Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review
Abstract
:1. Introduction
2. Effect of Metals on Microalgae: Growth Inhibition vs. Growth Enhancement
| Metal | Microalgae Strain | Cultivation Time | Concentration | Effect on Growth | Ref. |
|---|---|---|---|---|---|
| Hg | Chlorella sp. Scenedesmus acutus | 8 days | 2.5–5 mg/L | 100% growth inhibition | [58] |
| Hg | Selenastrum capricornutum | – | 0.027 mg/L | 50% inhibition | [59] |
| Pb | Phaeocystis antarctica | 10 days | 0.57 mg/L | 50% inhibition | [60] |
| Pb | Dunaliella tertiolecta | 48 h | 1.5–6.4 mg/L | 20% stimulation | [26] |
| 48 h | 7.29 mg/L | 25% inhibition | |||
| Cr(III) | Dyctiosphaerium chlorelloides | 72 h | 13–17 mg/L | 50% inhibition | [61] |
| Cr(III) | Scenedesmus sp. | 9 days | 0.75 µM | MMC | [62] |
| Geitlerinema sp. | 9 days | 0.25 µM | |||
| Cr(VI) | Chlorella pyrenoidosa | 72 h | 2 mg/L | 50% inhibition | [63] |
| Cr(VI) | Chlorella vulgaris | 96 h | 5 µmol/L | ~40% inhibition | [64] |
| As(III) | Chlorella sp. | 72 h | 25.2 mg/L | 50% inhibition | [65] |
| Monoraphidium arcuatum | 72 h | 14.6 mg/L | 50% inhibition | ||
| As(III) | Chlorella sp. | 72 h | 27 mg/L | 50% inhibition | [66] |
| As(V) | Chlorella sp. | 72 h | 1.1 mg/L | 50% inhibition | [66] |
| As(V) | Chlorella sp. | 72 h | 25.4 mg/L | 50% inhibition | [65] |
| Monoraphidium arcuatum | 72 h | 0.254 mg/L | 50% inhibition | ||
| As(V) | Oscillatoria tenuisa | 72 h | 3.8 mg/L | 50% inhibition | [67] |
| Anabaena affinis | 72 h | 2.6 mg/L | 50% inhibition | ||
| Microcystis aeruginosa | 72 h | 1.2 mg/L | 50% inhibition | ||
| As(III) | Nostoc minutum | 7 days | 5 mg/L | Cell death | [34] |
| As(V) | Nostoc minutum | 7 days | 1000 mg/L | 66% stimulation | [34] |
| Cu | Isochrysis galbana | 72 h | 0.01–0.018 mg/L T | 50% inhibition | [68] |
| Cu | Phaeocystis antarctica | 10 days | 0.0059 mg/L | 50% inhibition | [60] |
| Cd | Phaeocystis antarctica | 10 days | 1.5 mg/L | 50% inhibition | [60] |
| Cd | Scenedesmus armatus | 24 h | ~15–18 mg/L + or 0.46–0.54 mg/L +x | 50% inhibition | [69] |
| Cd | Thalassiosira weissflogii | – | 4.6 pM | ~30%–92% stimulation ZnL | [44] |
| Ni | Selenastrum capricornutum | – | 0.125 mg/L | 50% inhibition | [59] |
| Ni | Synechococcus sp. | 15 day | 25 mg/L | ~42% inhibition | [70] |
| Li | Chlorella vannielii | 12 h | 1000 mg/L | 48% inhibition | [30] |
| Li | Cyanothece sp. | 28 days | 70 mg/L | Cell death | [31] |
| Tl | Chlorella sp. | 72 h | 80 nmol | 100% inhibition | [71] |
| Tl | Synechocystis sp. | 72 h | 1 µM | 50% inhibition | [72] |
| Co | Monoraphidium minutum | 11 days | 0.5 ppm | 12% stimulation | [33] |
| 3 ppm | 44% inhibition | ||||
| Zn | Phaeocystis antarctica | 10 days | 1.11 mg/L | 50% inhibition | [60] |
| Zn | Anabaena sp. | 96 h | 0.38 mg/L | 50% inhibition | [73] |
| Al | Dunaliella tertiolecta | 48 h | 2.6–14.9 mg/L | 20% stimulation | [26] |
| 48 h | 22.42 mg/L | 25% inhibition | |||
| Al | Isochrysis galbana | 72 h | 2.57–3.23 mg/L T | 50% inhibition | [68] |
| V Met | Scenedesmus obliquus | 7 days | 20 µg/L | 534% stimulation * | [37] |
| V Met | Chlorella pyrenoidosa | 7 days | 1 µg/L | 67% stimulation | [38] |
| V Met | Chlorella pyrenoidosa | 7 days | >1 mg/L | Inhibitory threshold | [38] |
| V Ort | Haematococcus lacustris | 4 days | 2.5–5 mM | Full inhibition | [39] |
| V Oxi | Scenedesmus quadricauda | 12 days | 2.23 mg/L | 50% inhibition | [40] |
| Ce | Desmodesmus quadricauda | 3 days | 6 µmol/L | 16% stimulation A | [41] |
| Ce | Desmodesmus quadricauda | 3 days | 94 µmol/L | ~19% inhibition A | [41] |
| Ce | Desmodesmus quadricauda | 3 days | 5.74 µmol/L | 20% inhibition B | [41] |
| 60% stimulation C | |||||
| Ce | Desmodesmus quadricauda | 3 days | 1.14 µmol/L | 40% inhibition D | [41] |
| Ce | Anabaena flosaquae | 17 days | 0.1 mg/L | ~16% stimulation | [27] |
| 5–10 mg/L | ~33% inhibition | ||||
| La | Desmodesmus quadricauda | 3 days | 5.72 µmol/L | 10% inhibition B | [41] |
| 80% stimulation C | |||||
| La | Desmodesmus quadricauda | 3 days | 1.13 µmol/L | No change D | [41] |
| La | Scenedesmus quadricauda | 22–23 days | 72 µmol/L | 50% inhibition | [42] |
| La, Ce, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu | Skeletonema costatum | 96 h | 28–29 µmol/L | 50% inhibition | [43] |
| Nd | Desmodesmus quadricauda | 3 days | 5.76 µmol/L | 10% stimulation B | [41] |
| 120% stimulation C | |||||
| Nd | Desmodesmus quadricauda | 3 days | 1.09 µmol/L | ~5% inhibition D | [41] |
| TiO2-NPs | Nitzschia closterium | 96 h | 88–118 mg/L | 50% inhibition | [49] |
| TiO2-NPs | Pseudokirchneriella subcapitata | 72 h | 2.53 mg/L | 50% inhibition | [52] |
| TiO2-NPs | Chlorella vulgaris | – | 2.5–5 g/L | 42% inhibition | [74] |
| ZnO-NPs | Chlorella vulgaris | 72 h | 200 mg/L | 35% cell viability | [50] |
| ZnO-NPs | Dunaliella tertiolecta | 96 h | 2.4 mg/L | 50% inhibition | [56] |
| ZnO-NPs | Pseudokirchneriella subcapitata | 72 h | 0.1 mg/L | 80% inhibition | [52] |
| ZnO-NPs | Phaeodactylum tricornutum | – | 100 mg/L | 80% inhibition | [51] |
| Alexandrium minutum | 100 mg/L | 80% inhibition | |||
| Tetraselmis suecica | 100 mg/L | No effect | |||
| ZnO-NPs | Scenedesmus rubescens | 96 h | 14.27 mg/L or >810 mg/L CM | 50% inhibition | [53] |
| CeO2-NPs | Pseudokirchneriella subcapitata | 72 h | 4.1–6.2 mg/L AS | 50% inhibition | [55] |
| NiO-NPs | Chlorella vulgaris | 120 h | 44 mg/L | 50% inhibition | [75] |
| Y2O3-NPs | Phaeodactylum tricornutum | – | 100 mg/L | ~40% inhibition | [51] |
| Alexandrium minutum | 100 mg/L | ~40% inhibition | |||
| Tetraselmis suecica | 100 mg/L | 70% inhibition | |||
| BaTiO3-NPs | Chlorella vulgaris | 72 h | 1 mg/L | ~57% inhibition | [76] |
| Al2O3-NPs | Chlorella sp. | 72 h | 45.4 mg/L | 50% inhibition | [54] |
| Scenedesmus sp. | 72 h | 39.35 mg/L | 50% inhibition | ||
| Ag-NPs | Pseudokirchneriella subcapitata | 72 h | 1.63 mg/L | 50% inhibition | [77] |
| Pt-NPs | Pseudokirchneriella subcapitata | 72 h | 16.9 mg/L | 50% inhibition | [77] |
| nZVI-Nanofer 25 | Arthrospira maxima | 216 h | 5.1 mg/L | 19% stimulation | [57] |
| nZVI-Nanofer 25 | Desmodesmus subspicatus | 216 h | 5.1 mg/L | 73% stimulation | [57] |
| nZVI-Nanofer 25 | Parachlorella kessleri | 216 h | 5.1 mg/L | 38% stimulation | [57] |
3. Metal Stress as a Method for Stimulation of Bioproduct Synthesis
3.1. Pigments
| Chlorophyll Type | Microalgae Strain | Taxonomy | Reference |
|---|---|---|---|
| a, b | Chlorella vulgaris | Green microalgae | [98] |
| a, c1, c2 | Phaeodactylum tricornutum | Diatoms | [99] |
| a, c1, c2 | Kryptoperidinium foliaceum | Dinoflagellates | [100] |
| a, c2, c3 | Karenia mikimotoi | Dinoflagellates | [100] |
| a, d | Acaryochloris marina | Cyanobacteria | [101] |
| a, f | Halomicronema hongdechloris | Cyanobacteria | [102] |
3.2. Lipids
3.3. Exopolymers
3.4. Phytochelatin
| Strain | Metal | Metal Uplift | Phytochelatin Uplift | PCN A | Growth Rate C | Reference |
|---|---|---|---|---|---|---|
| Scenedesmus vacuolatus | Cd | 0.3→79 nM | ~3→25 amol/cell | PC2 | Reduced by 37% | [136] |
| ~1→44 amol/cell | PC3 | |||||
| ~0→17 amol/cell | PC4 | |||||
| Phaeodactylum tricornutum | Cd | 0→0.45 µM | ~0.16→3.6 amol/cell | PC2 | No change | [137] |
| ~0.5→1.3 amol/cell | PC3 | |||||
| ~0.05→1.5 amol/cell | PC4 | |||||
| Phaeodactylum tricornutum | Cu | 0.068 pM→0.4 µM | ~0.16→1.7 amol/cell | PC2 | No change | [137] |
| ~0.5→1.5 amol/cell | PC3 | |||||
| ~0.05→0.8 amol/cell | PC4 | |||||
| Phaeodactylum tricornutum | Cd | 0→10 µM | ~0→12.5 amol/cell | PC2 | Toxic effect avoided | [138] |
| ~0→25 amol/cell | PC4 | |||||
| ~0→5 amol/cell | PC5 | |||||
| Phaeodactylum tricornutum | Pb | 0→10 µM | ~0→50 amol/cell | PC2 | Toxic effect avoided | [138] |
| ~0→13 amol/cell | PC3 | |||||
| ~0→3 amol/cell | PC5 | |||||
| Phaeodactylum tricornutum | Cu | 0→10 µM | ~2→18 amol/cell | PC2 | – | [139] |
| ~0→38 amol/cell | PC3 | |||||
| ~0→5 amol/cell | PC6 | |||||
| Scenedesmus armatus | Cd | Const. 93 µM * | ~40→200 nmol-SH/g | PC2 | Reduced by 26% | [140] |
| ~80→1300 nmol-SH/g | PC3 | |||||
| ~20→280 nmol-SH/g | PC4 | |||||
| Stichococcus bacillaris | As(III) | Const. 100 µM ** | 0.07→0.15 µmol-SH/g | PC2 | Reduced by 20% | [141] |
| As(V) | Const. 100 µM ** | 0.14→0.38 µmol-SH/g | PC2 | Reduced by 30% |
3.5. Phytohormones
3.6. Organoarsenical Compounds
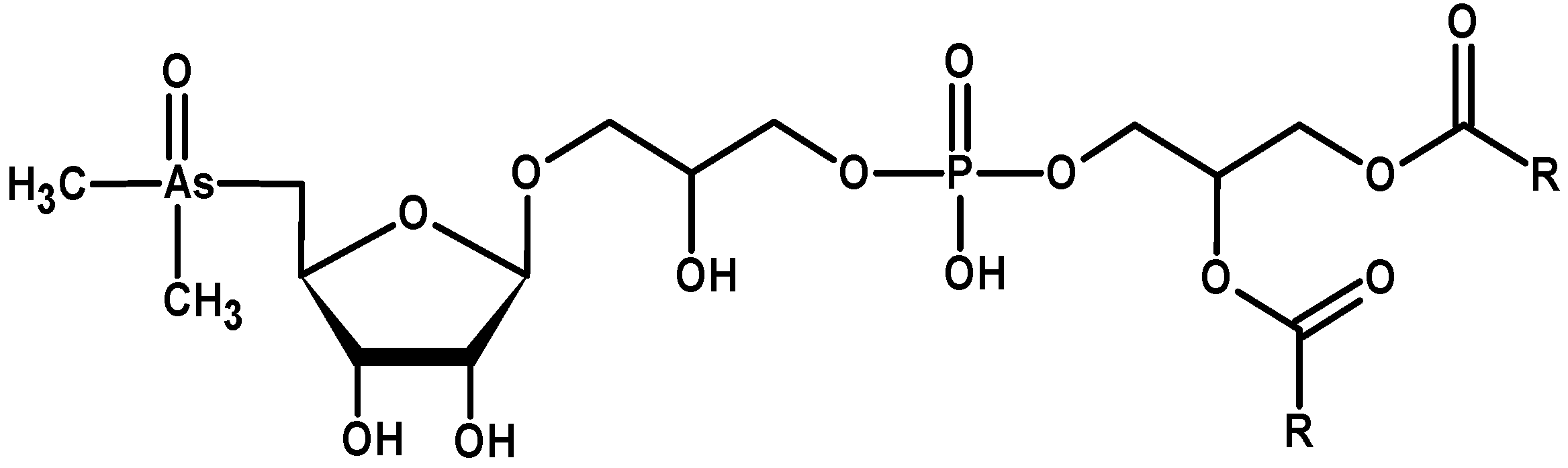
3.7. Nanoparticles and Nano-Needles
| Element NP | Source | Strain | Place of Synthesis | Average Particle Size (nm) | Reference |
|---|---|---|---|---|---|
| Gold (Au) | HAuCl4·3H2O | Chlorella vulgaris | Intracellularly | 40–60 | [170] |
| Gold (Au) | KAuCl4 | Eolimna minima | Intracellularly | 5–100 | [171] |
| Silver (Ag) | AgNO3 | Parachlorella kessleri | Extracellularly | 9, 14 or 18 | [172] |
