A Review of Pinealectomy-Induced Melatonin-Deficient Animal Models for the Study of Etiopathogenesis of Adolescent Idiopathic Scoliosis
Abstract
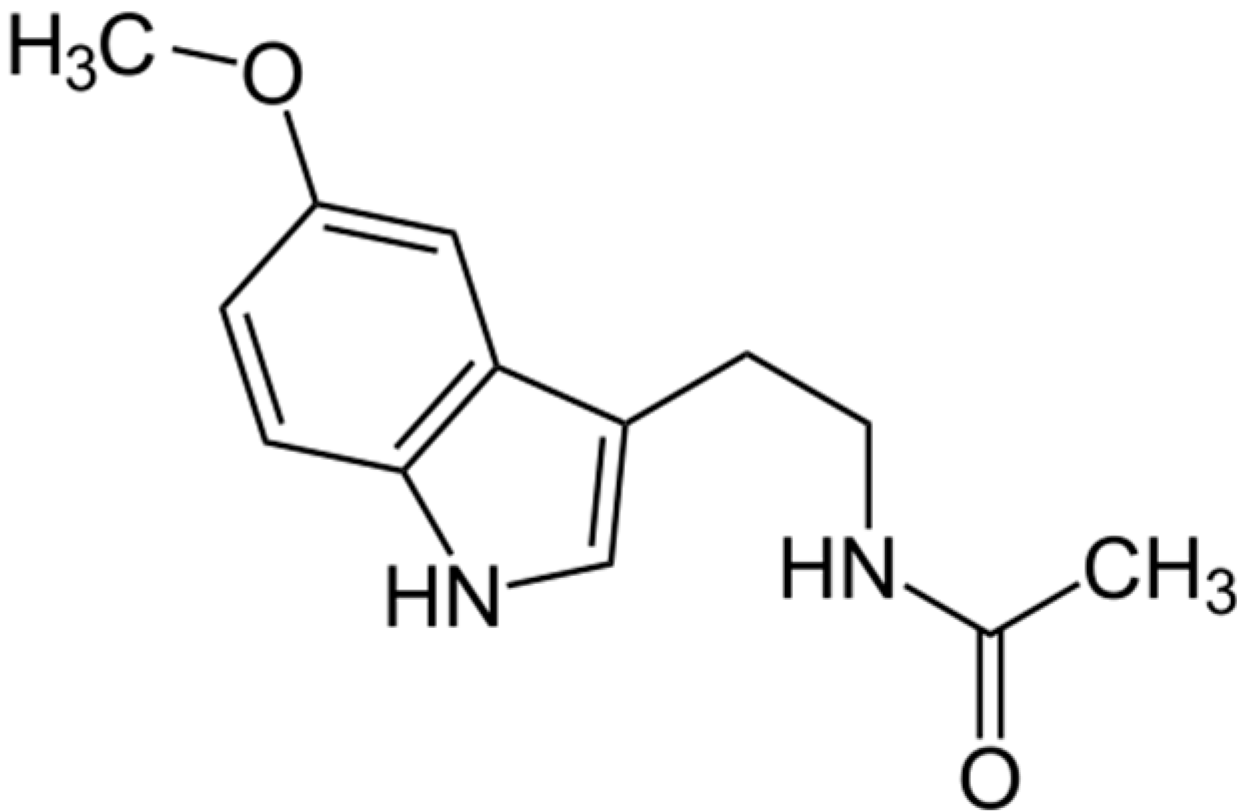
:1. Introduction
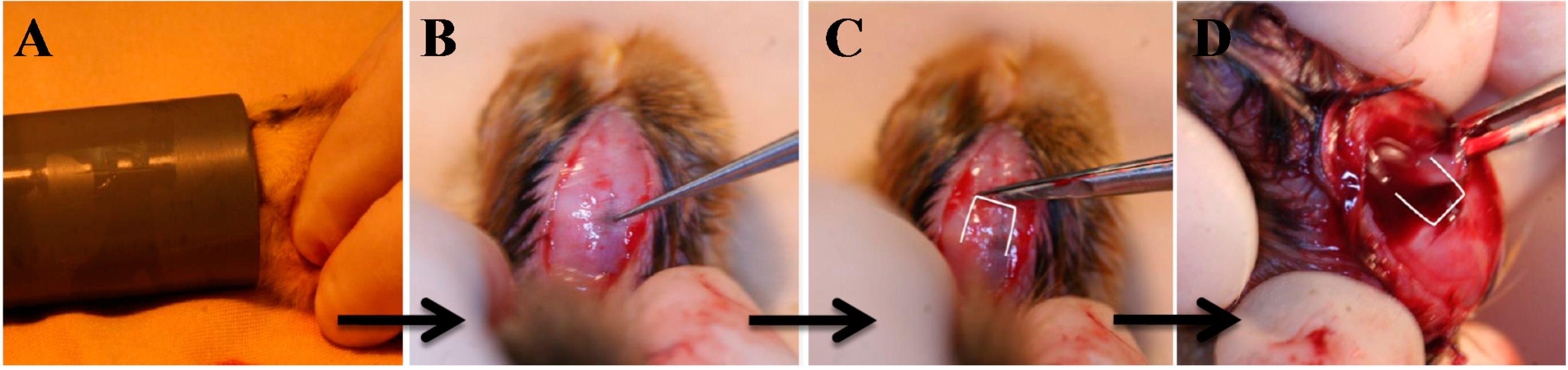
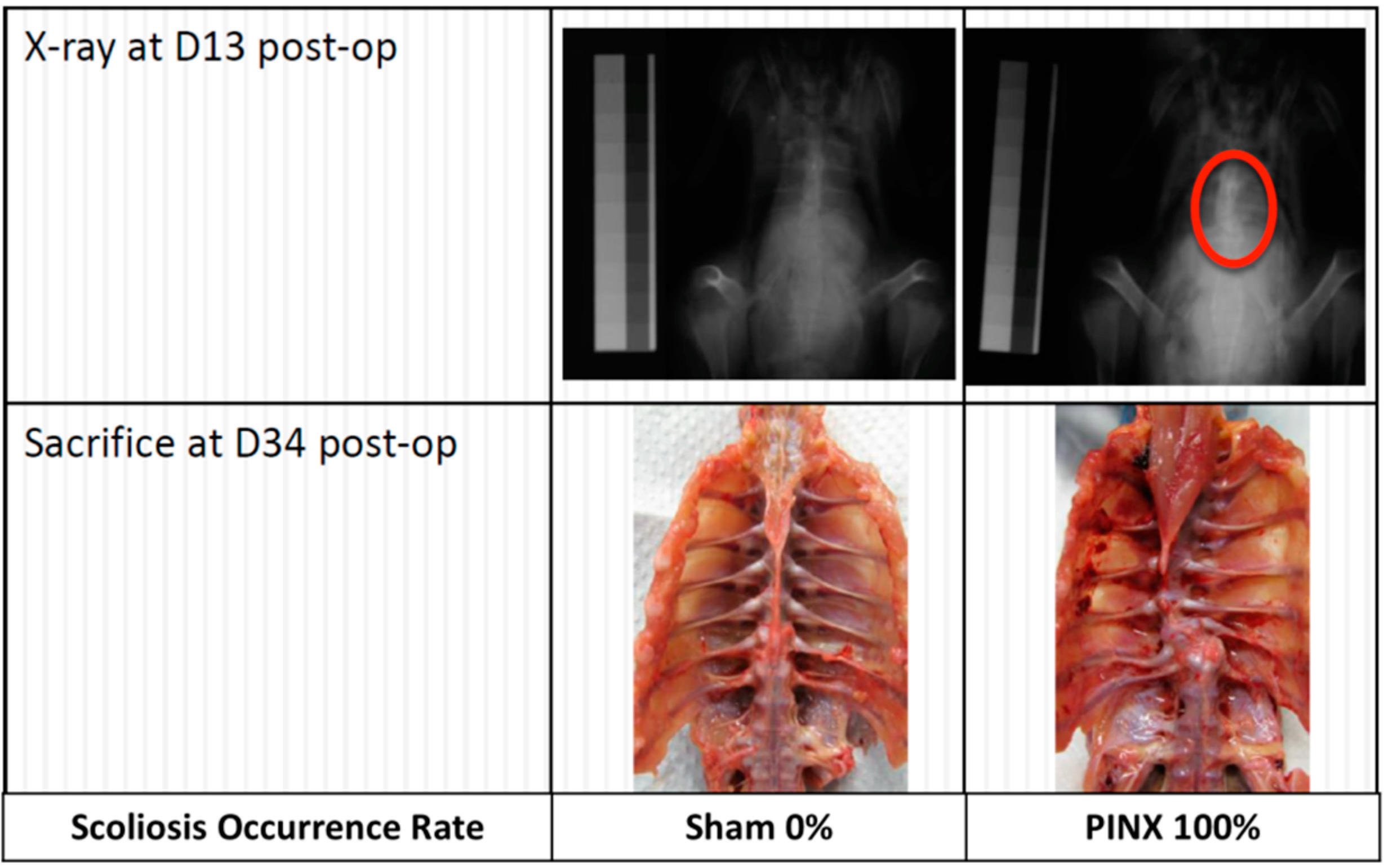
2. Pinealectomized Avian (Chicken) Model
| Procedure | Animal | Species/Variety | % of Animals with Scoliosis | Age at Operation | Time after Procedure at Which Scoliosis Was Diagnosed | Reference |
|---|---|---|---|---|---|---|
| Pinealectomy (PINX) | Chicken | Leghorn White | 100% (30/30) | / | 14 days | [12] |
| Leghorn White | 82% (36/44) in male, 100% (6/6) in female | 2 days | 90 days | [21] | ||
| Leghorn White | 100% (30/30) | 3 days | 14 days | [19] | ||
| Leghorn White | 48% (10/21) | 3 days | 14 days | [31] | ||
| / | 85% (17/20) | 3 days | 14 days | [13] | ||
| Leghorn White (Male) | 100% (40/40) | 2 days | 14 days | [23] | ||
| Leghorn White | 50% (15/30) | 3 days | 21 days | [33] | ||
| Leghorn White | 52% (17/33) | 3 days | 14 days | [34] | ||
| Mountain Hubbard | 57% (12/21) | 3 days | 21 days | [25] | ||
| Leghorn White | 26% (9/35) | 3 days | 14 days (increased to 60% (21/35) after 35 days) | [28] | ||
| Leghorn White | 25% (5/20) | 3–5 days | 14 days (increased to 55% (11/20) after 35 days) | [27] | ||
| Leghorn White (Female) | 45.5% (10/22) | 2 days | 28 days (increased to 63.6% (14/22) after 84 days) | [29] | ||
| 45.5% (10/22) | 4 days | 28 days (increased to 72.7% (16/22) after 84 days) | ||||
| 38.1% (8/21) | 11 days | 28 days (increased to 81% (17/21) after 84 days) | ||||
| 10% (2/20) | 18 days | 28 days (increased to 70% (14/20) after 84 days) | ||||
| Leghorn White | 95% (19/20) | 2 days | 14 days | [22] | ||
| / | 58% (21/36) | 7 days | 35 days | [26] | ||
| Mountain Hubbard | 50% (10/20) | / | 22 days | [30] | ||
