Stable Hydrogen Production from Ethanol through Steam Reforming Reaction over Nickel-Containing Smectite-Derived Catalyst
Abstract
:1. Introduction
2. Results and Discussion
| Entry | Catalyst | Ni Loading (wt %) | Surface Area (m2·g−1) | CO Uptake a (μmoL·g−1) | dNi b (nm) |
|---|---|---|---|---|---|
| 1 | SM(Ni35) | 35 | 493 | 30.5 | 5.4 |
| 2 | Ni05/SM | 5 | 478 | 2.4 | 6.6 |
| 3 | Ni10/SM | 10 | 457 | 7.3 | 7.5 |
| 4 | Ni35/SM | 35 | 293 | 41.0 | 21.0 |
| 5 | Ni35/MgO | 35 | 25 | 23.8 | 19.0 |
| 6 | Ni35/SiO2 | 35 | 323 | 20.2 | 37.0 |
| 7 | Ni35/Al2O3 | 35 | 100 | 35.9 | 22.0 |
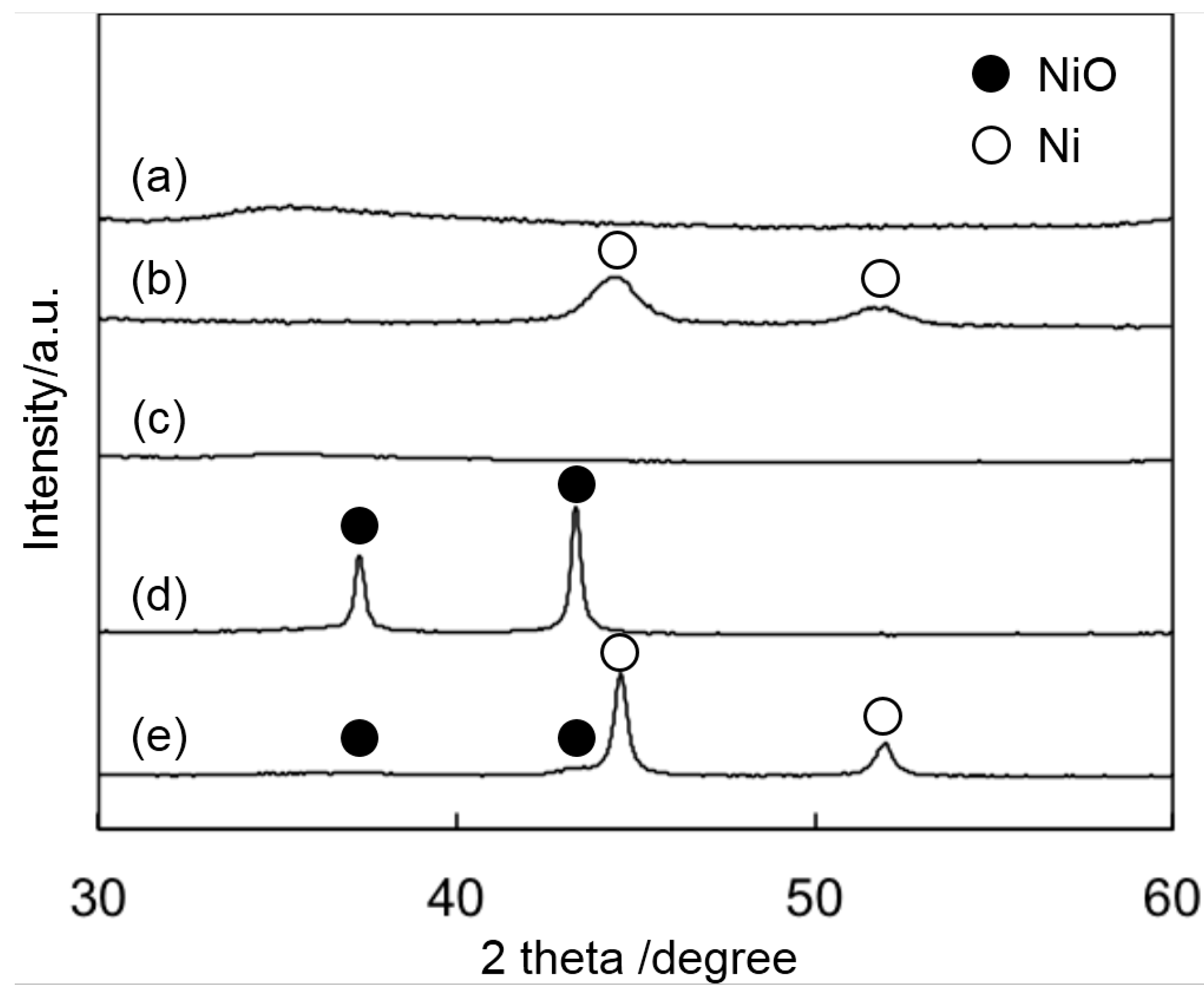
2.1. Catalytic Activity of Smectite-Derived and Conventional Supported Ni Catalysts
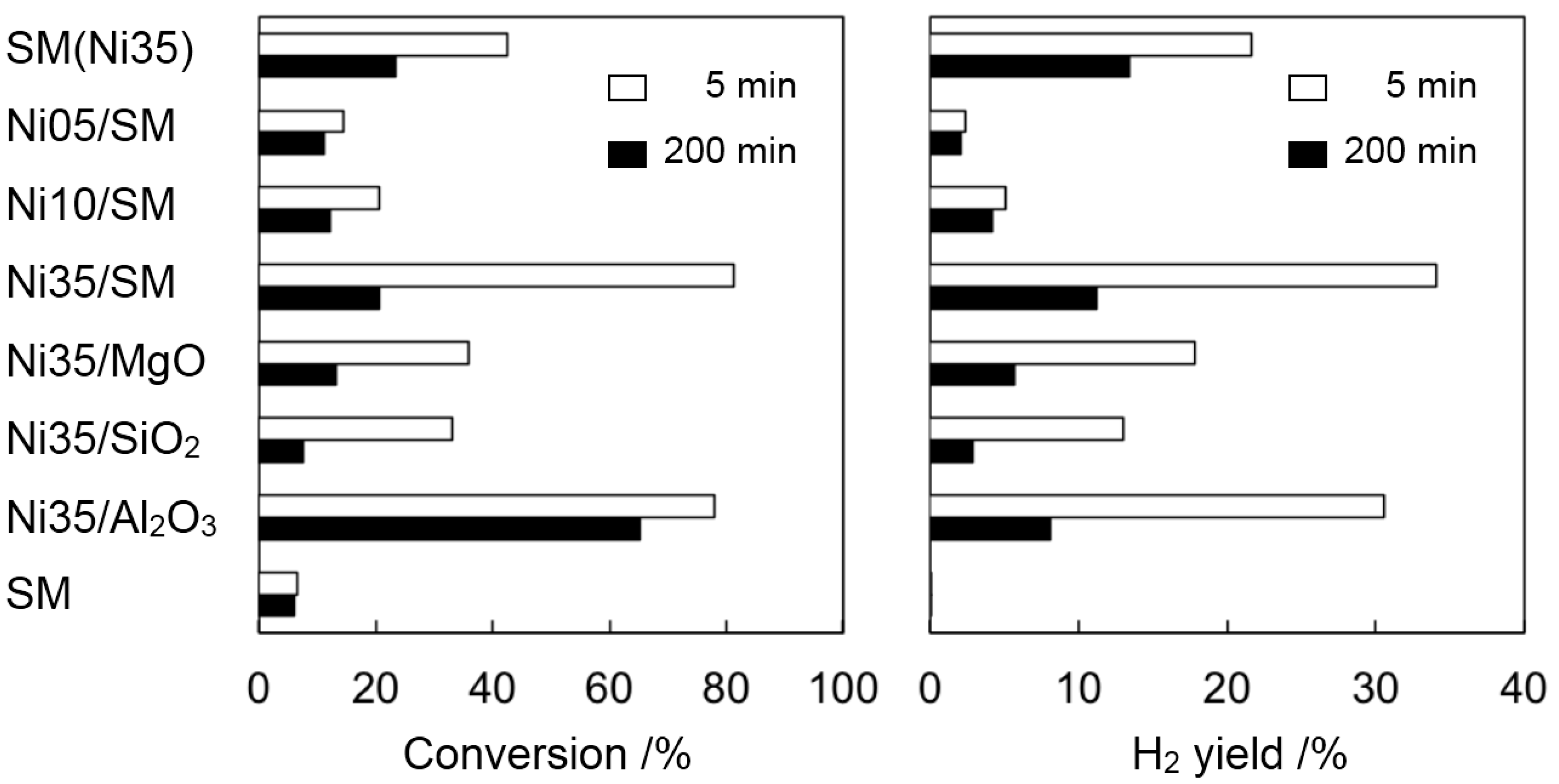
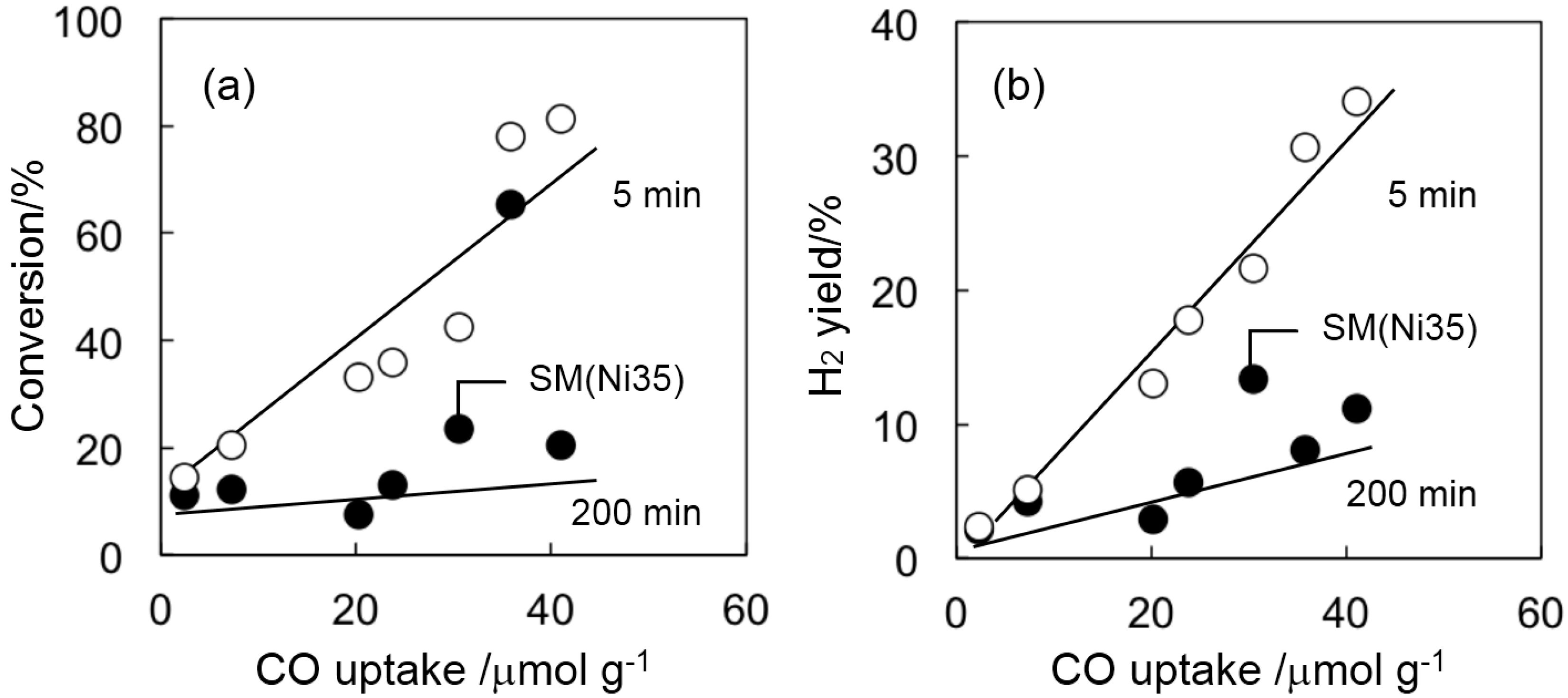
2.2. Catalyst Deactivation
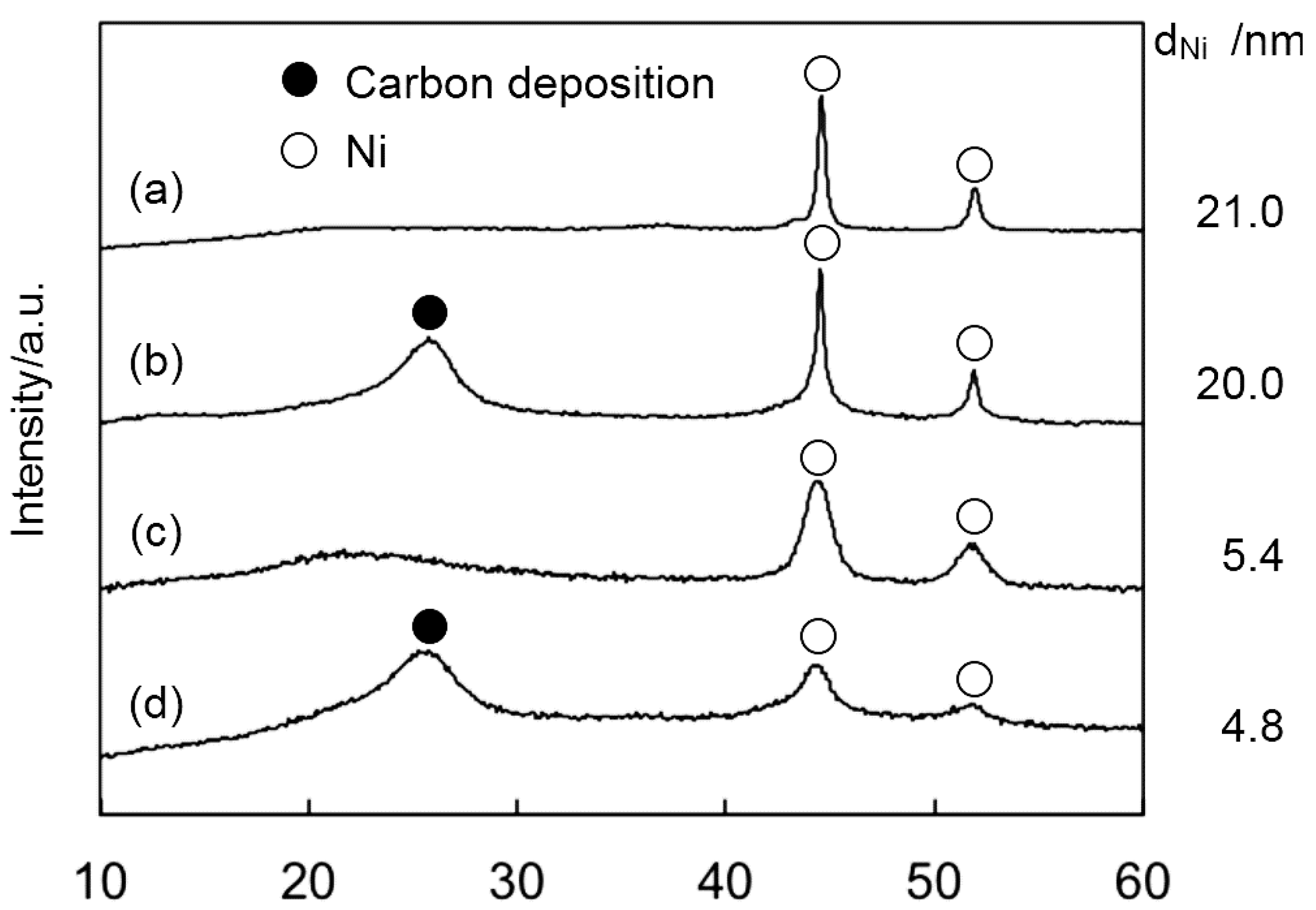
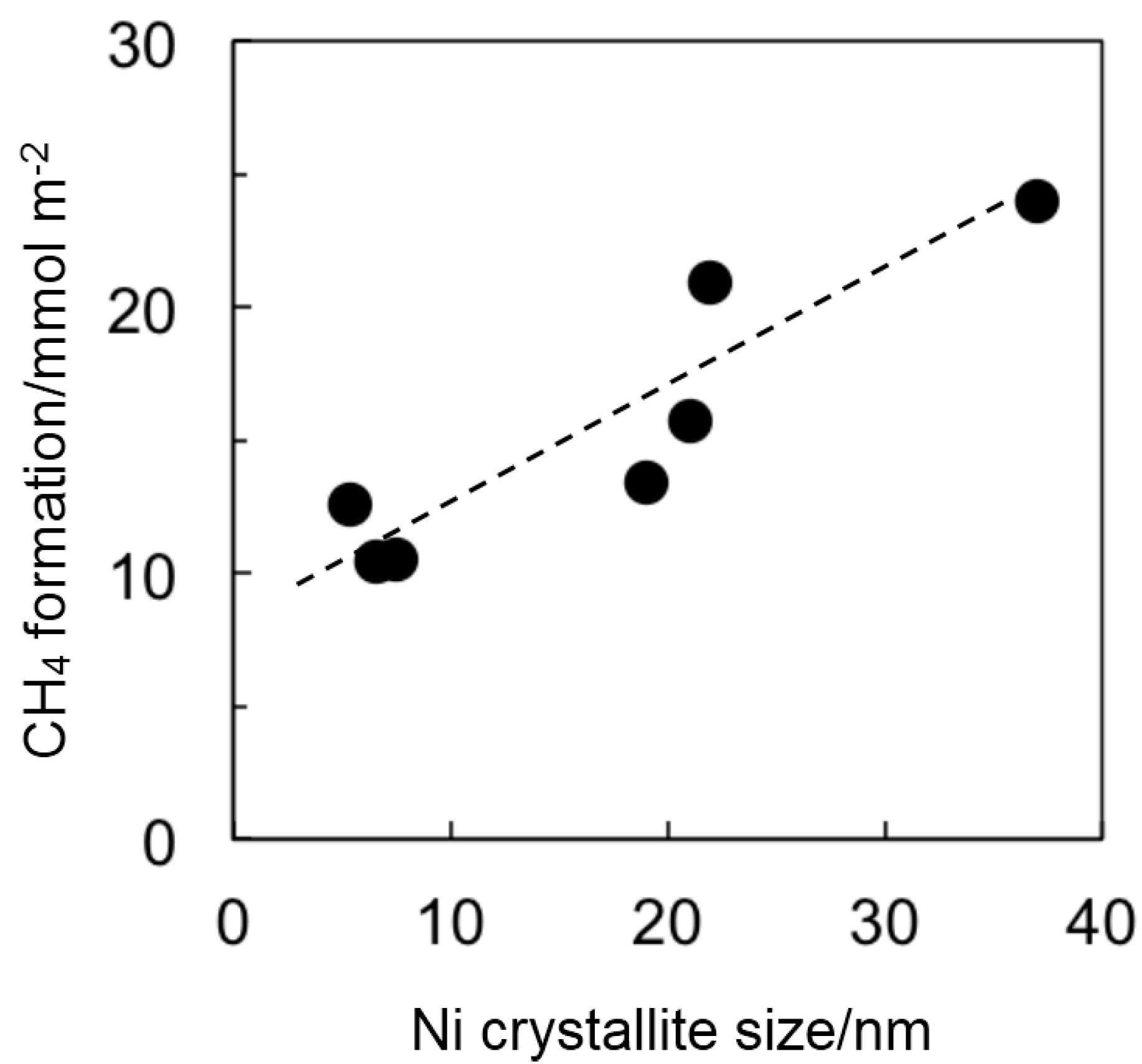
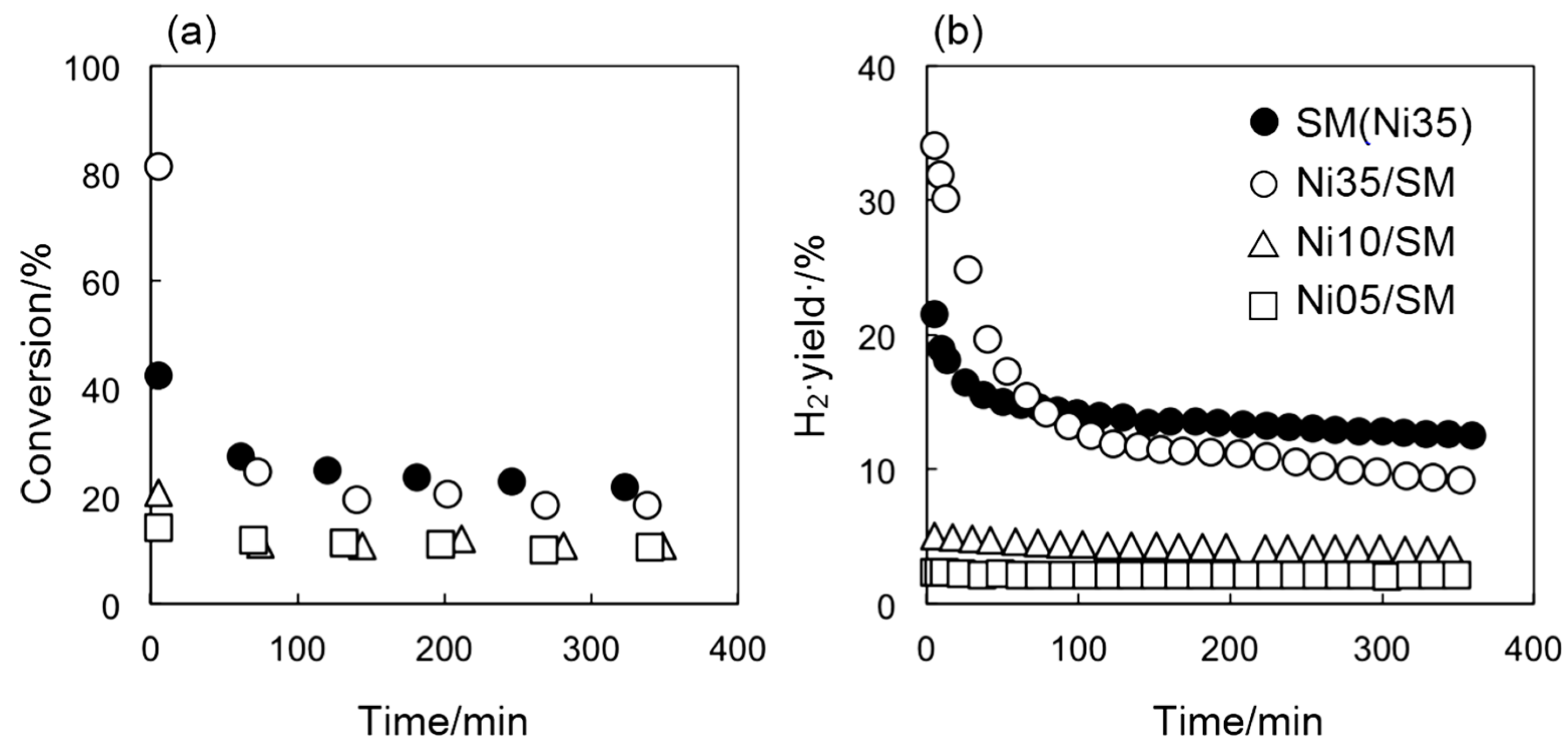
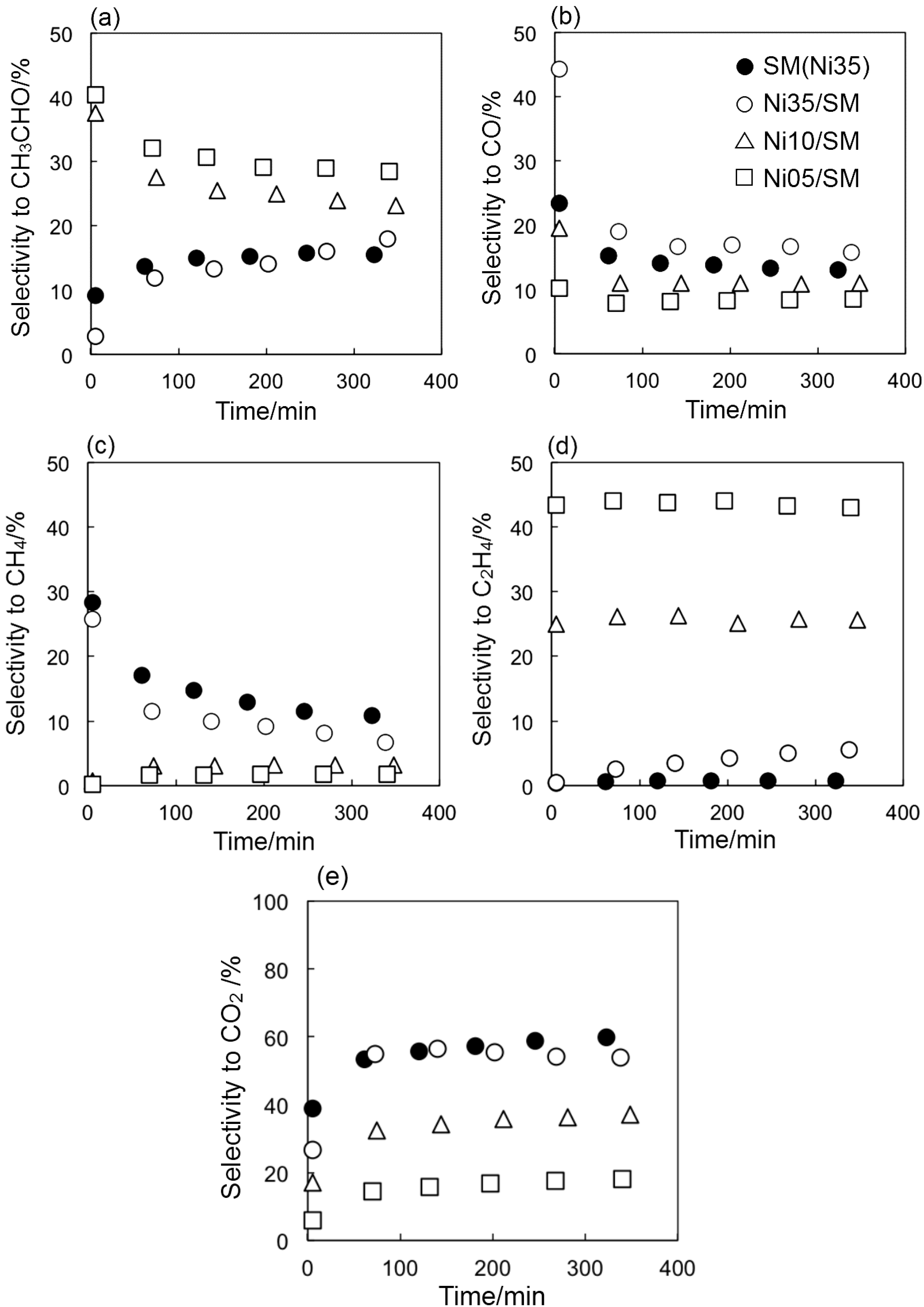
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Steam Reforming of Ethanol
3.4. Estimation of Carbon Deposition
