2.1. Strain Physiology
We cultivated the four recombinant
P. pastoris strains in dynamic batch cultivations with methanol pulses at different temperatures and analyzed the effect of temperature on strain physiology. A typical cultivation is exemplarily shown for strain wt
4/8 HRP in
Figure 1.
Figure 1.
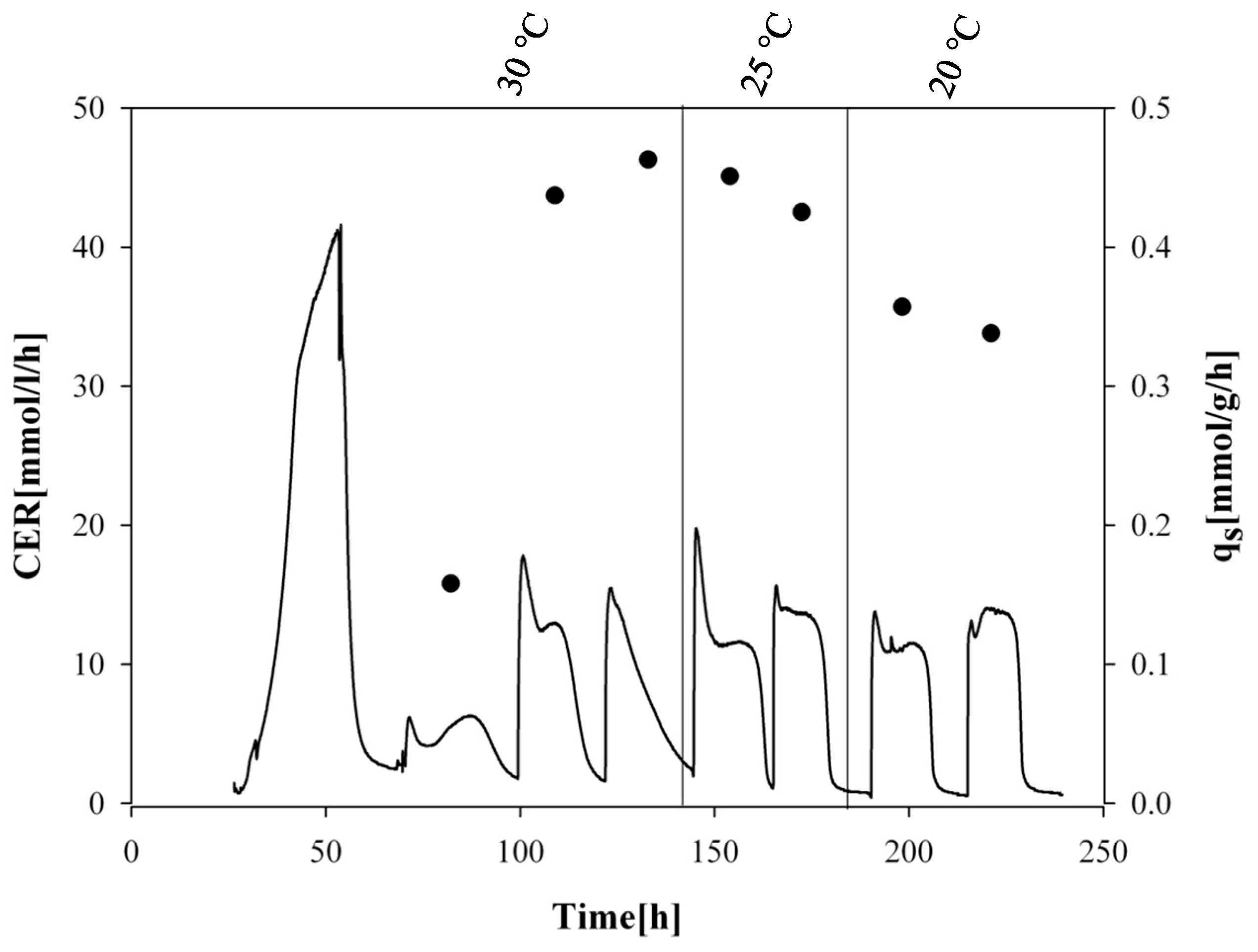
Schematic overview of the dynamic batch cultivation of strain wt4/8 HRP with methanol pulses at different temperatures. Black continuous line, carbon dioxide evolution rate (CER); black dots, specific methanol uptake rate (qs MeOH).
Figure 1.