| Leghorn White | 53.8% (7/13) | 3 days | 42 days | [35] | ||
| Leghorn White | 25% (13/25) | 3 days | 90 days | [36] | ||
| Hybro Broiler | 58% (7/12) | 2 days | 56 days | [32] | ||
| Leghorn White | 100% (15/15) | 2 days | 90 days | [24] | ||
| Hybro Broiler (Female) | 93.6% (87/93) | 3 days | 14 days | [42] | ||
| 90% (9/10) | 6 days | |||||
| Leghorn White | 50% (11/22) | 3 days | 42 days | [37] | ||
| Hybro Broiler | 93% (14/15) | 3 days | 56 days | [15] | ||
| Leghorn White | 42% (25/59) | 2 days | 35 days (increased to 45% (24/53) after 70 days | [40] | ||
| /(Female) | 95% (20/21) | 3 days | 35 days | [39] | ||
| Steggles | 75% (30/40) | 2 days | 14 days | [38] | ||
| Hybro Broiler (Female) | 84.2% (16/19) | 3 days | 7 days | [41] | ||
| 88.9% (16/18) | 14 days | |||||
| 89.5% (17/19) | 21 days | |||||
| Hybro Broiler (Female) | 100% (10/10) | 3 days | 60 days | [20] | ||
| Rat | Sprague-Dawley | 0% (0/32) | 2–4 days | 44 days | [31] | |
| Sprague-Dawley (Male) | 0% (0/10) | 21 days | 90 days | [14] | ||
| Hamster | Syrian | 0% (0/17) | 11–13 days | 43 days | [31] | |
| Salmon | Atlantic | 82% (71/86) | 3 years | 42 days | [43] | |
| Monkey | Rhesus | 0% (0/18) | 8–11 months | 300–1230 days | [44] | |
| Intense Continuous Lighting | Chicken | Mountain Hubbard | 15% (3/20) | / | 22 days | [30] |
| Leghorn White | 0% (0/41) | 3 days | 77 days | [35] | ||
| Intense Continuous Lighting + PINX | Chicken | Mountain Hubbard | 80% (16/20) | / | 22 days | [30] |
| Bipedalism + PINX | Rat | Sprague-Dawley (Male) | 100% (20/20) | 21 days | 90 days | [45] |
| 100% (10/10) | 21 days | 90 days | [14] | |||
| Mouse | C3H/HeJ (Male) | 70% | 35 days for bipedalism; 42 days for PINX | 315 days | [46] | |
| Bipedalism | Rat | Sprague-Dawley (Male) | 0 (0/5) | 21 days | 90 days | [45] |
| 0 (0/10) | 21 days | 90 days | [14] | |||