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Navarro, R.M.; Peña, M.A.; Fierro, J.L.G. Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
- Katikaneni, S.P.; Al-Muhaish, F.; Harale, A.; Pham, T.V. On-site hydrogen production from transportation fuels: An overview and techno-economic assessment. Int. J. Hydrog. Energy 2014, 39, 4331–4350. [Google Scholar] [CrossRef]
- Freni, S.; Cavallaro, S.; Mondello, N.; Spadaro, L.; Frusteri, F. Production of hydrogen for MC fuel cell by steam reforming of ethanol over MgO supported Ni and Co catalysts. Catal. Commun. 2003, 4, 259–268. [Google Scholar] [CrossRef]
- Mariño, F.; Baronetti, G.; Jobbagy, M.; Laborde, M. Cu-Ni-K/γ-Al2O3 supported catalysts for ethanol steam reforming: Formation of hydrotalcite-type compounds as a result of metal-support interaction. Appl. Catal. A Gen. 2003, 238, 41–54. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, X.P.; Wu, F.; Zhu, W.T. H2 from steam reforming of ethanol at low temperature over Ni/Y2O3, Ni/La2O3 and Ni/Al2O3 catalysts for fuel-cell application. Int. J. Hydrog. Energy 2005, 30, 437–445. [Google Scholar] [CrossRef]
