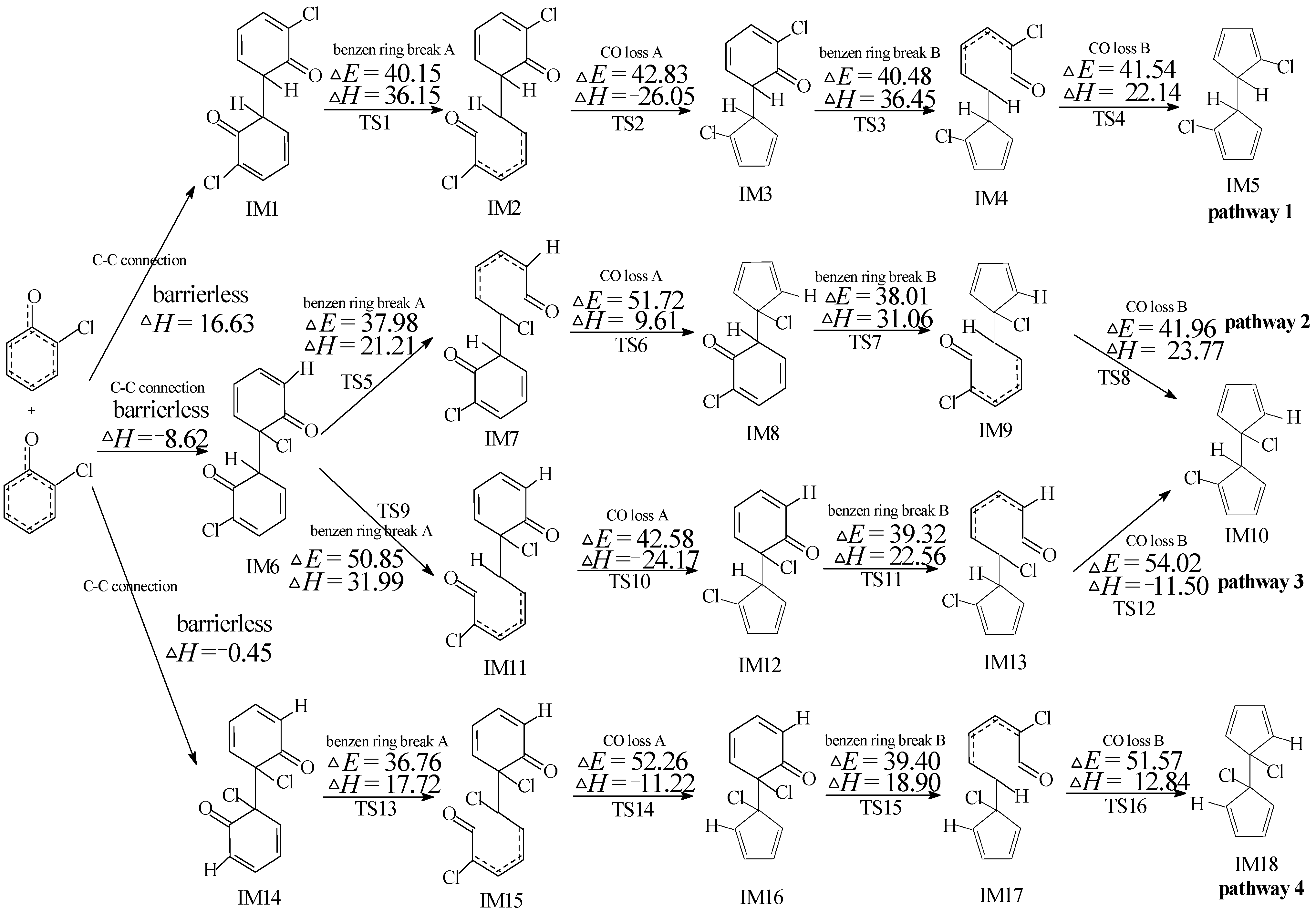
2.2. Chloro-Dihydrofulvalene Production from Dimerization of 2-Chlorophenoxy Radicals
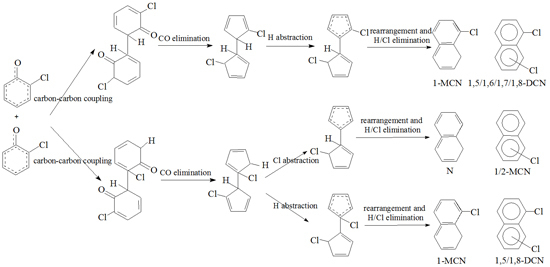
2.3. PCN Formation from the Following Reactions of IM5
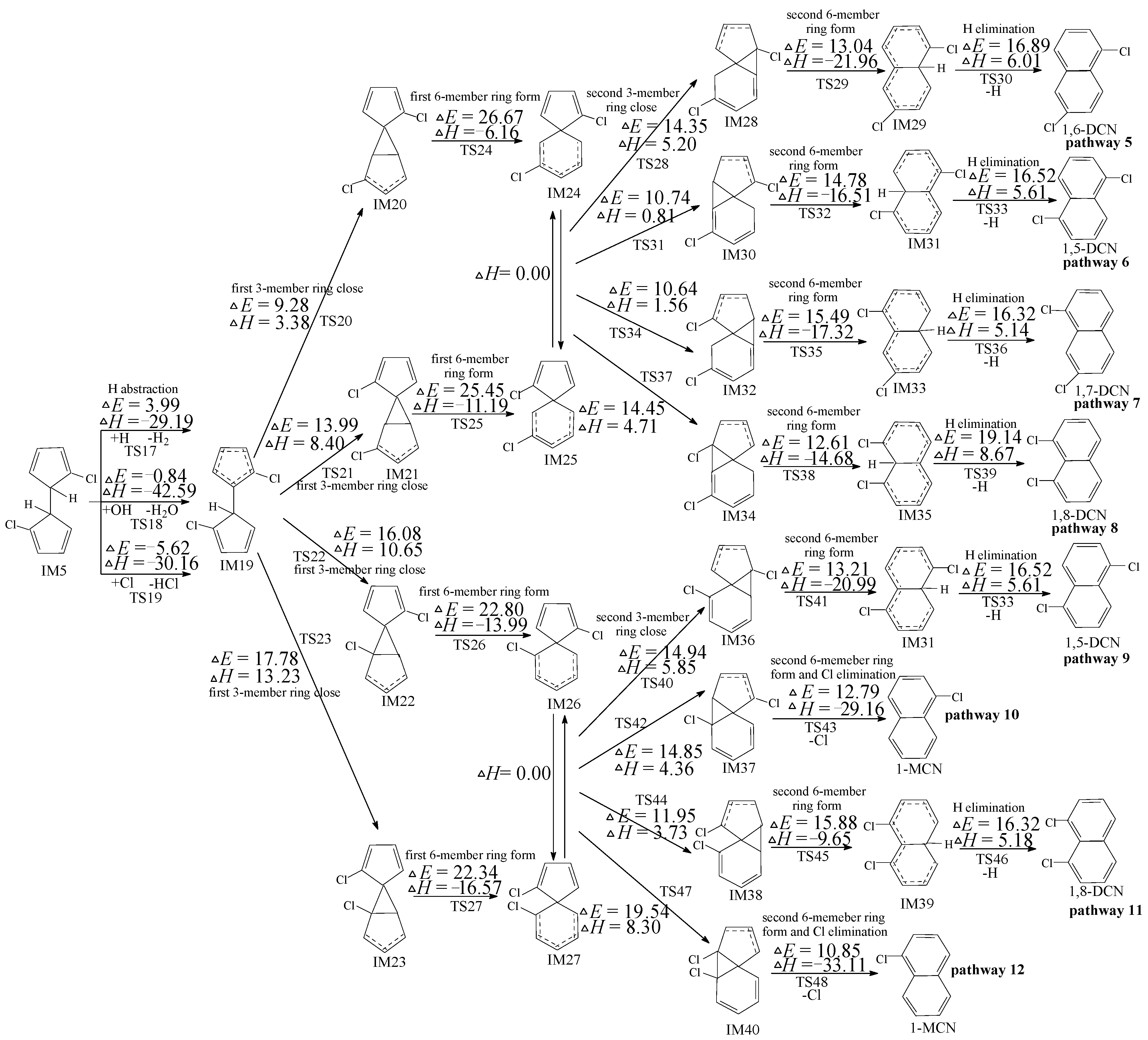
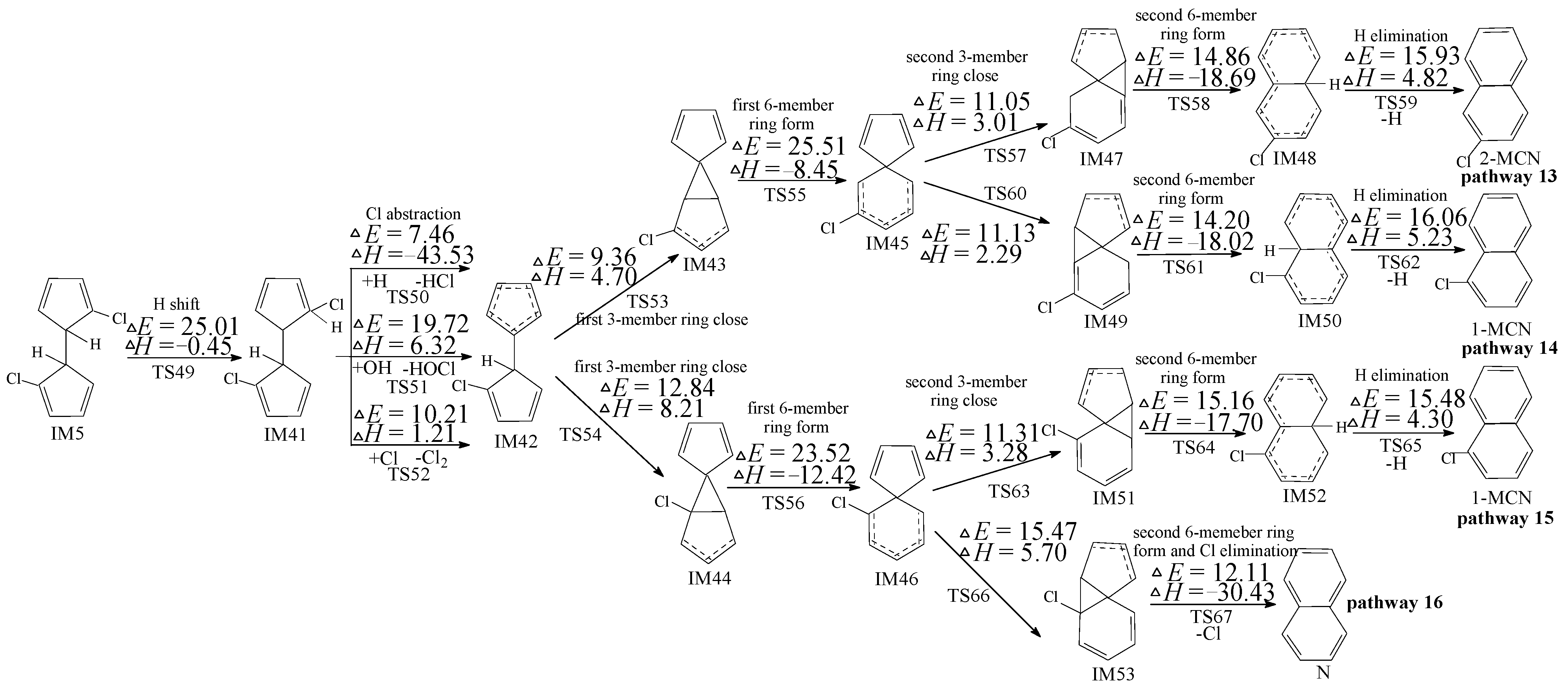
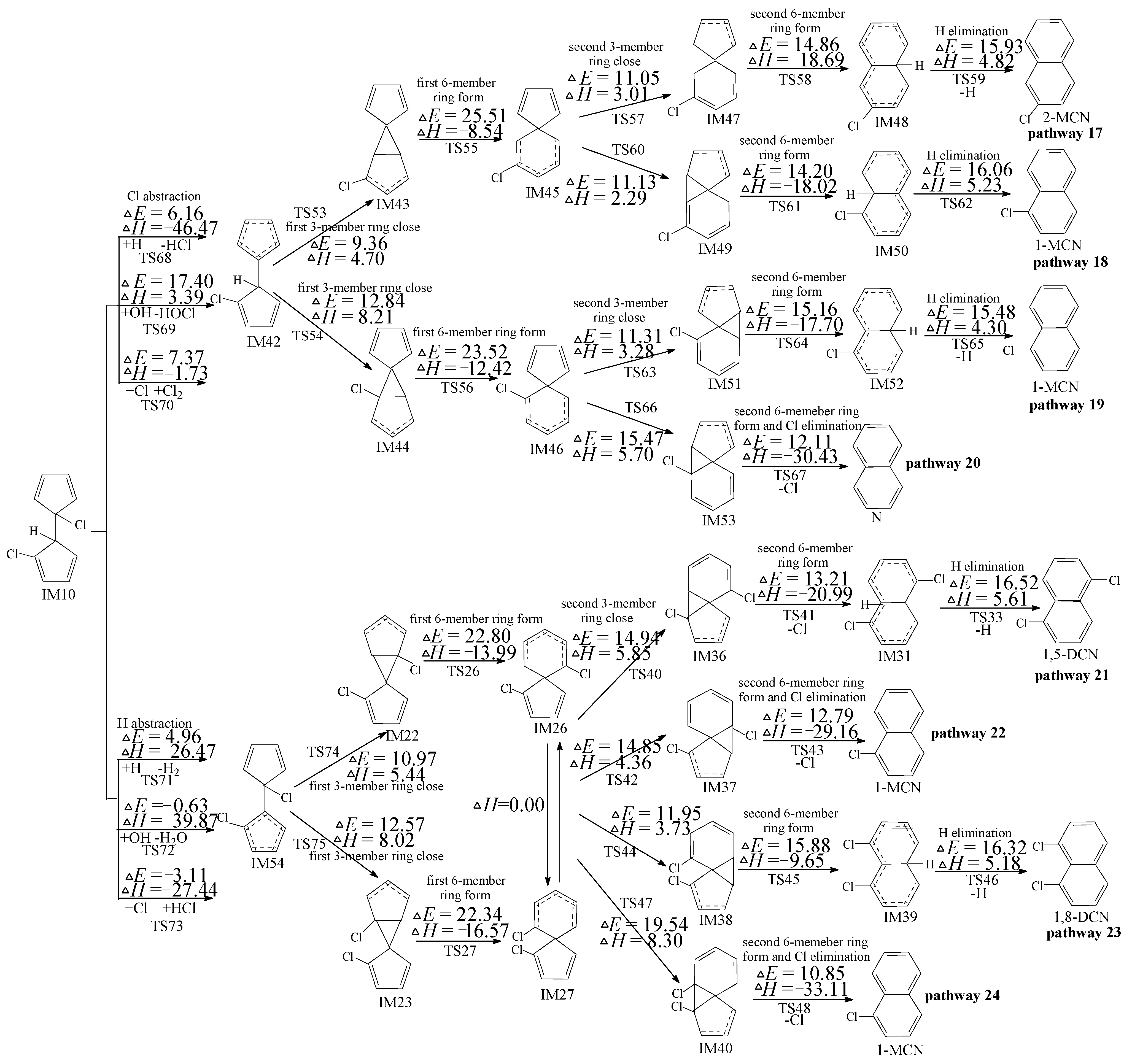
2.4. PCN Formation from the Following Reactions of IM10
2.5. Formation Comparison PCNs and PCDFs from 2-Chlorophenol
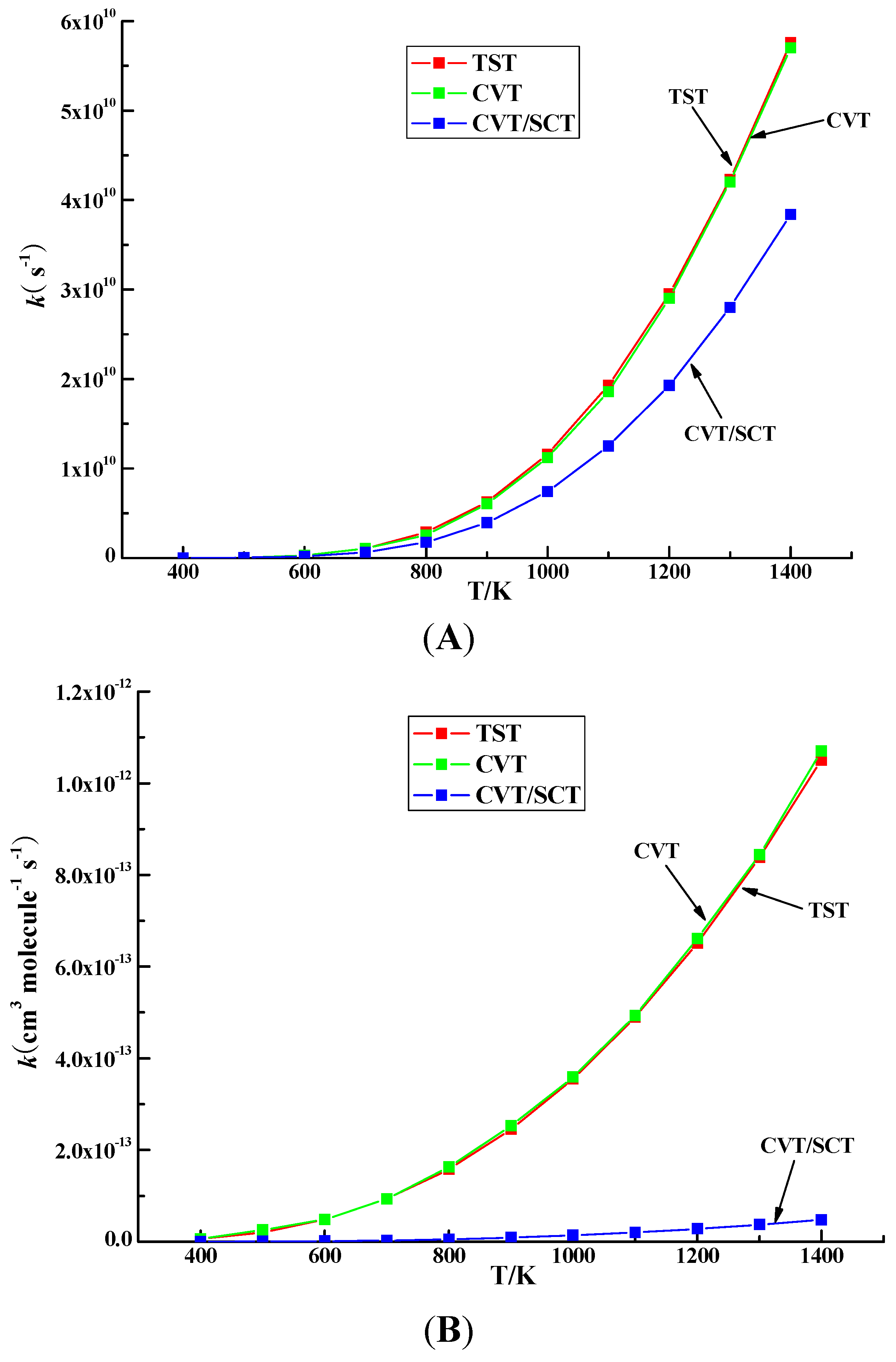
2.6. Rate Constant Calculations
| Reactions | Arrhenius Formulas |
|---|---|
| IM1 → IM2 via TS1 | k (T) = (3.12 × 1012) exp (−21,500.36/T) |
| IM2 → IM3 + CO via TS2 | k (T) = (2.32 × 1011) exp (−21,473.60/T) |
| IM3 → IM4 via TS3 | k (T) = (1.21 × 1013) exp (−21,309.58/T) |
| IM4 → IM5 + CO via TS4 | k (T) = (1.17 × 1011) exp (−21,746.24/T) |
| IM6 → IM7 via TS5 | k (T) = (1.94 × 1013) exp (−20,475.72/T) |
| IM7 → IM8 + CO TS6 | k (T) = (2.41 × 1011) exp (−24,764.15/T) |
| IM8 → IM9 via TS7 | k (T) = (5.43 × 1013) exp (−20,072.04/T) |
| IM9 → IM10 + CO via TS8 | k (T) = (3.33 × 1011) exp (−21,413.18/T) |
| IM6 → IM11 via TS9 | k (T) = (2.14 × 1013) exp (−26,398.65/T) |
| IM11 → IM12 + CO via TS10 | k (T) = (6.17 × 1011) exp (−21,840.02/T) |
| IM12 → IM13 via TS11 | k (T) = (1.37 × 1013) exp (−39,956.40/T) |
| IM13 → IM10 + CO via TS12 | k (T) = (3.70 × 1011) exp (−28,457.34/T) |
| IM5 + H → IM19 + H2 via TS17 | k (T) = (6.47 × 10−13) exp (−3826.40/T) |
| IM19 → IM20 via TS20 (0.93) | k (T) = (5.80 × 1012) exp (−4953.85/T) |
| IM19 → IM21 via TS21 (0.04) | k (T) = (2.76 × 1012) exp (−7312.34/T) |
| IM19 → IM22 via TS22 (0.02) | k (T) = (3.09 × 1012) exp (−8295.86/T) |
| IM19 → IM23 via TS23 (0.01) | k (T) = (4.04 × 1012) exp (−9165.93/T) |
| IM22 → IM26 via TS26 | k (T) = (1.70 × 1013) exp (−11,865.38/T) |
| IM23 → IM27 via TS27 | k (T) = (1.82 × 1013) exp (−11,673.73/T) |
| IM24/IM25 → IM28 via TS28 (0.06) | k (T) = (1.77 × 1012) exp (−7432.09/T) |