Schematic overview of the dynamic batch cultivation of strain wt4/8 HRP with methanol pulses at different temperatures. Black continuous line, carbon dioxide evolution rate (CER); black dots, specific methanol uptake rate (qs MeOH).
The most important strain physiological parameters are summarized in
Table 3. Closing C-balances for the benchmark strains underline data validity. Similar to our previous studies, we observed that the Δoch1 strains lost metabolic activity over time and were affected by cell lysis [
26]. Thus, C-balances did not close.
When comparing the recombinant benchmark strains, great differences in specific methanol uptake rates (q
s MeOH) were identified. While for both strains q
s MeOH increased with increasing temperature, a correlation we also had observed before [
6], the strain producing the 4/8 HRP variant showed a three-fold lower q
s MeOH compared to the strain producing the unmutated enzyme at the respective temperature. Apparently, production of the glyco-engineered 4/8 HRP caused a physiological burden for the yeast, decelerating metabolism and thus methanol uptake.
When comparing the recombinant Δoch1 strains, we observed that q
s MeOH for both strains decreased with increasing temperature, a phenomenon we also had described before [
6]. However, q
s MeOH of both Δoch1 strains were comparable at each temperature indicating that the mutated product did not cause any physiological burden. We speculate that the glyco-engineered 4/8 HRP variant might cause problems in the
P. pastoris benchmark strain during secretion as it might get stuck in the cell wall. On the contrary, the same enzyme variant can be secreted without any problems in the Δoch1 strain, which has a completely altered cell wall structure [
24]. However, this remains to be elucidated in detail.
Table 3.
Physiological parameters of strains wtwt HRP, benchmark strain expressing the unmutated HRP enzyme; wt4/8 HRP, benchmark strain expressing the mutated 4/8 HRP variant; OCH1wt HRP, deleted OCH1 gene strain expressing the unmutated HRP enzyme and OCH14/8 HRP delete OCH1 gene strain expressing the mutated 4/8 HRP variant were determined in dynamic batch cultivations.
Table 3.
Physiological parameters of strains wtwt HRP, benchmark strain expressing the unmutated HRP enzyme; wt4/8 HRP, benchmark strain expressing the mutated 4/8 HRP variant; OCH1wt HRP, deleted OCH1 gene strain expressing the unmutated HRP enzyme and OCH14/8 HRP delete OCH1 gene strain expressing the mutated 4/8 HRP variant were determined in dynamic batch cultivations.
| Strain | μmax gly (h−1) | Δtime adapt (h) | qs adapt (mmol/g/h) | qs MeOH 20 °C (mmol/g/h) | qs MeOH 25 °C (mmol/g/h) | qs MeOH 30 °C (mmol/g/h) | C-Balance |
|---|
| wtwt HRP | 0.271 | 11.1 | 0.272 | 0.931 | 1.190 | 1.32 | 0.96 |
| wt4/8 HRP | 0.200 | 16.0 | 0.158 | 0.354 | 0.438 | 0.450 | 0.97 |
| OCH1wt HRP | 0.199 | 4.5 | 0.370 | 0.891 | 0.780 | 0.632 | const. Decreasing |
| OCH14/8 HRP | 0.182 | 3.8 | 0.400 | 1.02 | 0.955 | 0.800 | const. Decreasing |
2.2. Protein Purification
After harvest, we purified the different enzyme variants by a 1-step hydrophobic charge interaction chromatography (HCIC) purification strategy [
8,
19]. As shown in
Table 4, the majority of each HRP variant was found in the respective flow-through fraction, where purification factors (PF) between 1.34 and 1.53 were achieved. However, enzyme OCH1
4/8 HRP showed a different result, as nearly 20% of the enzyme was retained on the resin. The eluate fraction showed a higher PF compared to the flow-through fraction (
Table 4). We ascribe this phenomenon to the fact that this HRP variant was the least glycosylated one, as the four remaining glycosylation sites mainly carried Man
8 instead of longer Man chains (respective detailed analyses had been performed in our previous study [
26]). Apparently, the reduced glycosylation of enzyme OCH1
4/8 HRP allows physico-chemical interactions of the enzyme variant and the resin. One might speculate that the amount of enzyme variant able to interact with the resin should be even higher. We think that the rather stressful manner of cultivation with pulses and temperature shifts might have caused very heterogeneous glycosylation on the four remaining
N-glycosylation sites and thus resulted in the still rather low fraction of only around 20% enzyme interacting with the resin. Detailed analysis of surface glycosylation by mass spectrometry, as we have done previously [
24], could shed light on this speculation. However, we did not perform this analysis in this study, since variant OCH1
4/8 HRP did not turn out to be interesting for further applications due to low catalytic activity (see below).