- Muroyama, H.; Nakase, R.; Matsui, T.; Eguchi, K. Ethanol steam reforming over Ni-based spinel oxide. Int. J. Hydrog. Energy 2010, 35, 1575–1581. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Longo, T.; Tosti, S.; Pinacci, P.; Basile, A. An experimental study on bio-ethanol steam reforming in a catalytic membrane reactor. Part II: Reaction pressure, sweep factor and WHSV effects. Int. J. Hydrog. Energy 2010, 35, 3159–3164. [Google Scholar] [CrossRef]
- Song, H.; Zhang, L.; Ozkan, U.S. Effect of synthesis parameters on the catalytic activity of Co-ZrO2 for bio-ethanol steam reforming. Green Chem. 2007, 9, 686–694. [Google Scholar] [CrossRef]
- Machocki, A.; Denis, A.; Grzegorczyk, W.; Gac, W. Nano- and micro-powder of zirconia and ceria-supported cobalt catalysts for the steam reforming of bio-ethanol. Appl. Surf. Sci. 2010, 256, 5551–5558. [Google Scholar] [CrossRef]
- Kazama, A.; Sekine, Y.; Oyama, K.; Matsukata, M.; Kikuchi, E. Promoting effect of small amount of Fe addition onto Co catalyst supported on α-Al2O3 for steam reforming of ethanol. Appl. Catal. A Gen. 2010, 383, 96–101. [Google Scholar] [CrossRef]