| Silver (Ag) | AgNO3 | Botryococcus braunii | Extracellularly | 15.67 | [173] |
| Silver (Ag) | AgNO3 | Scenedesmus sp. | Intracellularly | 15–20 | [174] |
| Palladium (Pd) | Na2(PdCl4) | Chlorella vulgaris | Microalga culture | 7 | [175] |
| Palladium (Pd) | PdCl2 | Chlorella vulgaris | Intracellularly | 5–12 | [170] |
| Palladium (Pd) | PdCl2 | Plectonema boryanum | Extracellularly | ≤30 | [176] |
| Cadmium sulphide (CdS) | Cd(NO3)2·4H2O | Scenedesmus | Intracellularly | 120–175 (described as nanoparticles) | [177] |
| Nickel (Ni) | NiO–NPs | Chlorella vulgaris | Microalga culture | – | [75] |
4. Influence of Growth Conditions on Microalgal Resistance Towards Metals
4.1. Growth Media Composition and Cultivation Conditions
4.2. Supportive Compounds
4.2.1. Phytohormones: Modulating Effect
4.2.2. Chelating Agents: Modulating Effect
| Chelating Agent | Metal | Uplift of Chelating Agent Concentration | Strain | Reduction of Growth Inhibition | Reference |
|---|---|---|---|---|---|
| Humic acid (Soil) | Ni2+ (0.5 mg/L) | 0→0.2 mg/L | Dunaliella salina Nannochloropsis salina | 40% A→25% C30% A→15% C | [118] |
| Humic acid (Soil) | Cd2+ (0.2 mg/L) | 0→5 mg/L | Pseudokirchneriella subcapitata | 52% A→28% C | [195] |
| Humic acid (Soil) | Zn2+ (0.39 mg/L) | 0→5 mg/L | Pseudokirchneriella subcapitata | 55% A→4% C | [195] |
| Humic acid (Peat) | Cd2+ (0.2 mg/L) | 0→5 mg/L | Pseudokirchneriella subcapitata | 52% A→8% C | [195] |
| Humic acid (Peat) | Zn2+ (0.39 mg/L) | 0→5 mg/L | Pseudokirchneriella subcapitata | 55% A→30% C | [195] |
| Humic acid | As(III) (100 µM) | 0→10 mg/L | Stichococcus bacillaris | 52% A→33% C | [141] |
| Humic acid (Sediment) | Hg2+ (10 ppb) | 0→10 ppm | Isochrysis galbana | Complete reduction in growth inhibition plus stimulation | [196] |
| Humic acid | ZnO–NPs (1 mg/L) | 0→3 mg/L | Anabaena sp. | 70% A→40% C | [197] |
| Fulvic acid (Sediment) | Cu2+ (~5 µM) | 1→5 mg/L | Scenedesmus subspicatus | 56% A1→30% C1 | [188] |
| Fulvic acid (Suwannee River) | Cd2+ (0.2 mg/L) | 0→5 mg/L | Pseudokirchneriella subcapitata | 52% A→45% C | [195] |
| Fulvic acid (Suwannee River) | Zn2+ (0.39 mg/L) | 0→5 mg/L | Pseudokirchneriella subcapitata | No reduction in growth inhibition | [195] |
| Fulvic acid (Soil) | Al i+o (6 µM) | 0→11 mg/L | Chlorella pyrenoidosa | Complete reduction in growth inhibition plus stimulation | [202] |
4.2.3. Nanoparticles: Modulating Effect
4.2.4. Macrocycles: Modulating Effect
4.3. Development of Strain Tolerance to Metals
5. Strategy for Microalgal Production in the Presence of Metals
| Microalgae Strain | Bioproduct | Metal/s | Bioproduct Synthesis Info | Growth | Reference |
|---|---|---|---|---|---|
| Pigments | |||||
| Chlamydomonas acidophilla | β-carotene | Cu2+ 0.1 g/L | 120% increase | – | [103] |
| Coccomyxa onubensis | Fe2+ | [104] | |||
| Lutein | 0.5 mM | ~33% increase | 35% increase | ||
| Zeaxanthin | 0.5 mM | ~93% increase | 35% increase | ||
| β-carotene | 0.5 mM | ~35% increase | 35% increase | ||
| Dunaliella salina | β-carotene | Fe2+ 0→450 µM Ac | 7-fold increase | 4-fold decrease | [105] |
| Nostoc minutum | As(V) | [34] | |||
| Chlorophyll a | 0→1000 mg/L | 75% increase | 66% increase | ||
| Carotenoids | 0→1000 mg/L | 40% increase | 66% increase | ||
| Allophycocyanin | 0→1000 mg/L | 24.7% increase | 66% increase | ||
| Anabaena doliolum | Ni2+ | [92] | |||
| Chlorophyll a | 0→10 µM | ~47% increase | 35% increase 24h | ||
| C-phycocyanin | 0→0.1 µM | 4.35-fold increase | 9% decrease 96h | ||
| Dunaliella salina | Carotenoids | Cu2+1 µM→20 µM | 131% increase | >50% decrease | [244] |
| Chlorophyll | 62% increase | ||||
| Dunaliella tertiolecta | Carotenoids | 133% increase | |||
| Chlorophyll | 152% increase | ||||
| Pseudokirchneriella subcapitata | Chlorophyll a | Cu2+0.5→60 µg/L | 10.3-fold increase | Decrease (20% in growth rate and 72% in biomass) | [248] |
| Chlorophyll b | 15.4-fold increase | ||||
| Carotenoids | 4.1-fold increase | ||||
| Scenedesmus obliquus | Chlorophyll | VO3− 0→20 µg/L | 100% increase | 34% increase | [37] |
| Chlorella fusca | Lutein | VO3− 0→20 µg/L SFeC | 18% increase | – | [266] |
| β-carotene | 400% increase | ||||
| Zeaxanthin | 130% increase | ||||
| Chlorella fusca | Lutein | VO3− 0→20 µg/L FeDC | 17% increase | – | [266] |
| β-carotene | 200% increase | ||||
| Zeaxanthin | 40% increase | ||||
| Haematococcus lacustris | Carotenoids | VO43−0→1.25 mM | 125% increase 2DE | 45% decrease 2DE | [39] |
| Haematococcus lacustris | Carotenoids | VO43− 0→1.25 mM | No increase 4DE | 40% decrease 4DE | [39] |
| Lipids | |||||
| Chlorella minutissima | Lipids | Cd2+ 0→0.4 mM | ~94% increase | ~12% increase | [115] |
| Euglena gracilis | Lipids | Cr6+ 0→1.3 µM 40%,1 | 44% increase 40%,1 | IC50 for 3.2 µM 1 | [116] |
| Euglena gracilis | Lipids | Cr6+ 0→9.84 µM 40%,2 | 28.5% increase 40%,2 | IC50 for 24.6 µM 2 | [116] |
| Euglena gracilis | Lipids | Cr6+ 0→36.16 µM 40%,3 | 100% increase 40%,3 | IC50 for 90.4 µM 3 | [116] |
| Euglena gracilis | Lipids | Cr6+ 0→48.2 µM 40%,4 | 10% increase 40%,4 | IC50 for 120.5 µM 4 | [116] |
| Chlorella vulgaris | Lipids | TiO2-NPs 0→0.1 g/L | 10% increase | No change | [74] |
| Arthrospira maxima | Lipids | nZVI-Nanofer 25 0→5.1 mg/L | 21% increase | 15% increase | [57] |
| Desmodesmus subspicatus | Lipids | nZVI-Nanofer 25 0→5.1 mg/L | 58% increase | 73% increase | [57] |
| Parachlorella kessleri | Lipids | nZVI-Nanofer 25 0→5.1 mg/L | 17% increase | 41% increase | [57] |
| Nannochloropsis limnetica | Eicosapentaenoic acid C20:5 | nZVI-Nanofer 25 0→5.1 mg/L | 58 % increase | 19% increase | [57] |
| Trachydiscus minutus | Eicosapentaenoic acid C20:5 | nZVI-Nanofer 25 0→5.1 mg/L | 34% increase | 31% increase | [57] |
| Scenedesmus obliquus | Lipids | (As, Cd, Co, Cr, Cu, Hg, Ni, Pb, Se, Zn) as a mixture | 61% increase 1x | 12% increase 1x | [255] |
| Neochloris sp. | Oleic acid C18:1 | Effluent from textile dyeing industry containing Pb Ut | Neutral lipid accumulation Oleic acid accumulation | – | [256] |
| Chlorella vulgaris | Lipids | Fe3+/EDTA0→12 µM | 7.25-fold increase | ~27% increase | [263] |
| Nannochloropsis oculata | Lipids | Fe3++EDTA 3.16→18.96 mg/L | 22% increase in production | – | [264] |
| Exopolymers | |||||
| Lyngbya putealis | Cu | 13% decrease | [131] | ||
| Exopolysaccharides | 0→2 mg/L | 2.43-fold increase | |||
| Exoproteins | 0→2 mg/L | 3.65-fold increase | |||
| Lyngbya putealis | Co | 21% decrease | [131] | ||
| Exopolysaccharides | 0→2 mg/L | 2.09-fold increase | |||
| Exoproteins | 0→2 mg/L | 2.64-fold increase | |||
| Thalassiosira weissflogii | Polysaccharides EPF | Ag RENP | ~3.5-fold increase NL if: Ag 0.03→0.11 nM | 50% decrease NL if: Ag 0.01 nM | [132] |
| Thalassiosira weissflogii | Polysaccharides EPF | Ag RENP | ~6-fold increase NE if: Ag0.01→6.14 pM | 50% decrease NE if: Ag 2.16 pM | [132] |
| Thalassiosira pseudonana | Proteins EPF | Cd RENP 0→0.05 nM | 50% increase CM,NE | No change NE | [133] |
| Thalassiosira pseudonana | Carbohydrates EPF | Cd RENP 0→0.05 nM | 2-fold increase CM,NE | No change NE | [133] |
| Cylindrotheca fusiformis | Exopolysaccharides | Cu2+ 0→0.5 mg/L | 100% increase RC | 57% decrease | [245] |
| Phytohormones | |||||
| Chlorella vulgaris | Indole-acetic acid | Cd | [154] | ||
| 0→10−4 M | ~147% increase Ct | ~35% decrease Ct | |||
| 0→10−4 M +B | 3.6-fold increase Ct | ~8% decrease Ct | |||
| Chlorella vulgaris | Zeatin | Pb | [154] | ||
| 0→10−4 M | ~35% increase Ct | ~40% decrease Ct | |||
| 0→10−4 M +B | ~85% increase Ct | ~16% decrease Ct | |||
| Chlorella vulgaris | Abscisic acid | Cu | [154] | ||
| 0→10−4 M | ~45% increaseCt | ~45% decrease Ct | |||
| 0→10−4 M +B | ~65% increaseCt | ~24% decrease Ct | |||
| Hydrogen | |||||
| Chlamydomonas reinhardtii | H2 | 16% leachate medium containing: (Cr, Mn, Fe, Co, Ni, Cu, Mo, Cd, Pb) | ~37% increase | ~50% increase | [257] |
| Anabaena variabilis | H2 | VO3− 0→0.023 mg/L M | 5.5-fold increase | Delayed FSC No change in growth PCT | [265] |
| Other products | |||||
| Dunaliella tertiolecta | Phenolics | Cu2+ 0→0.79 µM | 40% increase RC | 34% decrease | [246] |
| Chlorella vulgaris | Chlorophyll a | Cd2+ 0→0.1 µmol/L | ~4–fold increase | ~65% decrease | [247] |
| Protein | ~5–fold increase | ||||
| Lipids | ~3–fold increase | ||||
| Chlorella pyrenoidosa | Proline Total Amino Acids | Cr6+ 0→5 mg/L | 240% increase 66% increase | 60% decrease | [63] |
| Botryococcus braunii | Hydrocarbons | Modifications of culture media composition | 27% increase after: Fe and Mn uplift + Mo decrease + Ni addition (1.73 µM) | 34% increase after: Fe and Mn decrease + Mo uplift + Ni addition (3.38 µM) | [262] |
6. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; de Clerck, O. Phylogeny and molecular evolution of the green algae. CRC Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef]
