Transcriptional Responses of a Bicarbonate-Tolerant Monocot, Puccinellia tenuiflora, and a Related Bicarbonate-Sensitive Species, Poa annua, to NaHCO3 Stress
Abstract
:1. Introduction
2. Results
2.1. Bicarbonate Stress Tolerance Test of P. annua
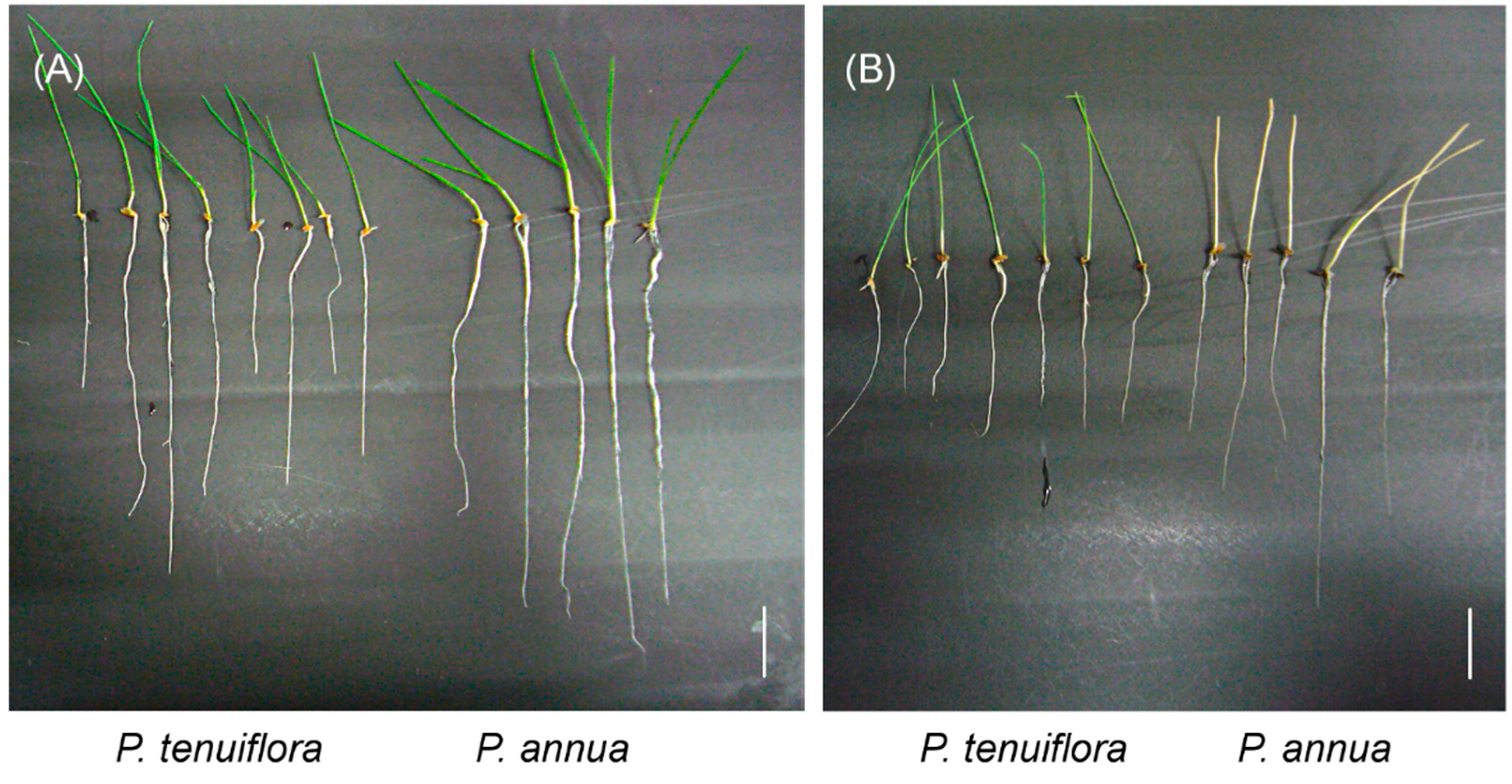
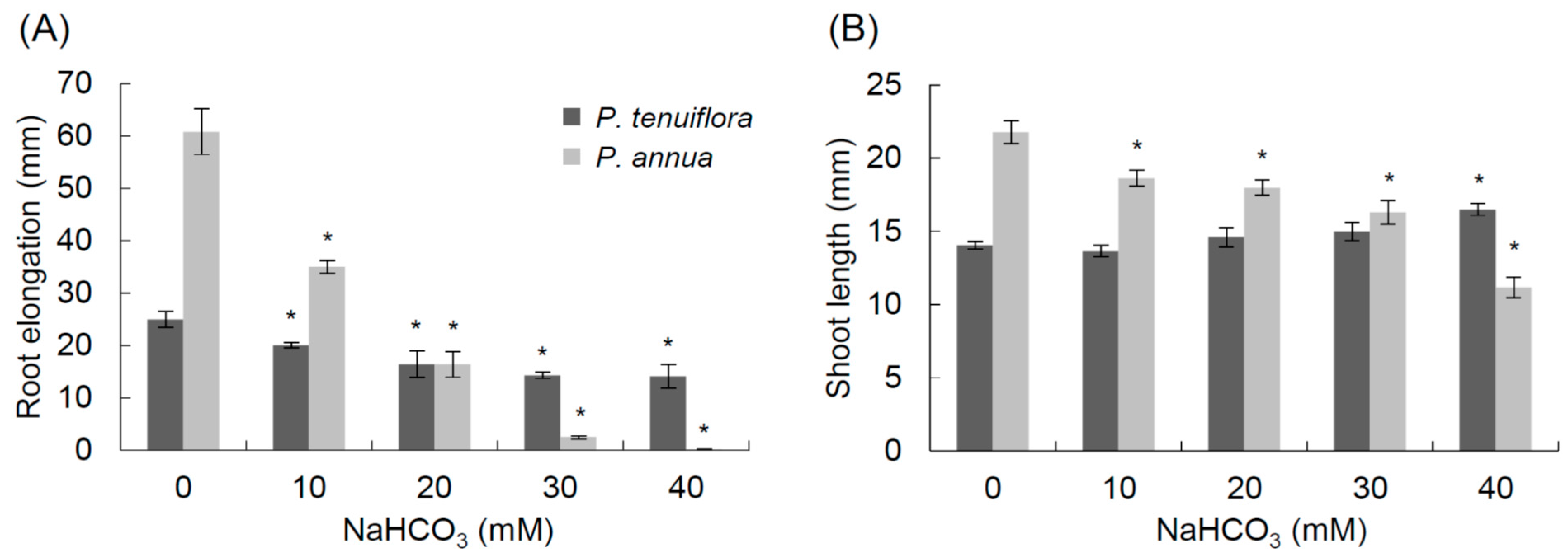
2.2. De Novo Assembly of P. tenuiflora and P. annua Transcripts
2.3. Read Mapping and Gene Annotation
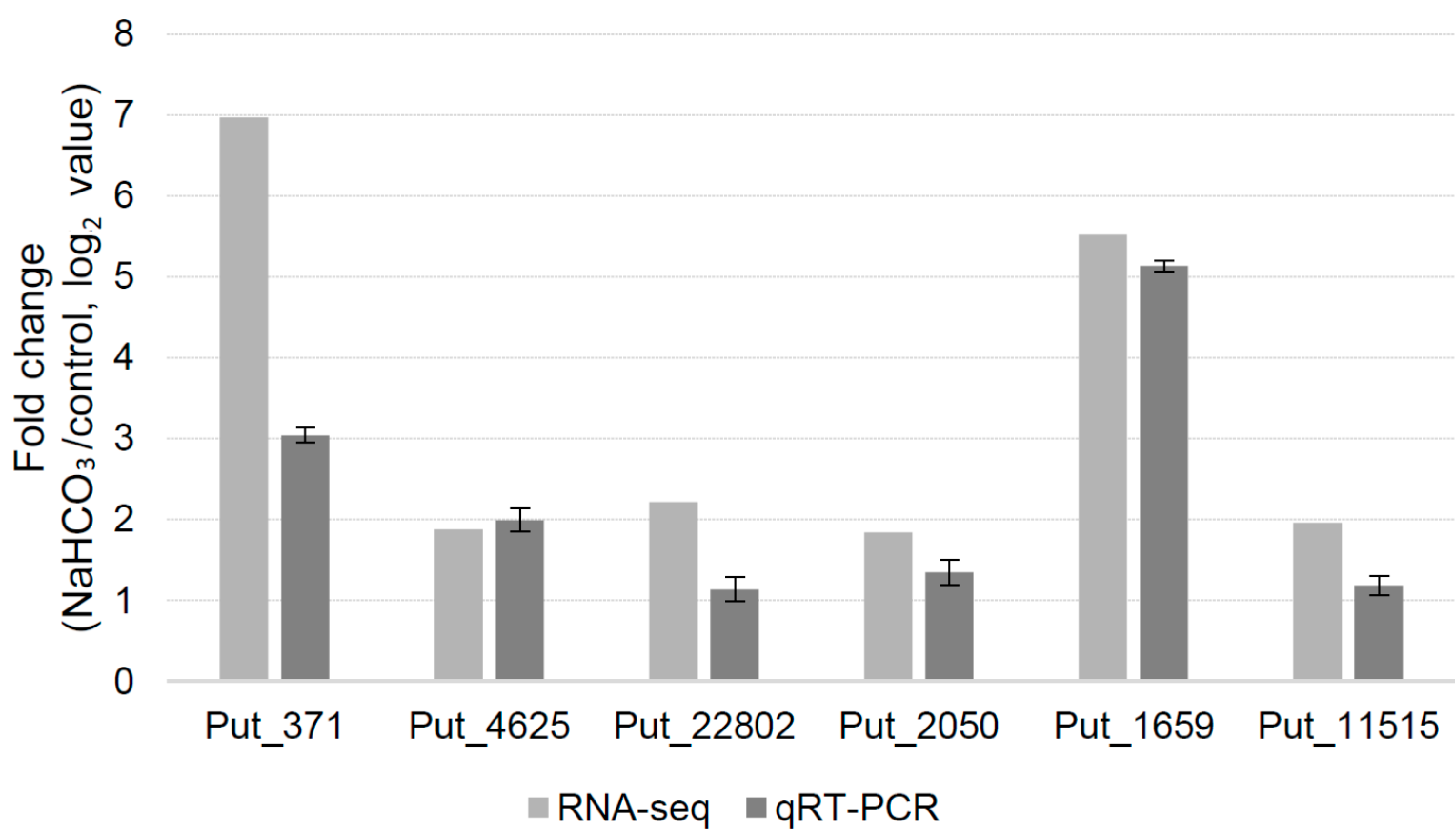
3. Discussion
3.1. Effects of NaHCO3 Stress on P. tenuiflora and P. annua
3.2. RNA-Seq Analysis and de Novo Assembly
3.2.1. Genes Differentially Regulated in P. annua under NaHCO3 Treatment
| Contig | Annotation | Fold Change (EDGE Test) |
|---|---|---|
| P. annua | ||
| Poa_27190 | Glutathione S-transferase | 11.34 |
| Poa_3657 | High affinity nitrate transporter | 53.21 |
| Poa_12387 | Anthocyanidin 5,3- O-glucosyltransferase-like | 5.09 |
| Poa_688 | ABC transporter b family member 4-like | 62.27 |
| Poa_2744 | Aspartic proteinase nepenthesin-2 | 7.68 |
| Poa_18988 | Cytochrome p450 | 54.50 |
| Poa_15731 | Disease resistance protein RPM1 | 18.52 |
| Poa_4898 | Flavonoid 3-monooxygenase-like | 14.47 |
| Poa_6299 | Mitochondrial chaperone BCS1-B | 2.93 |
| Poa_8595 | Patatin group A-3 | 3.97 |
| Poa_2480 | Phosphate transporter | 176.58 |
| Poa_8940 | Phosphoenolpyruvate carboxykinase | 54.00 |
| Poa_12214 | Potassium channel SKOR | 6.51 |
| Poa_25791 | Probable WRKY transcription factor 70-like | 16.62 |
| Poa_19228 | Subtilisin-like protease | 9.35 |
| P. tenuiflora | ||
| Put_2050 | Boron transporter | 3.58 |
| Put_11515 | Carbonic anhydrase | 3.90 |
| Put_1357 | High-affinity nitrate transporter-like | 3.53 |
| Put_763 | Leucine-rich repeat receptor-like protein kinase at2g19210-like | 3.48 |
| Put_2064 | Long chain acyl-CoA synthetase 4-like | 5.03 |
| Put_4625 | Metal-nicotianamine transporter YSL6 | 3.68 |
| Put_22802 | Nicotianamine aminotransferase A-like | 4.65 |
| Put_1383 | Phosphate transporter | 25.87 |
| Put_1973 | Phosphoenolpyruvate carboxykinase | 7.47 |
| Put_7021 | Sucrase-like protein | 6.08 |
| Put_3399 | Sulfate transporter | 3.21 |
| Put_4027 | Thionin-like peptide | 6.82 |
| Put_10418 | Zinc transporter | 5.74 |