| Mouse | C57BL/6J | 97% (29/30) | 21 days | 150 days | [16] | |
| C57BL/6J (Male) | 64.3% | 35 days | 315 days | [46] | ||
| C3H/HeJ (Male) | 25% | 35 days | 315 days | [46] | ||
| Quadrupedal + PINX | Rat | Sprague-Dawley (Male) | 0 (0/10) | 21 days | 90 days | [45] |
| Natural (Congenital) Model | Mouse | C57BL/6J | 25% (5/20) | / | 150 days | [16] |
3. Pinealectomized Bipedal Rodent (Rat) Model
4. Congenital Melatonin-Deficient Rodent (Mouse) Model
| Procedure | Animal | Strain/Variety | Treatment (Melatonin/Melatonin Precursor/Pineal Transplantation) | Dosage of Melatonin | Duration of Melatonin Treatment | % of Melatonin-Treated Animals with Scoliosis | % of Animals Without Melatonin Treatment Demonstrating Scoliosis | Reference |
|---|---|---|---|---|---|---|---|---|
| Pinealectomy (PINX) | Chicken | Leghorn White | Serotonin | 1.5 mg/100 mg/every other day; i.p. | 21 days | 73% (22/30) | 100% (30/30) | [19] |
| Melatonin | 2.5 mg/100 mg/every other day; i.p. | 20% (6/30) | ||||||
| Leghorn White (Male) | 5-hydroxytryptophan | 100 mg/100 mg/twice daily; i.p. | 84 days | 70% (28/40) | 100% (40/40) | [23] | ||
| Mountain Hubbard | Melatonin | 2.5 mg/100 mg/daily; i.p. | 35 days after PINX | 52% (12/23) | 57% (12/21) | [25] | ||
| 2.5 mg/100 mg/daily; i.p. | 21 days (started after PINX for 14 days) | 55% (12/22) | ||||||
| Hybro Broiler (Female) | Melatonin | 8 mg/kg BW/daily; s.c. | 56 days | 20% (2/10) | 100% (10/10) | [20] | ||
| Leghorn White | Transplantation of pineal gland; i.m. | / | 14 days | 10% (3/30) | 100% (30/30) | [12] | ||
| / | Transplantation of pineal gland; i.m. | / | 35 days | 46% (17/37) | 58% (21/36) | [26] | ||
| Hybro Broiler | Transplantation of pineal gland; i.m. | / | 56 days | 50% (6/12) | 58% (7/12) | [32] | ||
| Bipedalism | Rat | Sprague-Dawley (Male) | Melatonin pellet | 100/90 days release | 90 days | 10% (1/10) | 90% (9/10) | [45] |