- Scott, J.L.; Baca, B.; Ott, F.D.; West, J.A. Light and electron microscopic observations on Erythrolobus coxiae gen.et sp. nov. (Porphyrideophyceae, Rhodophyta) from Texas U.S.A. Algae 2006, 21, 407–416. [Google Scholar] [CrossRef]
- Mann, D.G. The species concept in diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef]
- Hackett, J.D.; Anderson, D.M.; Erdner, D.L.; Bhattacharya, D. Dinoflagellates: A remarkable evolutionary experiment. Am. J. Bot. 2004, 91, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Komarek, J. Cyanobacterial taxonomy: Current problems and prospects for the integration of traditional and molecular approaches. Algae 2006, 21, 349–375. [Google Scholar] [CrossRef]
- Lowrey, J.; Brooks, M.S.; McGinn, P.J. Heterotrophic and mixotrophic cultivation of microalgae for biodiesel production in agricultural wastewaters and associated challenges—A critical review. J. Appl. Phycol. 2015, 27, 1485–1498. [Google Scholar] [CrossRef]
- Shukla, S.P.; Kviderova, J.; Triska, J.; Elster, J. Chlorella mirabilis as a potential species for biomass production in low-temperature environment. Front. Microbiol. 2013, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Bleeke, F.; Rwehumbiza, V.M.; Winckelmann, D.; Klöck, G. Isolation and characterization of new temperature tolerant microalgal strains for biomass production. Energies 2014, 7, 7847–7856. [Google Scholar] [CrossRef]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Slaveykova, V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae—State of the art and knowledge gaps. Nanotoxicology 2013, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.; Vignati, D.A.L.; Leyval, C.; Giamberini, L. Environmental fate and ecotoxicity of lanthanides: Are they a uniform group beyond chemistry? Environ. Int. 2014, 71, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: San Diego, CA, USA, 2005; ISBN 978-0-12-088426-1. [Google Scholar]
- Moroney, J.V.; Bartlett, S.G.; Samuelsson, G. Carbonic anhydrases in plants and algae. Plant Cell Environ. 2001, 24, 141–153. [Google Scholar] [CrossRef]
- Sunda, W.G. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front. Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Bothe, H.; Schmitz, O.; Yates, M.G.; Newton, W.E. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.M.; Herrera, J.; Aparicio, P.J.; Paneque, A.; Losada, M. Role of molybdenum in nitrate reduction by Chlorella. Plant Physiol. 1971, 48, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Yamakoshi, Y.; Uemura, I.; Tominaga, N. Ultrastructural changes in Chlamydomonas acidophila (Chlorophyta) induced by heavy metals and polyphosphate metabolism. FEMS Microbiol. Ecol. 2003, 44, 253–259. [Google Scholar] [CrossRef]
- Carfagna, S.; Lanza, N.; Salbitani, G.; Basile, A.; Sorbo, S.; Vona, V. Physiological and morphological responses of lead and cadmium exposed Chlorella sorokiniana 211-8K (Chlorophyceae). Springerplus 2013, 2, 147. [Google Scholar] [CrossRef]
- Aoki, M.; Matsumoto, H.; Takahashi, T.; Sato, K.; Kumata, H.; Fujiwara, K. Thallium Induces Morphological Changes in the Photosynthetic Apparatus of Synechocystis sp. PCC6803. In Photosynthesis: Research for Food, Fuel and Future—15th International Conference on Photosynthesis; Zhejiang University Press: Hangzhou, China, 2013; pp. 586–589. [Google Scholar]
- Andosch, A.; Hoftberger, M.; Lutz, C.; Lutz-Meindl, U. Subcellular sequestration and impact of heavy metals on the ultrastructure and physiology of the multicellular freshwater alga Desmidium swartzii. Int. J. Mol. Sci. 2015, 16, 10389–10410. [Google Scholar] [CrossRef] [PubMed]
- Sacan, M.T.; Oztay, F.; Bolkent, S. Exposure of Dunaliella tertiolecta to lead and aluminum: Toxicity and effects on ultrastructure. Biol. Trace Elem. Res. 2007, 120, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Yingjun, W.; Jia, L.; Yun, L.; Hangbiao, J.; Shihuai, D.; Yunmin, Z. Effects of cerium on growth and physiological characteristics of Anabaena flosaquae. J. Rare Earths 2012, 30, 1287–1292. [Google Scholar]
- Periz, G.; Dharia, D.; Miller, S.H.; Keller, L.R. Flagellar elongation and gene expression in Chlamydomonas reinhardtii. Eukaryot. Cell 2007, 6, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Nordi, C.S.F.; Cavagliere, T.G.W.F.; Vieira, A.A.H.; Nascimento, O.R. Efeito caotrópico do íon lítio na permeabilidade da cápsula polissacarídica da microalga Ankistrodesmus gracilis (Reinsch) Korsikov (Chlorophyceae). Acta Bot. Bras. 2006, 20, 449–454. [Google Scholar] [CrossRef]
- Karlander, E.P.; Krauss, R.W. Absorption and toxicity of beryllium and lithium in Chlorella vannielii Shihira and Krauss. Chesap. Sci. 1972, 13, 245–253. [Google Scholar] [CrossRef]
- Mota, R.; Pereira, S.B.; Meazzini, M.; Fernandes, R.; Santos, A.; Evans, C.A.; de Philippis, R.; Wright, P.C.; Tamagnini, P. Effects of heavy metals on Cyanothece sp. CCY 0110 growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles. J. Proteom. 2015, 120, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Basharina, T.N.; Danilovtseva, E.N.; Zelinskiy, S.N.; Klimenkov, I.V.; Likhoshway, Y.V.; Annenkov, V.V. The effect of titanium, zirconium and tin on the growth of diatom Synedra acus and morphology of its silica valves. Silicon 2012, 4, 239–249. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; el-Naggar, A.H.; Osman, M.E.H.; el-Mazaly, E. Effect of cobalt on growth, pigments and the photosynthetic electron transport in Monoraphidium minutum and Nitzchia perminuta. Braz. J. Plant Physiol. 2003, 15, 159–166. [Google Scholar] [CrossRef]
- Ferrari, S.G.; Silva, P.G.; Gonzalez, D.M.; Navoni, J.A.; Silva, H.J. Arsenic tolerance of cyanobacterial strains with potential use in biotechnology. Rev. Argent. Microbiol. 2013, 45, 174–179. [Google Scholar] [CrossRef]
- Karadjova, I.B.; Slaveykova, V.I.; Tsalev, D.L. The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat. Toxicol. 2008, 87, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Knauer, K.; Hemond, H. Accumulation and reduction of arsenate by the freshwater green alga Chlorella sp. (Chlorophyta). J. Phycol. 2000, 36, 506–509. [Google Scholar] [CrossRef]
- Meisch, H.U.; Bielig, H.J. Effect of vanadium on growth, chlorophyll formation and iron metabolism in unicellular green algae. Arch. Microbiol. 1975, 105, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Meisch, H.U.; Benzschawel, H.; Bielig, H.J. The role of vanadium in green plants. Arch. Microbiol. 1977, 114, 67–70. [Google Scholar] [CrossRef]
- Tran, N.P.; Park, J.K.; Kim, Z.H.; Lee, C.G. Influence of sodium orthovanadate on the production of astaxanthin from green algae Haematococcus lacustris. Biotechnol. Bioprocess Eng. 2009, 14, 322–329. [Google Scholar] [CrossRef]
- Fargasova, A.; Bumbalova, A.; Havranek, E. Ecotoxicological effects and uptake of metals (Cu+, Cu2+, Mn2+, Mo6+, Ni2+, V5+) in freshwater alga Scenedesmus quadricauda. Chemosphere 1999, 38, 1165–1173. [Google Scholar] [CrossRef]
- Goecke, F.; Jerez, C.G.; Zachleder, V.; Figueroa, F.L.; Rezanka, T.; Bisova, K.; Vitova, M. Use of lanthanides to alleviate the effects of metal ion-deficiency in Desmodesmus quadricauda (Sphaeropleales, Chlorophyta). Front. Microbiol. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chu, Z.; Yan, F.; Zeng, Q. Effects of lanthanum(III) and EDTA on the growth and competition of Microcystis aeruginosa and Scenedesmus quadricauda. Limnologica 2009, 39, 86–93. [Google Scholar] [CrossRef]
- Tai, P.; Zhao, Q.; Su, D.; Li, P.; Stagnitti, F. Biological toxicity of lanthanide elements on algae. Chemosphere 2010, 80, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Roberts, S.B.; Morel, F.M.M. Cadmium: A nutrient for the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 1995, 40, 1056–1063. [Google Scholar] [CrossRef]
- Alterio, V.; Langella, E.; de Simone, G.; Monti, S.M. Cadmium-containing carbonic anhydrase CDCA1 in marine diatom Thalassiosira weissflogii. Mar. Drugs 2015, 13, 1688–1697. [Google Scholar] [PubMed]
- Rees, T.A.V.; Bekheet, I.A. The role of nickel in urea assimilation by algae. Planta 1982, 156, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Antia, N.J. Evidence of nickel ion requirement for autotrophic growth of a marine diatom with urea serving as nitrogen source. Br. Phycol. J. 1984, 19, 125–134. [Google Scholar] [CrossRef]
- Egleston, E.S.; Morel, F.M.M. Nickel limitation and zinc toxicity in a urea-grown diatom. Limnol. Oceanogr. 2008, 53, 2462–2471. [Google Scholar] [CrossRef]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef]