3.2.2. Genes Differentially Regulated in P. tenuiflora under NaHCO3 Treatment
| Contig | Annotation | Fold Change (EDGE Test) |
|---|---|---|
| P. annua | ||
| Poa_40361 | 2'-Deoxymugineic-acid 2'-dioxygenase-like | −3.90 |
| Poa_7047 | Actin | −3.31 |
| Poa_13081 | Ammonium transporter | −2.94 |
| Poa_24077 | β-Expansin 1a precursor | −8.33 |
| Poa_8466 | Cellulose synthase | −3.48 |
| Poa_9099 | GDSL esterase lipase At5g45910-like | −2.24 |
| Poa_2507 | High-affinity potassium transporter | −2.80 |
| Poa_21856 | Iron-phytosiderophore transporter | −3.51 |
| Poa_36815 | Mate efflux family protein chloroplastic-like | −6.77 |
| Poa_2955 | Nicotianamine aminotransferase A-like | −4.99 |
| Poa_64935 | Nicotianamine synthase 3 | −90.37 |
| Poa_58054 | Protein zinc induced facilitator-like 1-like | −50.8 |
| Poa_41522 | STOP1 | −9.44 |
| Poa_6256 | Urea active transporter 1 | −2.47 |
| Poa_9416 | Wall-associated receptor kinase 2-like | −3.47 |
| P. tenuiflora | ||
| Put_32521 | Cytochrome p450 716b1-like | −3.72 |
| Put_28631 | Sodium transporter | −8.04 |
| Put_52287 | WRKY transcription factor | −5.15 |
4. Experimental Section
4.1. Plant Materials, Growth Conditions and Stress Treatments
4.2. RNA Extraction and cDNA Library Construction
4.3. Next Generation Sequencing and Data Analysis
4.4. Real-Time PCR Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.; Seki, K.; Miyazaki, T.; Ishihama, Y. The causes of soil alkalinization in the Songnen Plain of Northeast China. Paddy Water Environ. 2009, 7, 259–270. [Google Scholar] [CrossRef]
- Zhang, L.M.; Liu, X.G.; Qu, X.N.; Yu, Y.; Han, S.P.; Dou, Y.; Xu, Y.Y.; Jing, H.C.; Hao, D.Y. Early transcriptomic adaptation to Na2CO3 stress altered the expression of a quarter of the total genes in the maize genome and exhibited shared and distinctive profiles with NaCl and high pH stresses. J. Integr. Plant Biol. 2013, 55, 1147–1165. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Plaha, P.; Park, J.Y.; Hong, C.P.; Lee, I.S.; Yang, Z.H.; Jiang, G.B.; Kwak, S.S.; Liu, S.K.; Lee, J.S.; et al. Comparative EST profiles of leaf and root of Leymus chinensis, a xerophilous grass adapted to high pH sodic soil. Plant Sci. 2006, 170, 1081–1086. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, Y.; Liu, G.; Wang, M.H.; Jiang, J.; Hou, Y.; Qu, G.; Yang, C. Identification of expressed sequence tags in an alkali grass (Puccinellia tenuiflora) cDNA library. J. Plant Physiol. 2007, 164, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Liu, G.; Jiang, J. Development of a cDNA microarray to identify gene expression of Puccinellia tenuiflora under saline-alkali stress. Plant Physiol. 2007, 45, 567–576. [Google Scholar]
- Wang, Y.; Yang, C.; Liu, G.; Zhang, G.; Ban, Q. Microarray and suppression subtractive hybridization analyses of gene expression in Puccinellia tenuiflora after exposure to NaHCO3. Plant Sci. 2007, 173, 309–320. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.; Wang, Z.; Wang, T. Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J. Integr. Plant Biol. 2012, 55, 262–276. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Liu, G.; Yang, C.; Jiang, J.; Li, H. Expression profiling of salinity-alkali stress responses by large-scale expressed sequence tag analysis in Tamarix hispid. Plant Mol. Biol. 2008, 66, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, C.; Wang, L.; Zheng, L.; Yang, C.; Wang, Y. Comprehensive transcriptional profiling of NaHCO3-stressed Tamarix hispida roots reveals networks of responsive genes. Plant Mol. Biol. 2013, 84, 145–157. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Liu, G.; Xu, C.; Zhang, D.; Ban, Q. Analysis of gene expression profile of Limonium bicolor under NaHCO3 stress using cDNA microarray. Plant Mol. Biol. Report. 2008, 26, 241–254. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Zhu, Y.M.; Bai, X.; Lv, D.K.; Guo, D.; Ji, W.; Cai, H. Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol. 2010, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Babuin, M.F.; Campestre, M.P.; Rocco, R.; Bordenave, C.D.; Escaray, F.J.; Antonelli, C.; Calzadilla, P.; Gárriz, A.; Serna, E.; Carrasco, P.; et al. Response to long-term NaHCO3-derived alkalinity in model Lotus japonicus ecotypes Gifu B-129 and Miyakojima MG-20: Transcriptomic profiling and physiological characterization. PLoS One 2014, 9, e97106. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, W.; Chen, H.; Wu, G.; Yuan, H.; Song, X.; Kang, Q.; Zhao, D.; Jiang, W.; Liu, Y.; et al. Identification of differentially expressed genes in flax (Linum usitatissimum L.) under saline-alkaline stress by digital gene expression. Gene 2014, 549, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Zhu, Y.F.; Mao, Y.Q.; Wang, S.M.; Su, W.A.; Tang, Z.C. Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+. J. Exp. Bot. 2004, 55, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Zhang, J.L.; Liu, X.S.; Li, Z.; Wu, G.Q.; Cai, J.Y.; Flowers, T.J.; Wang, S.M. Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant. Cell Environ. 2009, 32, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Guorong, S.; Yongzhen, P.; Hongbo, S.; Liye, C.; Xining, Z.; Haiyan, M.; Wenzhong, C.; Cunxu, W. Does Puccinelia tenuiflora have the ability of salt exudation? Coll. Surf. B. Biointerfaces 2005, 46, 197–203. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, G.; Suo, B.; Chen, G.; Wang, J.; Yan, Y. Effects of Na2CO3 and NaCl stresses on the antioxidant enzymes of chloroplasts and chlorophyll fluorescence parameters of leaves of Puccinellia tenuiflora (Turcz.) scribn.et Merr. Acta Physiol. Plant. 2007, 30, 143–150. [Google Scholar] [CrossRef]
- Guo, L.Q.; Shi, D.C.; Wang, D.L. The key physiological response to alkali stress by the alkali-resistant halophyte Puccinellia tenuiflora is the accumulation of large quantities of organic acids and into the rhyzosphere. J. Agron. Crop Sci. 2010, 196, 123–135. [Google Scholar] [CrossRef]
- Ardie, S.W.; Liu, S.; Takano, T. Expression of the AKT1-type K+ channel gene from Puccinellia tenuiflora, PutAKT1, enhances salt tolerance in Arabidopsis. Plant Cell Rep. 2010, 29, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ardie, S.W.; Nishiuchi, S.; Liu, S.; Takano, T. Ectopic expression of the K+ channel β subunits from Puccinellia tenuiflora (KPutB1) and rice (KOB1) alters K+ homeostasis of yeast and Arabidopsis. Mol. Biotechnol. 2011, 48, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ardie, S.W.; Xie, L.; Takahashi, R.; Liu, S.; Takano, T. Cloning of a high-affinity K+ transporter gene PutHKT2;1 from Puccinellia tenuiflora and its functional comparison with OsHKT2;1 from rice in yeast and Arabidopsis. J. Exp. Bot. 2009, 60, 3491–502. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Sun, B.; Zhou, A.; Zhang, X.; Lee, I.; Liu, S. Identification and characterization of a PutAMT1;1 gene from Puccinellia tenuiflora. PLoS One 2013, 8, e83111. [Google Scholar] [CrossRef] [PubMed]