| IM24/IM25 → IM30 via TS31 (0.50) | k (T) = (2.48 × 1012) exp (−5572.69/T) |
| IM24/IM25 → IM32 via TS34 (0.40) | k (T) = (2.24 × 1012) exp (−5708.95/T) |
| IM24/IM25 → IM34 via TS37 (0.11) | k (T) = (1.61 × 1012) exp (−7509.02/T) |
| IM26/IM27 → IM36 via TS40 (0.11) | k (T) = (1.98 × 1012) exp (−7696.65/T) |
| IM26/IM25 → IM37 via TS42 (0.18) | k (T) = (3.29 × 1012) exp (−7701.35/T) |
| IM37 → 1-MCN + Cl via TS43 | k (T) = (2.03 × 1013) exp (−6825.08/T) |
| IM26/IM27 → IM38 via TS44 (0.69) | k (T) = (2.77 × 1012) exp (−6154.47/T) |
| IM26/IM27 → IM40 via TS47 (0.03) | k (T) = (7.40 × 1011) exp (−8061.95/T) |
| IM40 → 1-MCN + Cl via TS48 | k (T) = (3.50 × 1013) exp (−5895.30/T) |
| IM10 + H → IM42 + HCl via TS68 | k (T) = (1.56 × 10−11) exp (−4151.85/T) |
| IM10 + OH → IM42 + H2O via TS69 | k (T) = (6.16 × 10−12) exp (−10,425.51/T) |
| IM10 + Cl → IM42 + Cl2 via TS70 | k (T) = (1.04 × 10−10) exp (−5075.97/T) |
| IM42 → IM43 via TS53 (0.92) | k (T) = (1.98 × 1012) exp (−7696.65/T) |
| IM42 → IM44 via TS54 (0.08) | k (T) = (1.09 × 1012) exp (−6633.06/T) |
| IM44 → IM46 via TS56 | k (T) = (2.16 × 1013) exp (−13,348.67/T) |
| IM45 → IM47 via TS57 (0.51) | k (T) = (2.90 × 1012) exp (−5798.88/T) |
| IM45 → IM49 via TS60 (0.49) | k (T) = (2.75 × 1012) exp (−5797.29/T) |
| IM46 → IM51 via TS63 (0.91) | k (T) = (3.40 × 1012) exp (−5853.60/T) |
| IM46 → IM53 via TS66 (0.09) | k (T) = (2.94 × 1012) exp (−8057.90/T) |
| IM53 → N + Cl via TS67 | k (T) = (1.93 × 1013) exp (−6496.42/T) |
| IM10 + H → IM54 + H2 via TS71 | k (T) = (6.32 × 10−11) exp (−3311.61/T) |
| IM54 → IM22 via TS74 (0.62) | k (T) = (2.34 × 1012) exp (−5539.60/T) |
| IM54 → IM23 via TS75 (0.38) | k (T) = (3.71 × 1012) exp (−6473.85/T) |
3. Experimental Section
3.1. Density Functional Theory