- Suman, T.Y.; Rajasree, S.R.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bugallo, A.; Gonzalez-Fernandez, A.; Guisande, C.; Barreiro, A. Comparative responses to metal oxide nanoparticles in marine phytoplankton. Arch. Environ. Contam. Toxicol. 2014, 67, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; An, Y.J. Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: No evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere 2013, 91, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Aravantinou, A.F.; Tsarpali, V.; Dailianis, S.; Manariotis, I.D. Effect of cultivation media on the toxicity of ZnO nanoparticles to freshwater and marine microalgae. Ecotoxicol. Environ. Saf. 2015, 114, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Manier, N.; Bado-Nilles, A.; Delalain, P.; Aguerre-Chariol, O.; Pandard, P. Ecotoxicity of non-aged and aged CeO2 nanomaterials towards freshwater microalgae. Environ. Pollut. 2013, 180, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Manzo, S.; Miglietta, M.L.; Rametta, G.; Buono, S.; Francia, G.D. Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tertiolecta. Sci. Total Environ. 2013, 445–446, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Padrova, K.; Lukavsky, J.; Nedbalova, L.; Cejkova, A.; Cajthaml, T.; Sigler, K.; Vitova, M.; Rezanka, T. Trace concentrations of iron nanoparticles cause overproduction of biomass and lipids during cultivation of cyanobacteria and microalgae. J. Appl. Phycol. 2015, 27, 1443–1451. [Google Scholar] [CrossRef]
- Capolino, E.; Tredici, M.; Pepi, M.; Baldi, F. Tolerance to mercury chloride in Scenedesmus strains. BioMetals 1997, 10, 85–94. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, K.C. Optimization and performance evaluation of the continuous algal toxicity test. Environ. Toxicol. Chem. 1997, 16, 1337–1344. [Google Scholar] [CrossRef]
- Gissi, F.; Adams, M.S.; King, C.K.; Jolley, D.F. Robust bioassay to assess the toxicity of metals to the Antarctic marine microalga Phaeocyctis antarctica. Environ. Toxicol. Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- D’ors, A.; Pereira, M.; Bartolome, M.C.; Lopez-Rodas, V.; Costas, E.; Sanchez-Fortun, S. Toxic effects and specific chromium acquired resistance in selected strains of Dyctiosphaerium chlorelloides. Chemosphere 2010, 81, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Millach, L.; Sole, A.; Esteve, I. Role of Geitlerinema sp. DE2011 and Scenedesmus sp. DE2009 as bioindicators and immobilizers of chromium in a contaminated natural environment. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Hörcsik, Z.; Oláh, V.; Balogh, A.; Mészáros, I.; Simon, L.; Lakatos, G. Effect of Chromium(VI) on growth, element and photosynthetic pigment composition of Chlorella pyrenoidosa. Acta Biol. Szeged. 2006, 50, 19–23. [Google Scholar]
- Ouyang, H.L.; Kong, X.Z.; He, W.; Qin, N.; He, Q.S.; Wang, Y.; Wang, R.; Xu, F.L. Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chin. Sci. Bull. 2012, 57, 3363–3370. [Google Scholar] [CrossRef]
- Levy, J.L.; Stauber, J.L.; Adams, M.S.; Maher, W.A.; Kirby, J.K.; Jolley, D.F. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ. Toxicol. Chem. 2005, 24, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hogan, B.; Duncan, E.; Doyle, C.; Krassoi, R.; Rahman, M.M.; Naidu, R.; Lim, R.P.; Maher, W.; Hassler, C. Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35). Ecotoxicol. Environ. Saf. 2014, 106, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Wu, C.C.; Chang, W.C. Bioaccumulation and toxicity of arsenic in cyanobacteria cultures separated from a eutrophic reservoir. Environ. Monit. Assess. 2014, 186, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, M.A.; van Dam, J.W.; Harford, A.J.; Parry, D.; Streten, C.; Gibb, K.; van Dam, R.A. Aluminium, gallium and molybdenum toxicity to the tropical marine microalga Isochrysis galbana. Environ. Toxicol. Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bascik-Remisiewicz, A.; Tukaj, Z. Toxicity of inorganic cadmium salts to the microalga Scenedesmus armatus (Chiorophyta) with respect to medium composition, pH and CO2 concentration. Acta Physiol. Plant. 2002, 24, 59–65. [Google Scholar] [CrossRef]
- Nohomovich, B.; Nguyen, B.T.; Quintanilla, M.; Lee, L.H.; Murray, S.R.; Chu, T.C. Physiological effects of nickel chloride on the freshwater cyanobacterium Synechococcus sp. IU 625. Adv. Biosci. Biotechnol. 2013, 4, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.S.; Chafin, R.D.; Klinger, M.B.; Twiss, M.R. Application of the biotic ligand model to explain potassium interaction with thallium uptake and toxicity to plankton. Environ. Toxicol. Chem. 2007, 26, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Suematsu, H.; Kumata, H.; Fujiwara, K. Physiological and photosynthetic toxicity of thallium in Synechocystis sp. PCC6803. In Photosynthesis. Energy from the Sun: 14th International Congress on Photosynthesis; Springer: Berlin, Germany, 2008; pp. 1399–1402. [Google Scholar]
- Tang, Y.; Li, S.; Qiao, J.; Wang, H.; Li, L. Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp. Int. J. Mol. Sci. 2013, 14, 14395–14407. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.K.; Lee, B.; Choi, G.G.; Moon, M.; Park, M.S.; Lim, J.K.; Yang, J.W. Enhancing lipid productivity of Chlorella vulgaris using oxidative stress by TiO2 nanoparticles. Korean J. Chem. Eng. 2014, 31, 861–867. [Google Scholar] [CrossRef]
- Gong, N.; Shao, K.; Feng, W.; Lin, Z.; Liang, C.; Sun, Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 2011, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Polonini, H.C.; Brandao, H.M.; Raposo, N.R.B.; Brandao, M.A.F.; Mouton, L.; Coute, A.; Yepremian, C.; Sivry, Y.; Brayner, R. Size-dependent ecotoxicity of barium titanate particles: The case of Chlorella vulgaris green algae. Ecotoxicology 2015, 24, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Ksiazyk, M.; Asztemborska, M.; Steborowski, R.; Bystrzejewska-Piotrowska, G. Toxic effect of silver and platinum nanoparticles toward the freshwater microalga Pseudokirchneriella subcapitata. Bull. Environ. Contam. Toxicol. 2015, 94, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Blaby-Haas, C.E.; Merchant, S.S. The ins and outs of algal metal transport. Biochim. Biophys. Acta 2012, 1823, 1531–1552. [Google Scholar] [CrossRef] [PubMed]
- Cassier-Chauvat, C.; Chauvat, F. Responses to oxidative and heavy metal stresses in cyanobacteria: Recent advances. Int. J. Mol. Sci. 2015, 16, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Perales-Vela, H.V.; Pena-Castro, J.M.; Canizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E. Chelation: Harnessing and enhancing heavy metal detoxification—A review. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Deuchi, K. Culture of a high-chlorophyll-producing and halotolerant Chlorella vulgaris. J. Biosci. Bioeng. 2014, 117, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, A.; Ladhari, N.; Hamadi, N.B.; Msaddek, M.; Sakli, F. First application of chlorophyll-a as biomordant: Sonicator dyeing of wool with betanin dye. J. Clean. Prod. 2013, 39, 97–104. [Google Scholar] [CrossRef]
- Park, S.J.; Park, Y.M. Eco-dyeing and antimicrobial properties of chlorophyllin copper complex extracted from Sasa veitchii. Fibers Polym. 2010, 11, 357–362. [Google Scholar] [CrossRef]
- Kupper, H.; Kupper, F.; Spiller, M. Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef]
- Droupadi, P.R.; Krishnan, V. An efficient method of preparation of pheophytin a—Divalent metal pheophytinates. Proc. Indian Acad. Sci. 1984, 93, 117–124. [Google Scholar]
- Karcz, D.; Boroń, B.; Matwijczuk, A.; Furso, J.; Staroń, J.; Ratuszna, A.; Fiedor, L. Lessons from chlorophylls: Modifications of porphyrinoids towards optimized solar energy conversion. Molecules 2014, 19, 15938–15954. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; Lima, A.; Soares, R.R.S.; Batistela, V.R.; Gerola, A.P.; Hioka, N.; Bonacin, J.A.; Severino, D.; Baptista, M.S.; da Hora Machado, A.E.; et al. Metallochlorophylls of magnesium, copper and zinc: Evaluation of the influence of the first coordination sphere on their solvatochromism and aggregation properties. J. Braz. Chem. Soc. 2009, 20, 1653–1658. [Google Scholar] [CrossRef]
- Petrovic, J.; Nikolic, G.; Markovic, D. In vitro complexes of copper and zinc with chlorophyll. J. Serb. Chem. Soc. 2006, 71, 501–512. [Google Scholar] [CrossRef]
- Ngo, T.; Zhao, Y. Formation of zinc-chlorophyll-derivative complexes in thermally processed green pears (Pyruscommunis L.). J. Food Sci. 2007, 72, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.K.; Tripathi, R.D.; Sharma, N.; Dwivedi, S.; Mishra, S.; Singh, R.; Shukla, O.P.; Rai, U.N. Responses of cyanobacterium Anabaena doliolum during nickel stress. J. Environ. Biol. 2009, 30, 871–876. [Google Scholar] [PubMed]