- Chang-Qing, Z.; Shunsaku, N.; Shenkui, L.; Tetsuo, T. Characterization of two plasma membrane protein 3 genes (PutPMP3) from the alkali grass, Puccinellia tenuiflora, and functional comparison of the rice homologues, OsLti6a/b from rice. BMB Rep. 2008, 41, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Abe, N.; Yoshida, K.T.; Liu, S.; Takano, T. Molecular cloning and characterization of plasma membrane- and vacuolar-type Na+/H+ antiporters of an alkaline-salt-tolerant monocot, Puccinellia tenuiflora. J. Plant Res. 2012, 125, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Takano, T.; Liu, S. Characterization of a PutCAX1 gene from Puccinellia tenuiflora that confers Ca2+ and Ba2+ tolerance in yeast. Biochem. Biophys. Res. Commun. 2009, 383, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, R.; Wang, B.; Liu, G.; Yang, C.; Cheng, Y. Functional characterization of a plasma membrane Na+/H+ antiporter from alkali grass (Puccinellia tenuiflora). Mol. Biol. Rep. 2010, 38, 4813–4822. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, S.; Wang, T.; Sun, G.; Dai, S. Comparative proteomic analysis of Puccinellia tenuiflora leaves under Na2CO3 stress. Int. J. Mol. Sci. 2013, 14, 1740–1762. [Google Scholar] [CrossRef] [PubMed]
- Zribi, K.; Gharsalli, M. Effect of bicarbonate on growth and iron nutrition of pea. J. Plant Nutr. 2002, 25, 2143–2149. [Google Scholar] [CrossRef]
- Yang, Y.; Smith, S.A. Optimizing de novo assembly of short-read RNA-seq data for phylogenomics. BMC Genomics 2013, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.D.; Lewis, O.A.M.; Lips, S.H. Inorganic carbon fixation and metabolism in maize roots as affected by nitrate and ammonium nutrition. Physiol. Plant. 1993, 89, 632–639. [Google Scholar] [CrossRef]
- Zioni, A.B.; Vaadia, Y.; Lips, S.H. Nitrate uptake by roots as regulated by nitrate reduction products of the shoot. Physiol. Plant. 1971, 15, 288–290. [Google Scholar] [CrossRef]
- Van der Westhuizen, M.M.; Cramer, M.D. The influence of elevated rhizosphere dissolved inorganic carbon concentrations on respiratory O2 and CO2 flux in tomato roots. J. Exp. Bot. 1998, 49, 1977–1985. [Google Scholar] [CrossRef]