- Liguras, D.K.; Kondarides, D.I.; Verykios, X.E. Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts. Appl. Catal. B Environ. 2003, 43, 345–354. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts. Catal. Commun. 2004, 5, 611–615. [Google Scholar] [CrossRef]
- Erdőhelyi, A.; Raskó, J.; Kecskés, T.; Tóth, M.; Dömök, M.; Baán, K. Hydrogen formation in ethanol reforming on supported noble metal catalysts. Catal. Today 2006, 116, 367–376. [Google Scholar] [CrossRef]
- Birot, A.; Epron, F.; Descorme, C.; Duprez, D. Ethanol steam reforming over Rh/CexZr1−xO2 catalysts: Impact of the CO-CO2-CH4 interconversion reactions on the H2 production. Appl. Catal. B Environ. 2008, 79, 17–25. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Torii, K.; Iwasaki, T. Synthesis of novel Ni-hectorite inorganic complexes. Chem. Lett. 1988, 17, 2045–2048. [Google Scholar] [CrossRef]
- Arai, M.; Guo, S.-L.; Shirai, M.; Nishiyama, Y.; Torii, K. The catalytic activity of platinum-loaded porous smectite-like clay minerals containing different divalent cations for butane hydrogenolysis and ethylene hydrogenation. J. Catal. 1996, 161, 704–712. [Google Scholar] [CrossRef]