3.2. Kinetic Calculation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, H.; Kang, J.H.; Baek, S.Y.; Chang, Y.S. Relative importance of polychlorinated naphthalenes compared to dioxins, and polychlorinated biphenyls in human serum from Korea: Contribution to TEQs and potential sources. Environ. Pollut. 2010, 158, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Imagawa, T.; Blankenship, A.L.; Giesy, J.P. Isomer-specific analysis and toxic evaluation of polychlorinated naphthalenes in soil, sediment, and biota collected near the site of a former chlor-alkali plant. Environ. Sci. Technol. 1998, 32, 2507–2514. [Google Scholar] [CrossRef]
- Kannan, K.; Kober, J.L.; Kang, Y.S.; Masunaga, S.; Nakanishi, J.; Ostaszewski, A.; Giesy, J.P. Polychlorinated naphthalenes, biphenyls, dibenzo-p-dioxins, and dibenzofurans as well as polycyclic aromatic hydrocarbons and alkylphenols in sediment from the Detroit and Rouge Rivers, Michigan, USA. Environ. Toxicol. Chem. 2001, 20, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Bidleman, T.F.; Helm, P.A.; Braune, B.M.; Gabrielsen, G.W. Polychlorinated naphthalenes in polar environments—A review. Sci. Total Environ. 2010, 408, 2919–2935. [Google Scholar] [CrossRef] [PubMed]
- Lerche, D.; van de Plassche, E.; Schwegler, A.; Balk, F. Selecting chemical substances for the UN-ECE POP protocol. Chemosphere 2002, 47, 617–630. [Google Scholar] [CrossRef]
- UNEP. Proposal to list chlorinated naphthalenes in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants. 2011. Available online: http://chm.pops.int/default.aspx?tabid=2267 (accessed on 22 October 2015).
- Olie, K.; Vermeulen, P.L.; Hutzinger, O. Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some incinerators in the Netherlands. Chemosphere 1977, 6, 455–459. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Clement, R.E.; Karasek, F.W. Analysis of fly ash from municipal incinerators for trace organic compounds. Anal. Chem. 1979, 51, 2343–2350. [Google Scholar] [CrossRef]