- Basaca-Loya, G.A.; Valdez, M.A.; Enriquez-Guevara, E.A.; Gutierrez-Millan, L.E.; Burboa, M.G. Extraction and purification of B-phycoerythrin from the red microalga Rhodosorus marinus. Cienc. Mar. 2009, 35, 359–368. [Google Scholar]
- Mishra, S.K.; Shrivastav, A.; Maurya, R.R.; Patidar, S.K.; Haldar, S.; Mishra, S. Effect of light quality on the C-phycoerythrin production in marine cyanobacteria Pseudanabaena sp. isolated from Gujarat coast, India. Protein Expr. Purif. 2012, 81, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 8, 1477–1491. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production and biotechnological applications of c-phycocyanin. Biomed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ratha, S.K.; Prasanna, R.; Gupta, V.; Dhar, D.W.; Saxena, A.K. Bioprospecting and indexing the microalgal diversity of different ecological habitats of India. World J. Microbiol. Biotechnol. 2012, 28, 1657–1667. [Google Scholar] [CrossRef]
- Kosakowska, A.; Lewandowska, J.; Ston, J.; Burkiewicz, K. Qualitative and quantitative composition of pigments in Phaeodactylum tricornutum (Bacillariophyceae) stressed by iron. BioMetals 2004, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.; Fraga, S.; Rodriguez, F.; Garrido, J.L. Pigment-based chloroplast types in dinoflagellates. Mar. Ecol. Prog. Ser. 2012, 465, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Mohr, R.; Voβ, B.; Schliep, M.; Kurz, T.; Maldener, I.; Adams, D.G.; Larkum, A.D.W.; Chen, M.; Hess, W.R. A new chlorophyll d-containing cyanobacterium: Evidence for niche adaptation in the genus Acaryochloris. ISME J. 2010, 4, 1456–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Birch, D.; Willows, R.D. A cyanobacterium that contains chlorophyll f—A red-absorbing photopigment. FEBS Lett. 2012, 586, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, I.; Cuaresma, M.; Vılchez, C.; Vega, J.M. Effect of abiotic stress on the production of lutein and β-carotene by Chlamydomonas acidophila. Process Biochem. 2008, 43, 1158–1161. [Google Scholar] [CrossRef]
- Garbayo, I.; Torronteras, R.; Forjan, E.; Cuaresma, M.; Casal, C.; Mogedas, B.; Ruiz-Domınguez, M.C.; Marquez, C.; Vaquero, I.; Fuentes-Cordero, J.L.; et al. Identification and physiological aspects of a novel carotenoid-enriched, metal-resistant microalga isolated from an acidic river in Huelva (Spain). J. Phycol. 2012, 48, 607–614. [Google Scholar] [CrossRef]
- Mojaat, M.; Pruvost, J.; Foucault, A.; Legrand, J. Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem. Eng. J. 2008, 39, 177–184. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Pal, R. Scope of phycoremediation of Arsenic using Phormidium tenue with special reference to modulation in cellular biochemistry. J. Algal Biomass Util. 2012, 3, 1–8. [Google Scholar]
- Arunakumara, K.K.I.U.; Xuecheng, Z. Effects of heavy metals (Pb2+ and Cd2+) on the ultrastructure, growth and pigment contents of the unicellular cyanobacterium Synechocystis sp. PCC 6803. Chin. J. Oceanol. Limnol. 2009, 27, 383–388. [Google Scholar] [CrossRef]
- Arunakumara, K.K.I.U.; Xuecheng, Z.; Xiaojin, S. Bioaccumulation of Pb2+ and its effects on growth, morphology and pigment contents of Spirulina (Arthrospira) platensis. J. Ocean Univ. Chin. 2008, 7, 397–403. [Google Scholar] [CrossRef]
- Shanab, S.; Essa, A.; Shalaby, E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates). Plant Signal. Behav. 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wong, K.H.; Yang, Y.; Li, X.; Jiang, J.; Zheng, W.; Wu, H.; Chen, T. Purification and in vitro antioxidant activities of tellurium-containing phycobiliproteins from tellurium-enriched Spirulina platensis. Drug Des. Dev. Ther. 2014, 8, 1789–1800. [Google Scholar]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Su, P.; Zhang, W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Iwama, D.; Liang, Y.; Murakami, N.; Ishikura, M.; Tanaka, T.; Matsunaga, T. Glycosylceramides from marine green microalga Tetraselmis sp. Phytochemistry 2013, 85, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yilancioglu, K.; Cokol, M.; Pastirmaci, I.; Erman, B.; Cetiner, S. Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS ONE 2014, 9, e91957. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Cao, J.; Xing, G.L.; Yuan, H.L. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 2015, 175, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Rocchetta, I.; Mazzuca, M.; Conforti, V.; Ruiz, L.; Balzaretti, V.; Molina, M.C.R. Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environ. Pollut. 2006, 141, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cheng, J.; Yang, Z.; Li, K.; Zhou, J.; Cen, K. Microstructures and functional groups of Nannochloropsis sp. cells with arsenic adsorption and lipid accumulation. Bioresour. Technol. 2015, 194, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mohammady, N.G.-E.D.; Fathy, A.A. Humic acid mitigates viability reduction, lipids and fatty acids of Dunaliella salina and Nannochloropsis salina grown under nickel stress. Int. J. Bot. 2007, 3, 64–70. [Google Scholar]
- Pinzi, S.; Rounce, P.; Herreros, J.M.; Tsolakis, A.; Dorado, M.P. The effect of biodiesel fatty acid composition on combustion and diesel engine exhaust emissions. Fuel 2013, 104, 170–182. [Google Scholar] [CrossRef]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef] [Green Version]
- Stansell, G.R.; Gray, V.M.; Sym, S.D. Microalgal fatty acid composition: Implications for biodiesel quality. J. Appl. Phycol. 2012, 24, 791–801. [Google Scholar] [CrossRef]
- Komprda, T. Eicosapentaenoic and docosahexaenoic acids as inflammation-modulating and lipid homeostasis influencing nutraceuticals: A review. J. Funct. Foods 2012, 4, 25–38. [Google Scholar] [CrossRef]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Villay, A.; Laroche, C.; Roriz, D.; Alaoui, H.E.; Delbac, F.; Michaud, P. Optimisation of culture parameters for exopolysaccharides production by the microalga Rhodella violacea. Bioresour. Technol. 2013, 146, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Jha, B. Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour. Technol. 2009, 100, 3382–3386. [Google Scholar] [CrossRef] [PubMed]
- Penna, A.; Berluti, S.; Penna, N.; Magnani, M. Influence of nutrient ratios on the in vitro extracellular polysaccharide production by marine diatoms from the Adriatic Sea. J. Plankton Res. 1999, 21, 1681–1690. [Google Scholar] [CrossRef]
- Mota, R.; Guimaraes, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; de Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A. Characterization and optimization of production of exopolysaccharide from Chlamydomonas reinhardtii. Carbohydr. Polym. 2013, 95, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; de Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.J.; de Morais, R.M.S.C.; Bernardo de Morais, A.M.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Kiran, B.; Thanasekaran, K. Metal tolerance of an indigenous cyanobacterial strain, Lyngbya putealis. Int. Biodeterior. Biodegradation 2011, 65, 1128–1132. [Google Scholar] [CrossRef]
- Miao, A.J.; Schwehr, K.A.; Xu, C.; Zhang, S.J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Jiang, Y.; Chen, C.S.; Creeley, D.; Schwehr, K.A.; Quigg, A.; Chin, W.C.; Santschi, P.H. Ameliorating effects of extracellular polymeric substances excreted by Thalassiosira pseudonana on algal toxicity of CdSe quantum dots. Aquat. Toxicol. 2013, 126, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Goo, B.G.; Baek, G.; Choi, D.J.; Park, Y.I.; Synytsya, A.; Bleha, R.; Seong, D.H.; Lee, C.G.; Park, J.K. Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Bioresour. Technol. 2013, 129, 343–350. [Google Scholar] [PubMed]
- Ahner, B.A.; Price, N.M.; Morel, F.M.M. Phytochelatin production by marine phytoplankton at low free metal ion concentrations: Laboratory studies and field data from Massachusetts Bay. Proc. Natl. Acad. Sci. USA 1994, 91, 8433–8436. [Google Scholar] [CrossRef] [PubMed]
- Faucheur, L.S.; Behra, R.; Sigg, L. Phytochelatin induction, cadmium accumulation, and algal sensitivity to free cadmium ion in Scenedesmus vacuolatus. Environ. Toxicol. Chem. 2005, 24, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.K.; Gledhill, M.; Achterberg, E.P. Effects of metal combinations on the production of phytochelatins and glutathione by the marine diatom Phaeodactylum tricornutum. BioMetals 2006, 19, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Morelli, E.; Scarano, G. Synthesis and stability of phytochelatins induced by cadmium and lead in the marine diatom Phaeodactylum tricornutum. Mar. Environ. Res. 2001, 52, 383–395. [Google Scholar] [CrossRef]
- Morelli, E.; Scarano, G. Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalga Phaeodactylum tricornutum. Plant Sci. 2004, 167, 289–296. [Google Scholar] [CrossRef]