- Van der Merwe, C.A.; Cramer, M.D. Effect of enriched rhizosphere carbon dioxide on nitrate and ammonium uptake in hydroponically grown tomato plants. Plant Soil 2000, 221, 5–11. [Google Scholar] [CrossRef]
- Gao, Z.F.; Lips, S.H. Effects of increasing inorganic carbon supply to roots on net nitrate uptake and assimilation in tomato seedlings. Physiol. Plant. 1997, 101, 206–212. [Google Scholar] [CrossRef]
- Alhendawi, R.A.; Römheld, V.; Kirkby, E.A.; Marschner, H. Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum, and maize. J. Plant Nutr. 1997, 20, 1731–1753. [Google Scholar] [CrossRef]
- Cai, C.; Wang, J.Y.; Zhu, Y.G.; Shen, Q.R.; Li, B.; Tong, Y.P.; Li, Z.S. Gene structure and expression of the high-affinity nitrate transport system in rice roots. J. Integr. Plant Biol. 2008, 50, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kolesch, H.; Oktay, M.; Höfner, W. Effect of iron chlorosis-inducing factors on the pH of the cytoplasm of sunflower (Helianthus annuus). Plant Soil 1984, 82, 215–221. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Ding, X.; Hu, Y.; Tian, S.; Yan, D.; Shao, S.; Gao, Y.; Liu, R.; Yang, Y. Effects of saline and alkaline stress on germination, seedling growth, and ion balance in wheat. Agron. J. 2010, 102, 1252. [Google Scholar] [CrossRef]
- Paz, R.C.; Rocco, R.A.; Reinoso, H.; Menéndez, A.B.; Pieckenstain, F.L.; Ruiz, O.A. Comparative study of alkaline, saline, and mixed saline-alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of Lotus tenuis. J. Plant Growth Regul. 2012, 31, 448–459. [Google Scholar] [CrossRef]
- Gong, B.; Zhang, C.; Li, X.; Wen, D.; Wang, S.; Shi, Q.; Wang, X. Identification of NaCl and NaHCO3 stress responsive proteins in tomato roots using iTRAQ-based analysis. Biochem. Biophys. Res. Commun. 2014, 446, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Mengel, K. Iron availability in plant tissues-iron chlorosis on calcareous soils. Plant Soil 1994, 165, 275–283. [Google Scholar] [CrossRef]
- Bavaresco, L.; Giachino, E.; Colla, R. Iron chlorosis paradox in grapevine. J. Plant Nutr. 1999, 22, 1589–1597. [Google Scholar] [CrossRef]