- Imagawa, T.; Takeuchi, M. Relation between isomer compositions of polychlorinated naphthalens and congener compositions of PCDDs/PCDFs from incinerators. Organohalogen Compd. 1995, 23, 487–490. [Google Scholar]
- Akki, U.; Mulholland, J.A. Gas-phase formation of dioxin and other aromatic products from 2,6-dichlorophenol pyrolysis. Organohalogen Compd. 1997, 31, 475–479. [Google Scholar]
- Yang, Y.; Mulholland, J.A.; Akki, U. Formation of furans by gasphase reactions of chlorophenols. Proc. Combust. Inst. 1998, 27, 1761–1768. [Google Scholar] [CrossRef]
- Evans, C.S.; Dellinger, B. Mechanisms of dioxin formation from the high-temperature pyrolysis of 2-chlorophenol. Environ. Sci. Technol. 2003, 37, 1325–1330. [Google Scholar] [CrossRef]
- Evans, C.S.; Dellinger, B. Mechanisms of dioxin formation from the high-temperature oxidation of 2-chlorophenol. Environ. Sci. Technol. 2005, 39, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.L.; Thomas, G.O.; Bailey, R.; Kerstiens, G.; Jones, K.C. Exchange of polychlorinated biphenyls (PCBs) and polychlorinated naphthalenes (PCNs) between air and a mixed pasture sward. Environ. Sci. Technol. 2004, 38, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.N.C.; Jansson, S.; Marklund, S. Effects of regional differences in waste composition on the thermal formation of polychlorinated aromatics during incineration. Chemosphere 2013, 93, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hiraoka, M.; Takeda, N.; Shiozaki, K. Behavior of coplanar PCNs and PCNs in oxidative conditions of municipal waste incineration. Chemosphere 1996, 32, 79–88. [Google Scholar] [CrossRef]
- Iino, F.; Imagawa, T.; Takeuchi, M.; Sadakata, M. De novo synthesis mechanism of polychlorinated dibenzofurans from polycyclic aromatic hydrocarbons and the characteristic isomers of polychlorinated naphthalenes. Environ. Sci. Technol. 1999, 33, 1038–1043. [Google Scholar] [CrossRef]