- Tukaj, Z.; Bascik-Remisiewicz, A.; Skowronski, T.; Tukaj, C. Cadmium effect on the growth, photosynthesis, ultrastructure and phytochelatin content of green microalga Scenedesmus armatus: A study at low and elevated CO2 concentration. Environ. Exp. Bot. 2007, 60, 291–299. [Google Scholar] [CrossRef]
- Pawlik-Skowronska, B.; Pirszel, J.; Kalinowska, R.; Skowronski, T. Arsenic availability, toxicity and direct role of GSH and phytochelatins in As detoxification in the green alga Stichococcus bacillaris. Aquat. Toxicol. 2004, 70, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Volland, S.; Schaumloffer, D.; Dobritzsch, D.; Krauss, G.J.; Lutz-Meindl, U. Identification of phytochelatins in the cadmium-stressed conjugating green alga Micrasterias denticulata. Chemosphere 2013, 91, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Srivastava, A.K.; Urmil, S.; Rai, L.C. Phytochelatin plays a role in UV-B tolerance in N2-fixing cyanobacterium Anabaena doliolum. J. Plant Physiol. 2005, 162, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Brautigam, A.; Bomke, S.; Pfeifer, T.; Karst, U.; Krauss, G.J.; Wesenberg, D. Quantification of phytochelatins in Chlamydomonas reinhardtii using ferrocene-based derivatization. Metallomics 2010, 2, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy-metal ion biosensor. Sensors 2005, 5, 70–84. [Google Scholar] [CrossRef]
- Politi, J.; Spadavecchia, J.; Iodice, M.; de Stefano, L. Oligopeptide—Heavy metal interaction monitoring by hybrid gold nanoparticle based assay. Analyst 2015, 140, 149. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Ross, C.W.; Chastain, C.J.; Koomanoff, N.; Hendrix, J.E.; Volkenburgh, E.V. Cytokinin-induced wall extensibility in excised cotyledons of radish and cucumber. Plant Physiol. 1981, 68, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Wareing, P.F.; Bradbeer, J.W. Abscisic acid as a natural growth regulator (and discussion). Philos. Trans. R. Soc. Lond. B 1978, 284, 483–498. [Google Scholar] [CrossRef]
- Prusty, R.; Grisafi, P.; Fink, G.R. The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. PNAS 2004, 101, 4153–4157. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S.; Sodagam, L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res. 2005, 8, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Berezniak, T.; Panek, J.J.; Jezierska-Mazzarello, A. Theoretical study of zeatin—A plant hormone and potential drug for neural diseases—On the basis of DFT, MP2 and target docking. Chem. Phys. Lett. 2013, 557, 140–144. [Google Scholar] [CrossRef]
- Kawano, T. Possible use of indole-3-acetic acid and its antagonist tryptophan betaine in controlled killing of horseradish peroxidase-labeled human cells. Med. Hypotheses 2003, 60, 664–666. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch. Environ. Contam. Toxicol. 2011, 60, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Liu, M.; Wang, Y.; Wang, C.; Zhang, C.; Ge, Y. Review of arsenic speciation, toxicity and metabolism in microalgae. Rev. Environ. Sci. Biotechnol. 2015. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Z.; Luo, Z. Arsenic Efflux from Microcystis aeruginosa under different phosphate regimes. PLoS ONE 2014, 9, e116099. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.M.; Raber, G.; Foster, S.; Chen, S.C.; Francesconi, K.A.; Zhu, Y.G. Biosynthesis of arsenolipids by the cyanobacterium Synechocystis sp. PCC 6803. Environ. Chem. 2014, 11, 506–513. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Z.; Yan, C. Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2013, 20, 7286–7295. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Levitsky, D.O. Arsenolipids. Prog. Lipid Res. 2004, 43, 403–448. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, S.I.; Fujiwara, S.; Tsuzuki, M.; Kaise, T. Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii. Biosci. Biotechnol. Biochem. 2011, 75, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, S.I.; Fujiwara, S.; Tsuzuki, M.; Kaise, T. Cyanobacteria produce arsenosugars. Environ. Chem. 2012, 9, 474–484. [Google Scholar] [CrossRef]
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F. The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta. Mar. Chem. 2013, 157, 78–85. [Google Scholar] [CrossRef]
- Foster, S.; Thomson, D.; Maher, W. Uptake and metabolism of arsenate by axenic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar. Chem. 2008, 108, 172–183. [Google Scholar] [CrossRef]
- Chen, B.; Liu, Q.; Popowich, A.; Shen, S.; Yan, X.; Zhang, Q.; Li, X.F.; Weinfeld, M.; Cullen, W.R.; Le, X.C. Therapeutic and analytical applications of arsenic binding to proteins. Metallomics 2015, 7, 39. [Google Scholar] [CrossRef]
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Schrofel, A.; Kratosova, G.; Safarik, I.; Safarikova, M.; Raska, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A Review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Płaza, G.A.; Chojniak, J.; Banat, I.M. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int. J. Mol. Sci. 2014, 15, 13720–13737. [Google Scholar] [CrossRef] [PubMed]
- Luangpipat, T.; Beattie, I.R.; Chisti, Y.; Haverkamp, R.G. Gold nanoparticles produced in a microalga. J. Nanopart. Res. 2011, 13, 6439–6445. [Google Scholar] [CrossRef]
- Feurtet-Mazel, A.; Mornet, S.; Charron, L.; Mesmer-Dudons, N.; Maury-Brachet, R.; Baudrimont, M. Biosynthesis of gold nanoparticles by the living freshwater diatom Eolimna minima, a species developed in river biofilms. Environ. Sci. Pollut. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kadukova, J.; Velgosova, O.; Mrazikova, A.; Marcincakova, R. The effect of culture age and initial silver concentration on biosynthesis of Ag nanoparticles. Nova Biotechnol. Chim. 2014, 13, 28–37. [Google Scholar] [CrossRef]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2014, 20, 7286–7295. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Microalga Scenedesmus sp.: A potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. 2014, 24, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Chen, X.; Bradshaw, M.; Agarwal, V.; Zou, J.; Stewart, S.G.; Duan, X.; Lamb, R.N.; Smith, S.M.; Raston, C.L.; et al. Biogenic production of palladium nanocrystals using microalgae and their immobilization on chitosan nanofibers for catalytic applications. RSC Adv. 2013, 3, 1009. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial biomass with a palladium(II) chloride complex. Langmuir 2007, 23, 8982–8987. [Google Scholar] [CrossRef] [PubMed]
- Jena, J.; Pradhan, N.; Aishvarya, V.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Biological sequestration and retention of cadmium as CdS nanoparticles by the microalga Scenedesmus-24. J. Appl. Phycol. 2014. [Google Scholar] [CrossRef]
- Santomauro, G.; Srot, V.; Bussmann, B.; van Aken, P.A.; Brümmer, F.; Strunk, H.; Bill, J. Biomineralization of zinc-phosphate-based nano needles by living microalgae. J. Biomater. Nanobiotechnol. 2012, 3, 362–370. [Google Scholar] [CrossRef]
- Schulze, H.; Brand, J.J. Lead toxicity and phosphate deficiency in Chlamydomonas. Plant Physiol. 1978, 62, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Mera, R.; Torres, E.; Abalde, J. Sulphate, more than a nutrient, protects the microalga Chlamydomonas moewusii from cadmium toxicity. Aquat. Toxicol. 2014, 148, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.D.; Beatty, J.C.; Loiselle, J.B.R.; Vlassov, K.A.; Lefebvre, D.D. Aerobic transformation of cadmium through metal sulfide biosynthesis in photosynthetic microorganisms. BMC Microbiol. 2013, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Lavoie, M.; Fan, X.; Bai, X.; Qian, H. Ammonium reduces chromium toxicity in the freshwater alga Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2015, 99, 3249–3258. [Google Scholar] [CrossRef]
- Deleebeeck, N.M.E.; de Schamphelaere, K.A.C.; Janssen, C.R. Effects of Mg2+ and H+ on the toxicity of Ni2+ to the unicellular green alga Pseudokirchneriella subcapitata: Model development and validation with surface waters. Sci. Total Environ. 2009, 407, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Stauber, J.L.; Markich, S.J.; Lim, R.P. pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.). Aquat. Toxicol. 2000, 48, 275–289. [Google Scholar] [CrossRef]
- Volland, S.; Bayer, E.; Baumgartner, V.; Andosch, A.; Lutz, C.; Sima, E.; Lutz-Meindl, U. Rescue of heavy metal effects on cell physiology of the algal model system Micrasterias by divalent ions. J. Plant Physiol. 2014, 171, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Cheloni, G.; Cosio, C.; Slaveykova, V.I. Antagonistic and synergistic effects of light irradiation on the effects of copper on Chlamydomonas reinhardtii. Aquat. Toxicol. 2014, 155, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Zylkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhu, W.; Wang, Z.; Witkamp, G.J. Accumulation, assimilation and growth inhibition of copper on freshwater alga (Scenedesmus subspicatus 86.81 SAG) in the presence of EDTA and fulvic acid. Aquat. Toxicol. 2003, 63, 221–228. [Google Scholar] [CrossRef]