- Römheld, V. The chlorosis paradox: Fe inactivation as a secondary event in chlorotic leaves of grapevine. J. Plant Nutr. 2000, 23, 1629–1643. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.S.; Jeon, U.S.; Kim, Y.K.; Schjoerring, J.K.; An, G. Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol. Cells 2012, 33, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yamaguchi, H.; Nakanishi, H.; Shioiri, T.; Nishizawa, N.K.; Mori, S. Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol. 1999, 121, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Navarro, A.; Rubio, F. High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 2006, 57, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Cobbett, C.S. A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiol. 2007, 143, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Wakuta, S.; Takada, S.; Ide, K.; Takano, J.; Naito, S.; Omori, H.; Matsunaga, T.; Fujiwara, T. Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiol. 2013, 163, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Forster, H. Boron sorption on calcareous soils and reference calcites. Soil Sci. 1991, 152, 304–310. [Google Scholar] [CrossRef]
- Epple, P.; Apel, K.; Bohlmann, H. Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell 1997, 9, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, S.; Satone, H.; Tan, E.; Kurokochi, H.; Asakawa, S.; Liu, S.; Takano, T. Transcriptional Responses of a Bicarbonate-Tolerant Monocot, Puccinellia tenuiflora, and a Related Bicarbonate-Sensitive Species, Poa annua, to NaHCO3 Stress. Int. J. Mol. Sci. 2015, 16, 496-509. https://doi.org/10.3390/ijms16010496
Kobayashi S, Satone H, Tan E, Kurokochi H, Asakawa S, Liu S, Takano T. Transcriptional Responses of a Bicarbonate-Tolerant Monocot, Puccinellia tenuiflora, and a Related Bicarbonate-Sensitive Species, Poa annua, to NaHCO3 Stress. International Journal of Molecular Sciences. 2015; 16(1):496-509. https://doi.org/10.3390/ijms16010496
Chicago/Turabian StyleKobayashi, Shio, Hina Satone, Engkong Tan, Hiroyuki Kurokochi, Shuichi Asakawa, Shenkui Liu, and Tetsuo Takano. 2015. "Transcriptional Responses of a Bicarbonate-Tolerant Monocot, Puccinellia tenuiflora, and a Related Bicarbonate-Sensitive Species, Poa annua, to NaHCO3 Stress" International Journal of Molecular Sciences 16, no. 1: 496-509. https://doi.org/10.3390/ijms16010496