- Imagawa, T.; Lee, C.W. Correlation of polychlorinated naphthalenes with polychlorinated dibenzofurans formed from waste incineration. Chemosphere 2001, 44, 1511–1520. [Google Scholar] [CrossRef]
- Weber, R.; Iino, F.; Imagawa, T.; Takeuchi, M.; Sakurai, T.; Sadakata, M. Formation of PCDF, PCDD, PCB, and PCN in de novo synthesis from PAH: Mechanistic aspects and correlation to fluidized bed incinerators. Chemosphere 2001, 44, 1429–1438. [Google Scholar] [CrossRef]
- Oh, J.E.; Gullett, B.; Ryan, S.; Touati, A. Mechanistic relationships among PCDDs/Fs, PCNs, PAHs, CIPhs, and CIBzs in municipal waste incineration. Environ. Sci. Technol. 2007, 41, 4705–4710. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Mulholland, J.A. Temperature-dependent formation of polychlorinated naphthalenes and dihenzofurans from chlorophenols. Environ. Sci. Technol. 2005, 39, 5831–5836. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Mulholland, J.A.; Ryu, J.Y. Formation of polychlorinated naphthalenes from chlorophenols. Proc. Combust. Inst. 2005, 30, 1245–1253. [Google Scholar] [CrossRef]
- Kim, D.H.; Mulholland, J.A.; Ryu, J.Y. Chlorinated naphthalene formation from the oxidation of dichlorophenols. Chemosphere 2007, 67, S135–S143. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Meares, J.; Brooks, L. Priority pollutant PAH analysis of incinerator emission particles using HPLC and optimized fluorescence detection. Int. J. Environ. Anal. Chem. 1994, 54, 299–314. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kim, D.H.; Jang, S.H. Is chlorination one of the major pathways in the formation of polychlorinated naphthalenes (PCNs) in municipal solid waste. Environ. Sci. Technol. 2013, 47, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.J.; Russell, D.K. Role of hydrogen abstraction acetylene addition mechanisms in the formation of chlorinated naphthalenes. 1. A quantum chemical investigation. J. Phys. Chem. A 2014, 118, 12192–12204. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.J.; Russell, D.K. Role of hydrogen abstraction acetylene addition mechanisms in the formation of chlorinated naphthalenes. 2. Kinetic modeling and the detailed mechanism of ring closure. J. Phys. Chem. A 2014, 118, 12205–12220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Li, S.Q.; Qu, X.H.; Shi, X.Y.; Wang, W.X. A quantum mechanical study on the formation of PCDD/Fs from 2-chlorophenol as forerunner. Environ. Sci. Technol. 2008, 42, 7301–7308. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.H.; Wang, H.; Zhang, Q.Z.; Shi, X.Y.; Xu, F.; Wang, W.X. Mechanistic and kinetic studies on the homogeneous gas-phase formation of PCDD/Fs from 2,4,5-trichlorophenol. Environ. Sci. Technol. 2009, 43, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, W.N.; Gao, R.; Zhou, Q.; Zhang, Q.Z.; Wang, W.X. Dioxin formations from the radical/radical cross-condensation of phenoxy radicals with 2-chlorophenoxy radicals and 2,4,6-trichlorophenoxy radicals. Environ. Sci. Technol. 2010, 44, 6745–6751. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, W.N.; Zhou, Q.; Gao, R.; Sun, X.Y.; Zhang, Q.Z.; Wang, W.X. Mechanism and direct kinetic study of the polychlorinated dibenzo-p-dioxin and dibenzofuran formations from the radical/radical cross-condensation of 2,4-dichlorophenoxy with 2-chlorophenoxy and 2,4,6-trichlorophenoxy. Environ. Sci. Technol. 2011, 45, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Melius, C.F.; Colvin, M.E.; Marinov, N.M.; Pitz, W.J.; Senkan, S.M. Reaction mechanisms in aromatic hydrocarbon formation involving the C5H5 cyclopentadienyl moiety. Symp. Int. Combust. Proc. 1996, 26, 685–692. [Google Scholar] [CrossRef]
- Okajima, T.; Imafuku, K. Theoretical study on chlorine and hydrogen shift in cycloheptatriene and cyclopentadiene derivatives. J. Org. Chem. 2002, 67, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Qu, X.H.; Xu, F.; Shi, X.Y.; Wang, W.X. Mechanism and thermal rate constants for the complete series reactions of chlorophenols with H. Environ. Sci. Technol. 