| Mice | C57BL/6J | Melatonin | 8 mg/kg BW/daily; s.c. | 140 days | 0 (0/30) | 97% (29/30) | [16] | |
| Natural Model | Mice | C57BL/6J | Melatonin | 8 mg/kg BW/daily; s.c. | 140 days | 0 (0/20) | 25% (5/20) | [16] |
5. Pinealectomized Fish (Salmon) Model
6. Pinealectomized Non-Human Primate (Monkey) Model
7. Discussion
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Brooks, H.L.; Azen, S.P.; Gerberg, E.; Brooks, R.; Chan, L. Scoliosis: A prospective epidemiological study. J. Bone Jt. Surg. Am. 1975, 57, 968–972. [Google Scholar]
- Weinstein, S.L. Natural history. Spine 1999, 24, 2592–2600. [Google Scholar]
- Rogala, E.J.; Drummond, D.S.; Gurr, J. Scoliosis: Incidence and natural history. A prospective epidemiological study. J. Bone Jt. Surg. Am. 1978, 60, 173–176. [Google Scholar]
- Weinstein, S.L.; Ponseti, I.V. Curve progression in idiopathic scoliosis. J. Bone Jt. Surg. Am. 1983, 65, 447–455. [Google Scholar]
- Lawton, J.O.; Dickson, R.A. The experimental basis of idiopathic scoliosis. Clin. Orthop. Relat. Res. 1986, 210, 9–17. [Google Scholar]
- Schwab, F.; Patel, A.; Lafage, V.; Farcy, J.P. A porcine model for progressive thoracic scoliosis. Spine 2009, 34, E397–E404. [Google Scholar]
- Newton, P.O.; Faro, F.D.; Farnsworth, C.L.; Shapiro, G.S.; Mohamad, F.; Parent, S.; Fricka, K. Multilevel spinal growth modulation with an anterolateral flexible tether in an immature bovine model. Spine 2005, 30, 2608–2613. [Google Scholar]
- Sadat-Ali, M.; Al-Habdan, I.; Al-Othman, A. Adolescent idiopathic scoliosis. Is low melatonin a cause? Jt. Bone Spine 2000, 67, 62–64. [Google Scholar]
- Machida, M.; Dubousset, J.; Yamada, T.; Kimura, J. Serum melatonin levels in adolescent idiopathic scoliosis prediction and prevention for curve progression—A prospective study. J. Pineal Res. 2009, 46, 344–348. [Google Scholar]