After HCIC, flow-through fractions showing highest PFs were pooled and concentrated to around 1.5 mL. To assess purity of the different enzyme preparations Reinheitszahl values (RZ; A
404/A
280) were determined (
Table 4). Highly pure HRP preparations are known to have RZ values of more than 3.0 [
16]. Although we did not get pure enzyme preparations by the one-step HCIC purification in this study, the RZ values of the different enzyme variants were in the same range. We also analyzed the different enzyme variants on SDS-PAGE gels, to identify potential differences in apparent size (
Figure 2). Furthermore, interesting protein bands were excised and analyzed by mass spectrometry (respective bands are indicated in black boxes in
Figure 2).
Table 4.
Results of the hydrophobic charged interaction chromatography (HCIC) purification and Reinheitszahl (RZ) measurements for the four different HRP enzyme variants.
Table 4.
Results of the hydrophobic charged interaction chromatography (HCIC) purification and Reinheitszahl (RZ) measurements for the four different HRP enzyme variants.
| HCIC | Concentrated Fraction |
|---|
| Enzyme Variant | R% Total | R% FT | PF FT | Specific Activity (U/mg) | RZ (A404/A280) |
|---|
| wtwt HRP | 87.2 | 87.2 | 1.49 | 273.3 | 0.50 |
| wt4/8 HRP | 97.4 | 95.6 | 1.34 | 36.4 | 0.51 |
| OCH1wt HRP | 99.8 | 98.7 | 1.53 | 59.8 | 0.63 |
| OCH14/8 HRP | 93.1 | 76.1 | 1.47 | 11.5 | 0.19 |
| Variant | R% Total | R% Eluate | PF Eluate | Specific Activity (U/mg) | RZ (A404/A280) |
| OCH14/8 HRP | 93.1 | 17.5 | 2.43 | 19.1 | 0.31 |
Figure 2.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of different horseradish peroxidase (HRP) variants. Black boxes indicate protein bands analyzed by mass spectrometry. Lane 1, BLUeye Prestained Protein Ladder; lanes 2 and 3, HRP from plant in two different concentrations (4 μg protein and 8 μg protein, respectively); lane 4, wtwt HRP, unmutated HRP enzyme expressed in the benchmark strain; lane 5, wt4/8 HRP, mutated 4/8 HRP variant expressed in the benchmark strain; lane 6, OCH14/8 HRP, mutated 4/8 HRP variant expressed in the deleted OCH1 gene strain.
Figure 2.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of different horseradish peroxidase (HRP) variants. Black boxes indicate protein bands analyzed by mass spectrometry. Lane 1, BLUeye Prestained Protein Ladder; lanes 2 and 3, HRP from plant in two different concentrations (4 μg protein and 8 μg protein, respectively); lane 4, wtwt HRP, unmutated HRP enzyme expressed in the benchmark strain; lane 5, wt4/8 HRP, mutated 4/8 HRP variant expressed in the benchmark strain; lane 6, OCH14/8 HRP, mutated 4/8 HRP variant expressed in the deleted OCH1 gene strain.
The enzyme preparation from plant showed a rather distinct band at an apparent size of around 40 kDa on the SDS gel (lanes 2 and 3 in
Figure 2), whereas the recombinant enzyme wt
wt HRP showed a smear at an apparent size of around 65 kDa (lane 4 in
Figure 2). Both proteins were identified as HRP by mass spectrometry (
Table 5). The apparent size difference of around 20 kDa and the smeary appearance of wt
wt HRP result from the heterogeneous yeast-derived glycosylation [
17,
20,
21]. The second prominent protein band in lane 4 at an apparent size of around 30 kDa was identified as a glucosidase from
P. pastoris (
Table 5).
As shown in
Figure 2, the preparation of enzyme wt
4/8 HRP showed a different protein pattern on the SDS gel, as the band at an apparent size of 65 kDa disappeared, whereas a prominent band at an apparent size of around 50 kDa appeared. In fact, this protein was identified as HRP (
Table 5). Apparently, the mutation of four of the eight
N-glycosylation sites resulted in the absence of glycans there and thus a size reduction of around 15 kDa. This nicely underlines the feasibility of reducing the vast and heterogeneous glycosylation of recombinant proteins from yeast by protein design. The second prominent band at an apparent size of around 30 kDa was again identified as a glucosidase (
Table 5).