- Hao, S.; Xiaorong, W.; Liansheng, W.; Lemei, D.; Zhong, L.; Yijun, C. Bioconcentration of rare earth elements lanthanum, gadolinium and yttrium in algae (Chlorella Vulgarize Beijerinck): Influence of chemical species. Chemosphere 1997, 34, 1753–1760. [Google Scholar] [CrossRef]
- Errecalde, O.; Seidl, M.; Campbell, P.G.C. Influence of a low molecular weight metabolite (citrate) on the toxicity of cadmium and zinc to the unicellular green alga Selenastrum capricornutum: An exception to the free-ion model. Water Res. 1998, 32, 419–429. [Google Scholar] [CrossRef]
- Gerringa, L.J.A.; de Baar, H.J.W.; Timmermans, K.R. A comparison of iron limitation of phytoplankton in natural oceanic waters and laboratory media conditioned with EDTA. Mar. Chem. 2000, 68, 335–346. [Google Scholar] [CrossRef]
- Lewin, J.; Chen, C.H. Available iron: A limiting factor for marine phytoplankton. Limnol. Oceanogr. 1971, 16, 670–675. [Google Scholar] [CrossRef]
- Botebol, H.; Sutak, R.; Scheiber, I.F.; Blaiseau, P.L.; Bouget, F.Y.; Camadro, J.M.; Lesuisse, E. Different iron sources to study the physiology and biochemistry of iron metabolism in marine micro-algae. Biometals 2014, 27, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kean, M.A.; Brons Delgado, E.; Mensink, B.P.; Bugter, M.H.J. Iron chelating agents and their effects on the growth of Pseudokirchneriella subcapitata, Chlorella vulgaris, Phaeodactylum tricornutum and Spirulina platensis in comparison to Fe-EDTA. J. Algal Biomass Util. 2015, 6, 56–73. [Google Scholar]
- Koukal, B.; Gueguen, C.; Pardos, M.; Dominik, J. Influence of humic substances on the toxic effects of cadmium and zinc to the green alga Pseudokirchneriella subcapitata. Chemosphere 2003, 53, 953–961. [Google Scholar] [CrossRef]
- Kaladharan, P.; Leela Bhai, K.S. Effect of humic acids on mercury toxicity to marine algae. Fish. Technol. 2007, 44, 93–98. [Google Scholar]
- Tang, Y.; Li, S.; Lu, Y.; Li, Q.; Yu, S. The Influence of humic acid on the toxicity of nano-ZnO and Zn2+ to the Anabaena sp. Environ. Toxicol. 2014. [Google Scholar] [CrossRef]
- Sanchez-Marin, P.; Beiras, R. Adsorption of different types of dissolved organic matter to marine phytoplankton and implications for phytoplankton growth and Pb bioavailability. J. Plankton Res. 2011, 33, 1396–1409. [Google Scholar] [CrossRef]
- Lamelas, C.; Wilkinson, K.J.; Slaveykova, V.I. Influence of the composition of natural organic matter on Pb bioavailability to microalgae. Environ. Sci. Technol. 2005, 39, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tanji, Y.; Unno, H. Influences of iron and humic acid on the growth of the cyanobacterium Anabaena circinalis. Biochem. Eng. J. 2005, 24, 195–201. [Google Scholar] [CrossRef]
- Worms, I.A.M.; Adenmatten, D.; Mieville, P.; Traber, J.; Slaveykova, V.I. Photo-transformation of pedogenic humic acid and consequences for Cd(II), Cu(II) and Pb(II) speciation and bioavailability to green microalga. Chemosphere 2015, 138, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Parent, L.; Twiss, M.R.; Campbell, P.G.C. Influences of natural dissolved organic matter on the interaction of aluminum with the microalga Chlorella: A Test of the free-ion model of trace metal toxicity. Environ. Sci. Technol. 1996, 30, 1713–1720. [Google Scholar] [CrossRef]
- Tang, Y.; Tian, J.; Li, S.; Xue, C.; Xue, Z.; Yin, D.; Yu, S. Combined effects of graphene oxide and Cd on the photosynthetic capacity and survival of Microcystis aeruginosa. Sci. Total Environ. 2015, 532, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Miao, A.J.; Yang, L.Y. Cd2+ Toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS ONE 2012, 7, e32300. [Google Scholar] [CrossRef] [PubMed]
- Worms, I.A.M.; Boltzman, J.; Garcia, M.; Slaveykova, V.I. Cell-wall-dependent effect of carboxyl-CdSe/ZnS quantum dots on lead and copper availability to green microalgae. Environ. Pollut. 2012, 167, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Comprehensive Supramolecular Chemistry; Pergamon Press: Oxford, UK, 1996; Volume 3. [Google Scholar]
- Bender, M.L.; Momiyama, M. Cyclodextrin Chemistry; Springer: Berlin, Germany, 1978. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Zia, V.; Rajewski, R.A.; Stella, V.J. Effect of cyclodextrin charge on complexation of neutral and charged substrates: Comparison of (SBE)7M-β-CD to HP-β-CD. Pharm. Res. 2001, 18, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, R.; Banerjee, U.C. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, 329–357. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; la Mendola, D.; Pedone, C.; Rizzarelli, E.; Saviano, M.; Vecchio, G. Selectively functionalized cyclodextrins and their metal complexes. Chem. Soc. Rev. 2009, 38, 2756–2781. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Pan, Q.; Liu, Y. Preparation and properties of phytosterols with hydroxypropyl β-cyclodextrin inclusion complexes. Eur. Food Res. Technol. 2012, 235, 1039–1047. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Z.; Xu, X. Inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin: UV, FTIR, 1H NMR and molecular modeling studies. Carbohydr. Polym. 2012, 89, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Anderson, P.P.; Schubert, C.M.; Gault, M.B.; Blanford, W.J.; Sandrin, T.R. Carboxymethyl-β-cyclodextrin mitigates toxicity of cadmium, cobalt and copper during naphthalene biodegradation. Bioresour. Technol. 2010, 101, 2672–2677. [Google Scholar] [CrossRef] [PubMed]
- Bi Fai, P.; Grant, A.; Reid, B.J. Compatibility of hydroxypropyl-β-cyclodextrin with algal toxicity bioassays. Environ. Pollut. 2009, 157, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C. Calixarenes, Monographs in Supramolecular Chemistry; Stoddart, J., Ed.; The Royal Society of Chemisfry: Cambridge, UK, 1989. [Google Scholar]
- Gutsche, C.D.; Iqbal, M. p-tert-Butylcalix[4]arene. Org. Synth. 1990, 68, 234–237. [Google Scholar]
- Gutsche, C.D.; Dhawan, B.; Leonis, M.; Stewart, D. p-tert-Butylcalix[6]arene. Org. Synth. 1990, 68, 238–242. [Google Scholar]
- Munch, J.H.; Gutsche, C.D. p-tert-Butylcalix[8]arene. Org. Synth. 1990, 68, 243–245. [Google Scholar]
- Cram, D.J.; Cram, J.M. Container Molecules and Their Guests; The Royal Society of Chemistry: Cambridge, UK, 1997. [Google Scholar]
- Sliwa, W.; Kozlowski, C. Calixarenes and Resorcinarenes: Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Schneider, H.J.; Güttes, D.; Schneider, U. A macrocyclic polyphenolate as receptor analogue for cholin and related ammonium compounds. Angew. Chem. Int. Ed. Engl. 1986, 25, 647–649. [Google Scholar] [CrossRef]
- Schneider, H.J.; Güttes, D.; Schneider, U. Host-guest complexes with water soluble macrocyclic polyphenolates including induced fit and simple elements of a proton pump. J. Am. Chem. Soc. 1988, 110, 6449–6454. [Google Scholar] [CrossRef]
- Casnati, A.; Sciotto, D.; Arena, G. Calixarenes 2001; Asfari, Z., Behmer, V., Harrowfield, J., Vicens, J., Eds.; Kluwer Academic Pubhshers: Dordrecht, The Netherlands, 2001; pp. 440–457. [Google Scholar]
- Arena, G.; Cali, R.; Lombardo, G.G.; Rizzarelli, E.; Sciotto, D.; Ungaro, R.; Casnati, A. Water soluble calix[4]arenes. A thermodynamic investigation of proton complex formation. Supramol. Chem. 1992, 1, 19–24. [Google Scholar] [CrossRef]
- Johnson, C.P.; Atwood, J.L.; Steed, J.W.; Bauer, C.B.; Rogers, R.D. Transition metal complexes of p-sulfonatocalix[5]arene. Inorg. Chem. 1996, 35, 2602–2610. [Google Scholar] [PubMed]
- Gangemi, C.M.A.; Pappalardo, A.; Sfrazzetto, G.T. Applications of supramolecular capsules derived from resorcin[4]arenes, calix[n]arenes and metallo-ligands: From biology to catalysis. RSC Adv. 2015, 5, 51919–51933. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tominaga, M.; Asakawa, Y.; Aoyama, Y. Binding of amino acids in water to a highly electron-rich aromatic cavity of pyrogallol or resorcinol cyclic tetramer as π-base. Tetrahedron Lett. 1993, 34, 5121–5124. [Google Scholar] [CrossRef]
- Kobayashi, K.; Asakawa, Y.; Aoyama, Y. Complexation of methylammonium salts and sugar-related alcohols with resorcinol cyclic tetramer in water: An implication of the CH-π interaction on polar guest binding. Supramol. Chem. 1993, 2, 133–135. [Google Scholar] [CrossRef]
- Kobayashi, K.; Asakawa, Y.; Kato, Y.; Aoyama, Y. Complexation of hydrophobic sugars and nucleosides in water with tetrasulfonate derivatives of resorcinol cyclic tetramer having a polyhydroxy aromatic cavity: Importance of guest–host CH–π interaction. J. Am. Chem. Soc. 1992, 114, 10307–10313. [Google Scholar] [CrossRef]