- Azeddine, B.; Letellier, K.; Wang da, S.; Moldovan, F.; Moreau, A. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin. Orthop. Relat. Res. 2007, 462, 45–52. [Google Scholar]
- Moreau, A.; Wang, D.S.; Forget, S.; Azeddine, B.; Angeloni, D.; Fraschini, F.; Labelle, H.; Poitras, B.; Rivard, C.H.; Grimard, G. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine 2004, 29, 1772–1781. [Google Scholar]
- Machida, M.; Dubousset, J.; Imamura, Y.; Iwaya, T.; Yamada, T.; Kimura, J. An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine 1993, 18, 1609–1615. [Google Scholar]
- Kanemura, T.; Kawakami, N.; Deguchi, M.; Mimatsu, K.; Iwata, H. Natural course of experimental scoliosis in pinealectomized chickens. Spine 1997, 22, 1563–1567. [Google Scholar]
- Machida, M.; Saito, M.; Dubousset, J.; Yamada, T.; Kimura, J.; Shibasaki, K. Pathological mechanism of idiopathic scoliosis: Experimental scoliosis in pinealectomized rats. Eur. Spine J. 2005, 14, 843–848. [Google Scholar]
- Turgut, M.; Basaloglu, H.K.; Yenisey, C.; Ozsunar, Y. Surgical pinealectomy accelerates intervertebral disc degeneration process in chicken. Eur. Spine J. 2006, 15, 605–612. [Google Scholar]
- Machida, M.; Dubousset, J.; Yamada, T.; Kimura, J.; Saito, M.; Shiraishi, T.; Yamagishi, M. Experimental scoliosis in melatonin-deficient C57BL/6J mice without pinealectomy. J. Pineal Res. 2006, 41, 1–7. [Google Scholar]
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar]
- Thillard, M.J. Vertebral column deformities following epiphysectomy in the chick. C. R. Hebd. Seances Acad. Sci. 1959, 248, 1238–1240. [Google Scholar]
- Machida, M.; Dubousset, J.; Imamura, Y.; Iwaya, T.; Yamada, T.; Kimura, J. Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J. Bone Jt. Surg. Br. 1995, 77, 134–138. [Google Scholar]