Lane 6 in
Figure 2 shows the protein bands of enzyme preparation OCH1
4/8 HRP. Again, we observed a different protein pattern. The prominent band at an apparent size of around 70 kDa was identified as an oxidase and the band at an apparent size of around 30 kDa again as a glucosidase (
Table 5). We expected to see a protein band at an apparent size of around 45 kDa representing enzyme OCH1
4/8 HRP. However, no respective band was visible on the SDS gel. We ascribe this absence to the extremely low protein production in the Δoch1 strain [
6,
26]. Furthermore, cell lysis during bioreactor cultivation resulted in a rather high impurity pattern, which is also demonstrated by the low RZ value for this enzyme variant (
Table 4). However, we still measured enzymatic activity for OCH1
4/8 HRP and thus included this enzyme variant in the comparative biochemical characterization.
Table 5.
Identification of prominent protein bands by mass spectrometry.
Table 5.
Identification of prominent protein bands by mass spectrometry.
| Lane | Apparent Size [kDa] | Rank | Peptides | Scores | Protein | Accession |
|---|
| 3 | 45 | 1 | 11 | 608.9 | Peroxidase C1A Organism species (OS)=Armoracia rusticana | PER1A_ARMRU |
| 4 | 65 | 1 | 6 | 386.9 | 1,3-β-glucanosyltransferase OS=Komagataella pastoris | Q0QCW1_PICPA |
| 2 | 9 | 368.0 | Peroxidase C1A OS=Armoracia rusticana | PER1A_ARMRU |
| 30 | 1 | 9 | 411.5 | Glucan 1,3-β-glucosidase OS=Komagataella pastoris | F2QPL8_PICP7 |
| 5 | 50 | 1 | 9 | 454.5 | Alpha-1-antichymotrypsin 2 OS=Sus scrofa | Q9GMA6_PIG |
| 2 | 9 | 386.6 | Keratin, type II cytoskeletal 1 OS=Homo sapiens | K2C1_HUMAN |
| 3 | 9 | 317.4 | Peroxidase C1A OS=Armoracia rusticana | PER1A_ARMRU |
| 30 | 1 | 9 | 411.5 | Glucan 1,3-β-glucosidase OS=Komagataella pastoris | F2QPL8_PICP7 |
| 6 | 70 | 1 | 20 | 1136.6 | Primary-amine oxidase OS=Komagataella pastoris | F2QTE6_PICP7 |
| 2 | 10 | 685.6 | 1,3-β-glucanosyltransferase OS=Komagataella pastoris | Q0QCW1_PICPA |
| 3 | 9 | 548.8 | 1,3-β-glucanosyltransferase OS=Komagataella pastoris | F2QQJ2_PICP7 |
| 30 | 1 | 9 | 411.5 | Glucan 1,3-β-glucosidase OS=Komagataella pastoris | F2QPL8_PICP7 |
2.3. Biochemical Enzyme Characterisation
After purification, we biochemically characterized the different HRP variants. The kinetic parameters for ABTS and H
2O
2 are summarized in
Table 6. We also included plant HRP for comparison. Introducing the four mutations N13D, N57S, N255D and N268D into HRP did not affect the affinity towards ABTS and H
2O
2 as
Km values of enzymes wt
wt HRP and wt
4/8 HRP were comparable. However, the catalytic efficiency was reduced seven-fold for ABTS and six-fold for H
2O
2, respectively. Apparently, the introduced mutations did not only reduce surface glycosylation (
Figure 2) but also affected the active site and reduced catalytic activity.
When we produced the HRP variants in the Δoch1 strain, we found that reduced surface glycosylation, caused by the absence of the native enzyme OCH1 [
26], did not alter substrate affinity either. In fact,
Km values of all four HRP variants for both ABTS and H
2O
2 were comparable. However, reducing surface glycosylation affected catalytic efficiency. Enzyme OCH1
wt HRP showed a nearly six-fold reduced
vmax/
Km compared to enzyme wt
wt HRP. Apparently, the benefit of having a more homogeneously glycosylated enzyme produced in the Δoch1 strain comes at cost of catalytic activity. Finally, we characterized the mutated 4/8 HRP produced in the Δoch1 strain (enzyme OCH1
4/8 HRP). This enzyme is the least glycosylated one in this study, only providing four out of eight
N-glycosylation sites, which are mainly occupied by Man
8-glycan structures [
26]. However, compared to enzyme wt
wt HRP the catalytic efficiency for ABTS was reduced 119-fold and for H
2O
2 76-fold, respectively. Although, enzyme OCH1
4/8 HRP is basically fit for medical applications, as it misses the heterogeneous yeast-derived glycan structures, the highly reduced catalytic activity as well as low productivity in the bioreactor will most likely prevent future applications.