2009, 43, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, H.; Zhang, Q.Z.; Zhang, R.X.; Qu, X.H.; Wang, W.X. Kinetic properties for the complete series reactions of chlorophenols with OH radicals-relevance for dioxin formation. Environ. Sci. Technol. 2010, 44, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.; Altarawneh, M.; Dlugogorski, B.Z. Quantum chemical study on formation of PCDT/TA from 2-Chlorothiophenol forerunner. Environ. Sci. Technol. 2013, 47, 11040–11047. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Quantum chemical investigation of formation of polychlorodibenzo-p-dioxins and dibenzofurans from oxidation and pyrolysis of 2-chlorophenol. J. Phys. Chem. A 2007, 111, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Hybrid meta density functional theory methods for therochemistry, thermochemical kinetics, and noncovalent interactions: The MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Baldridge, M.S.; Gordon, R.; Steckler, R.; Truhlar, D.G. Ab initio reaction paths and direct dynamics calculations. J. Phys. Chem. 1989, 93, 5107–5119. [Google Scholar] [CrossRef]
- Gonzalez-Lafont, A.; Truong, T.N.; Truhlar, D.G. Interpolated variational transition-state theory: Practical methods for estimating variational transition-state properties and tunneling contributions to chemical reaction rates from electronic structure calculations. J. Chem. Phys. 1991, 95, 8875–8894. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Generalized transition state theory. Classical mechanical theory and applications to collinear reactions of hydrogen molecules. J. Phys. Chem. 1979, 83, 1052–1079. [Google Scholar] [CrossRef]
- Fernandez-Ramos, A.; Ellingson, B.A.; Garret, B.C.; Truhlar, D.G. Variational Transition State Theory with Multidimensional Tunneling. In Reviews in Computational Chemistry; Lipkowitz, K.B., Cundari, T.R., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2007. [Google Scholar]