- Arai, M.; Kanno, M.; Nishiyama, Y.; Torii, K.; Shirai, M. Synthetic smectite-like materials containing different divalent cations as catalysts and supports for gas-phase hydrogenation of acetonitrile. J. Catal. 1999, 182, 507–510. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Fujita, S.; Ikushima, Y.; Torii, K.; Arai, M. Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides and methanol using heterogeneous Mg containing smectite catalysts: effect of reaction variables on activity and selectivity performance. Green Chem. 2003, 5, 71–75. [Google Scholar] [CrossRef]
- Fujita, S.; Bhanage, B.M.; Aoki, D.; Ochiai, Y.; Iwasa, N.; Arai, M. Mesoporous smectites incorporated with alkali metal cations as solid base catalysts. Appl. Catal. A Gen. 2006, 313, 151–159. [Google Scholar] [CrossRef]
- Iwasa, N.; Yamane, T.; Arai, M. Influence of alkali metal modification and reaction conditions on the catalytic activity and stability of Ni containing smectite-type material for steam reforming of acetic acid. Int. J. Hydrog. Energy 2011, 36, 5904–5911. [Google Scholar] [CrossRef]
- Iwasa, N.; Takizawa, M.; Arai, M. Preparation and application of nickel-containing smectite-type clay materials for methane reforming with carbon dioxide. Appl. Catal. A Gen. 2006, 314, 32–39. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, K.; Iwasa, N.; Fujita, S.; Arai, M. Selective methanation of CO in H2-rich gas stream by synthetic nickel-containing smectite based catalysts. Appl. Catal. B Environ. 2015, 162, 93–97. [Google Scholar] [CrossRef]
- Fujita, S.; Yamanishi, Y.; Arai, M. Synthesis of glycerol carbonate from glycerol and urea using zinc-containing solid catalysts: A homogeneous reaction. J. Catal. 2013, 297, 137–141. [Google Scholar] [CrossRef]
- Fujita, S.; Tanaka, M.; Arai, M. Synthesis of quinazoline-2,4(1H,3H)-dione from carbon dioxide and 2-aminobenzonitrile using mesoporous smectites incorporated with alkali hydroxide. Catal. Sci. Technol. 2014, 4, 1563–1569. [Google Scholar] [CrossRef]