- Lamartinea, R.; Tsukadab, M.; Wilson, D.; Shirata, A. Antimicrobial activity of calixarenes. C. R. Chim. 2002, 5, 163–169. [Google Scholar] [CrossRef]
- Mouradzadegun, A.; Elahi, S.; Abadast, F.; Motamedi, H. A straightforward route for covalently anchored pyridinium salt onto upper rim of c-methylcalix[4]resorcinarene with selective antibacterial activity against Gram-positive bacteria. Res. Chem. Intermed. 2015. [Google Scholar] [CrossRef]
- Yoshida, N.; Ikeda, R.; Okuno, T. Identification and characterization of heavy metal-resistant unicellular alga isolated from soil and its potential for phytoremediation. Bioresour. Technol. 2006, 97, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, R.; Pawlik-Skowronska, B. Metal resistance of soil algae (Chlorophyta) occurring in post-flotation Zn/Pb- and Cu-tailing ponds. Pol. J. Ecol. 2008, 56, 415–430. [Google Scholar]
- Kalinowska, R.; Pawlik-Skowronska, B. Response of two terrestrial green microalgae (Chlorophyta, Trebouxiophyceae) isolated from Cu-rich and unpolluted soils to copper stress. Environ. Pollut. 2010, 158, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Halter, D.; Casiot, C.; Heipieper, H.J.; Plewniak, F.; Marchal, M.; Simon, S.; Arsène-Ploetze, F.; Bertin, P.N. Surface properties and intracellular speciation revealed an original adaptive mechanism to arsenic in the acid mine drainage bio-indicator Euglena mutabilis. Appl. Microbiol. Biotechnol. 2012, 93, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Marva, F.; Garcıa-Balboa, C.; Baselga-Cervera, B.; Costas, E. Rapid adaptation of some phytoplankton species to osmium as a result of spontaneous mutations. Ecotoxicology 2014, 23, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ipatova, V.I.; Spirkina, N.E.; Dmitrieva, A.G. Resistance of microalgae to colloidal silver nanoparticles. Russ. J. Plant Physiol. 2015, 62, 253–261. [Google Scholar] [CrossRef]
- Baos, R.; Garcia-Villada, L.; Agrelo, M.; Lopez-Rodas, V.; Hiraldo, F.; Costas, E. Short-term adaptation of microalgae in highly stressful environments: An experimental model analysing the resistance of Scenedesmus intermedius (Chlorophyceae) to the heavy metals mixture from the Aznalcóllar mine spill. Eur. J. Phycol. 2002, 37, 593–600. [Google Scholar] [CrossRef]
- Fathi, A.A.; Zaki, F.T.; Ibraheim, H.A. Response of tolerant and wild type strains of Chlorella vulgaris to copper with special references to copper uptake system. Protistology 2005, 4, 73–78. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Nikookar, K.; Moradshahi, A.; Hosseini, L. Physiological responses of Dunaliella salina and Dunaliella tertiolecta to copper toxicity. Biomol. Eng. 2005, 22, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Pistocchi, R.; Guerrini, F.; Balboni, V.; Boni, L. Copper toxicity and carbohydrate production in the microalgae Cylindrotheca fusiformis and Gymnodinium sp. Eur. J. Phycol. 1997, 32, 125–132. [Google Scholar] [CrossRef]
- Lopez, A.; Rico, M.; Santana-Casiano, J.M.; Gonzalez, A.G.; Gonzalez-Davila, M. Phenolic profile of Dunaliella tertiolecta growing under high levels of copper and iron. Environ. Sci. Pollut. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chia, M.A.; Lombardi, A.T.; da Graca Gama Melao, M.; Parrish, C.C. Combined nitrogen limitation and cadmium stress stimulate total carbohydrates, lipids, protein and amino acid accumulation in Chlorella vulgaris (Trebouxiophyceae). Aquat. Toxicol. 2015, 160, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, B.T.A.; Janssen, C.R. Long-term acclimation of Pseudokirchneriella subcapitata (Korshikov) Hindak to different copper concentrations: Changes in tolerance and physiology. Aquat. Toxicol. 2004, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Radzun, K.A.; Wolf, J.; Jakob, G.; Zhang, E.; Stephens, E.; Ross, I.; Hankamer, B. Automated nutrient screening system enables high-throughput optimisation of microalgae production conditions. Biotechnol. Biofuels 2015, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.; Kinney, K.; Katz, L.; Berberoglu, H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012, 114, 542–548. [Google Scholar] [CrossRef] [PubMed]
- El-Enany, A.E.; Issa, A.A. Cyanobacteria as a biosorbent of heavy metals in sewage water. Environ. Toxicol. Pharmacol. 2000, 8, 95–101. [Google Scholar] [CrossRef]
- Harrison, E.Z.; Oakes, S.R.; Hysell, M.; Hay, A. Organic chemicals in sewage sludges. Sci. Total Environ. 2006, 367, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, E.M.; Phang, S.M.; Chu, W.L. Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J. Appl. Phycol. 2012, 24, 953–963. [Google Scholar] [CrossRef]
- Richards, R.G.; Mullins, B.J. Using microalgae for combined lipid production and heavy metal removal from leachate. Ecol. Modell. 2013, 249, 59–67. [Google Scholar] [CrossRef]
- Napan, K.; Teng, L.; Quinn, J.C.; Wood, B.D. Impact of heavy metals from flue gas integration with microalgae production. Algal Res. 2015, 8, 83–88. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Ramamurthy, D. Dyeing industry effluent system as lipid production medium of Neochloris sp. for biodiesel feedstock preparation. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Anandraj, A.; Trois, C. The effect of landfill leachate on hydrogen production in Chlamydomonas reinhardtii as monitored by PAM Fluorometry. Int. J. Hydrog. Energy 2013, 38, 14214–14222. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Lubian, L.M.; Soares, A.M.V.M. Influence of cellular density on determination of EC50 in microalgal growth inhibition tests. Ecotoxicol. Environ. Saf. 2000, 47, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Stauber, J.L.; Apte, S.C.; Lim, R.P. Effect of initial cell density on the bioavailability and toxicity of copper in microalgal bioassays. Environ. Toxicol. Chem. 2002, 21, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, N.G.J.; Fu, B.; Li, S.F.Y. Metallomics and NMR-based metabolomics of Chlorella sp. reveal the synergistic role of copper and cadmium in multi-metal toxicity and oxidative stress. Metallomics 2015. [Google Scholar] [CrossRef]
- Song, L.; Qin, J.G.; Su, S.; Xu, J.; Clarke, S.; Shan, Y. Micronutrient requirements for growth and hydrocarbon production in the oil producing green alga Botryococcus braunii (Chlorophyta). PLoS ONE 2012, 7, e41459. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Lu, X.H.; Lu, M.Z.; Yu, L.S.; Xue, R.; Ji, J.B. The effects of trace elements on the lipid productivity and fatty acid composition of Nannochloropis oculata. J. Renew. Energy 2013. [Google Scholar] [CrossRef]
- Berberoglu, H.; Jay, J.; Pilon, L. Effect of nutrient media on photobiological hydrogen production by Anabaena variabilis ATCC 29413. Int. J. Hydrog. Energy 2008, 33, 1172–1184. [Google Scholar] [CrossRef]
- Becker, L.J.M.; Meisch, H.U. Effect of vanadate and iron stress on the pigment composition of Chlorella fusca. Z. Naturforsch. 1981, 36, 207–209. [Google Scholar]
- Salama, E.S.; Kabra, A.N.; Ji, M.K.; Kim, J.R.; Min, B.; Jeon, B.H. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour. Technol. 2014, 172, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Josephine, A.; Niveditha, C.; Radhika, A.; Shali, A.B.; Kumar, T.S.; Dharani, G.; Kirubagaran, R. Analytical evaluation of different carbon sources and growth stimulators on the biomass and lipid production of Chlorella vulgaris—Implications for biofuels. Biomass Bioenergy 2015, 75, 170–179. [Google Scholar] [CrossRef]
- Pradeep, V.; van Ginkel, S.W.; Park, S.; Igou, T.; Yi, C.; Fu, H.; Johnston, R.; Snell, T.; Chen, Y. Use of copper to selectively inhibit Brachionus calyciflorus (Predator) growth in Chlorella kessleri (Prey) mass cultures for algae biodiesel production. Int. J. Mol. Sci. 2015, 16, 20674–20684. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. Int. J. Mol. Sci. 2015, 16, 23929-23969. https://doi.org/10.3390/ijms161023929
Miazek K, Iwanek W, Remacle C, Richel A, Goffin D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. International Journal of Molecular Sciences. 2015; 16(10):23929-23969. https://doi.org/10.3390/ijms161023929
Chicago/Turabian StyleMiazek, Krystian, Waldemar Iwanek, Claire Remacle, Aurore Richel, and Dorothee Goffin. 2015. "Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review" International Journal of Molecular Sciences 16, no. 10: 23929-23969. https://doi.org/10.3390/ijms161023929
APA StyleMiazek, K., Iwanek, W., Remacle, C., Richel, A., & Goffin, D. (2015). Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. International Journal of Molecular Sciences, 16(10), 23929-23969. https://doi.org/10.3390/ijms161023929