- Corchado, J.C.; Chuang, Y.Y.; Fast, P.L.; Villa, J.; Hu, W.P.; Liu, Y.P.; Lynch, G.C.; Nguyen, K.A.; Jackels, C.F.; Melissas, V.S.; et al. POLYRATE, version 9.7; University of Minnesota: Minneapolis, MN, USA, 2007. [Google Scholar]
- Zhang, C.X.; Sun, T.L.; Sun, X.M. Mechanism for OH-initiated degradation of 2,3,7,8-tetrachlorinated dibenzo-p-dioxins in the presence of O2 and NO/H2O. Environ. Sci. Technol. 2011, 45, 4756–4762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Zhao, Y.Y.; Bai, J.; Gong, C.; Sun, X.M. Mechanism and kinetic study on the OH-initiated degradation of 2,3,7,8-tetrachlorinated dibenzofuran in atmosphere. Sci. Total Environ. 2012, 435, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, C.X.; Yang, W.B.; Hu, J.T.; Sun, X.M. Mechanism and kinetics study on the OH-initiated oxidation of organophosphorus pesticide trichlorfon in atmosphere. Sci. Total Environ. 2012, 419, 144–150. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Zhang, R.; Li, Y.; Zhang, Q.; Wang, W. Theoretical Mechanistic and Kinetic Studies on Homogeneous Gas-Phase Formation of Polychlorinated Naphthalene from 2-Chlorophenol as Forerunner. Int. J. Mol. Sci. 2015, 16, 25641-25656. https://doi.org/10.3390/ijms161025641
Xu F, Zhang R, Li Y, Zhang Q, Wang W. Theoretical Mechanistic and Kinetic Studies on Homogeneous Gas-Phase Formation of Polychlorinated Naphthalene from 2-Chlorophenol as Forerunner. International Journal of Molecular Sciences. 2015; 16(10):25641-25656. https://doi.org/10.3390/ijms161025641
Chicago/Turabian StyleXu, Fei, Ruiming Zhang, Yunfeng Li, Qingzhu Zhang, and Wenxing Wang. 2015. "Theoretical Mechanistic and Kinetic Studies on Homogeneous Gas-Phase Formation of Polychlorinated Naphthalene from 2-Chlorophenol as Forerunner" International Journal of Molecular Sciences 16, no. 10: 25641-25656. https://doi.org/10.3390/ijms161025641