- Vicente, J.; Montero, C.; Ereña, J.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Coke deactivation of Ni and Co catalysts in ethanol steam reforming at mild temperatures in a fluidized bed reactor. Int. J. Hydrog. Energy 2014, 39, 12586–12596. [Google Scholar] [CrossRef]
- Vicente, J.; Ereña, J.; Montero, C.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Reaction pathway for ethanol steam reforming on a Ni/SiO2 catalyst including coke formation. Int. J. Hydrog. Energy 2014, 39, 18820–18834. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Arai, M.; Guo, S.-L.; Sonehara, N.; Naito, T.; Torii, K. Catalytic properties of hectorite-like smectites containing nickel. Appl Catal. A Gen. 1993, 95, 171–181. [Google Scholar] [CrossRef]
- Shirai, M.; Aoki, K.; Torii, K.; Arai, M. Acidity and 1-butene isomerization of synthesized smectite-type catalysts containing different divalent cations. Appl. Catal. A Gen. 1999, 187, 141–146. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Hojlund, N. Deactivation and Poisoning of Catalyst; Oudar, J., Wise, H., Dekker, M., Eds.; CRC Press: Boca Raton, FL, USA, 1985; p. 57. [Google Scholar]
- Aupretre, F.; Descorme, C.; Duprez, D.; Casanave, D.; Uzio, D. Ethanol steam reforming over MgxNi1−xAl2O3 spinel oxide-supported Rh catalysts. J. Catal. 2005, 233, 464–477. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts. Int. J. Hydrog. Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, H.; Yamaoka, R.; Arai, M. Stable Hydrogen Production from Ethanol through Steam Reforming Reaction over Nickel-Containing Smectite-Derived Catalyst. Int. J. Mol. Sci. 2015, 16, 350-362. https://doi.org/10.3390/ijms16010350
Yoshida H, Yamaoka R, Arai M. Stable Hydrogen Production from Ethanol through Steam Reforming Reaction over Nickel-Containing Smectite-Derived Catalyst. International Journal of Molecular Sciences. 2015; 16(1):350-362. https://doi.org/10.3390/ijms16010350
Chicago/Turabian StyleYoshida, Hiroshi, Ryohei Yamaoka, and Masahiko Arai. 2015. "Stable Hydrogen Production from Ethanol through Steam Reforming Reaction over Nickel-Containing Smectite-Derived Catalyst" International Journal of Molecular Sciences 16, no. 1: 350-362. https://doi.org/10.3390/ijms16010350