Table 6.
Kinetic constants of four different HRP enzyme variants and plant HRP.
Table 6.
Kinetic constants of four different HRP enzyme variants and plant HRP.
| Enzyme | ABTS | H2O2 |
|---|
| Variant | Km (mM) | vmax (U/mg) | vmax/Km (U/mg/mM) | Km (mM) | vmax (U/mg) | vmax/Km (U/mg/mM) |
|---|
| wtwt HRP | 1.50 | 152.9 | 101.9 | 0.009 | 55.2 | 6133 |
| wt4/8 HRP | 0.99 | 13.6 | 13.7 | 0.015 | 15.5 | 1030 |
| OCH1wt HRP | 1.56 | 26.8 | 17.2 | 0.008 | 10.4 | 1300 |
| OCH14/8 HRP | 1.34 | 1.28 | 0.96 | 0.016 | 1.29 | 80.7 |
| plant HRP | 1.75 | 567.2 | 324.8 | 0.033 | 377.8 | 11,589 |
Compared to the commercially available HRP preparation from plant, all recombinant HRP variants showed comparable Km values but reduced catalytic activity. This might be a result from incomplete heme incorporation or different surface glycosylation. However, the plant preparation contains a mixture of different HRP isoenzymes with varying surface glycosylation, which has to be isolated from its natural source in a rather cumbersome way. Furthermore, seasonal variation in the isoenzyme content and thus the variable production scenario describe only some of the disadvantages of plant HRP. Thus, even though recombinant HRP variants show lower catalytic activity, they are still interesting for industry, as they only describe a single isoenzyme that can be produced in a predictable manner in the controlled environment of a bioreactor. Furthermore, less glycosylated enzymes might allow more controlled and efficient conjugation to antibodies and lectins, which outweighs reduced catalytic activity.
2.4. Thermal Stability
Since we had determined mutations N13D, N57S and N268D to positively affect thermal stability of HRP before (
Table 2; [
27]), we also analyzed thermal half-life times of the four HRP variants at 60 °C. We again included plant HRP as standard and normalized all enzyme preparations to a protein concentration of 0.1 mg/mL before incubation to guarantee comparability. In
Table 7 the respective results are summarized.
Table 7.
Thermal half-life times of four different HRP variants and the HRP plant preparation at 60 °C. All enzymes were normalized to a protein concentration of 0.1 mg/mL before incubation.
Table 7.
Thermal half-life times of four different HRP variants and the HRP plant preparation at 60 °C. All enzymes were normalized to a protein concentration of 0.1 mg/mL before incubation.
| Enzyme Variant | Protein Concentration (mg/mL) | τ1/2 60 °C (min) |
|---|
| wtwt HRP | 0.1 | 31.5 |
| wt4/8 HRP | 173.2 |
| OCH1wt HRP | 3.3 |
| OCH14/8 HRP | 19.3 |
| plant HRP | 53.3 |
As shown in
Table 7, the introduction of the four mutations into HRP caused a significant increase in thermal stability. Enzyme wt
4/8 HRP had a 5.5-fold higher τ
1/2 60 °C than enzyme wt
wt HRP and was even 3.3-fold more stable than the enzyme preparation from plant. The same HRP variants produced in the Δoch1 strain showed a 10-fold reduced thermal stability compared to their respective counterparts from the benchmark strain. Reducing glycosylation to Man
8 structures apparently affected stability to a greater extent than completely removing four
N-glycosylation sites. In fact, mutating the four
N-glycosylation sites significantly increased stability instead of decreasing it. It is remarkable that enzyme OCH1
4/8 HRP actually showed a similar thermal half-life time as enzyme wt
wt HRP (
Table 7).
Considering both, kinetic parameters and thermal stability, enzyme wt4/8 HRP might be interesting for future applications in medical diagnostics. This enzyme variant can be efficiently produced in bioreactor cultivations, is less glycosylated, which might allow more controlled and efficient conjugation to antibodies and lectins, still shows considerable catalytic activity and a 5.5-fold higher thermal stability compared to enzyme wtwt HRP. In fact, higher stability and reduced glycosylation could compensate for reduced catalytic activity.