- Kono, H.; Machida, M.; Saito, M.; Nishiwaki, Y.; Kato, H.; Hosogane, N.; Chiba, K.; Miyamoto, T.; Matsumoto, M.; Toyama, Y. Mechanism of osteoporosis in adolescent idiopathic scoliosis: Experimental scoliosis in pinealectomized chickens. J. Pineal Res. 2011, 51, 387–393. [Google Scholar]
- Machida, M.; Dubousset, J.; Imamura, Y.; Iwaya, T.; Yamada, T.; Kimura, J.; Toriyama, S. Pathogenesis of idiopathic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patients with idiopathic scoliosis. J. Pediatr. Orthop. 1994, 14, 329–335. [Google Scholar]
- Machida, M.; Dubousset, J.; Satoh, T.; Murai, I.; Wood, K.B.; Yamada, T.; Ryu, J. Pathologic mechanism of experimental scoliosis in pinealectomized chickens. Spine 2001, 26, E385–E391. [Google Scholar]
- Machida, M.; Miyashita, Y.; Murai, I.; Dubousset, J.; Yamada, T.; Kimura, J. Role of serotonin for scoliotic deformity in pinealectomized chicken. Spine 1997, 22, 1297–1301. [Google Scholar]
- Machida, M.; Yamada, H.; Yamada, T.; Kimura, J.; Saito, M.; Shibasaki, K. Rib length in experimental scoliosis induced by pinealectomy in chickens. Spine 2005, 30, E692–E696. [Google Scholar]
- Bagnall, K.; Raso, V.J.; Moreau, M.; Mahood, J.; Wang, X.; Zhao, J. The effects of melatonin therapy on the development of scoliosis after pinealectomy in the chicken. J. Bone Jt. Surg. Am. 1999, 81, 191–199. [Google Scholar]
- Bagnall, K.M.; Beuerlein, M.; Johnson, P.; Wilson, J.; Raso, V.J.; Moreau, M. Pineal transplantation after pinealectomy in young chickens has no effect on the development of scoliosis. Spine 2001, 26, 1022–1027. [Google Scholar]
- Beuerlein, M.; Wang, X.; Moreau, M.; Raso, J.; Mahood, J.; Bagnall, K. Development of scoliosis following pinealectomy in young chickens is not the result of an artifact of the surgical procedure. Microsc. Res. Tech. 2001, 53, 81–86. [Google Scholar]
- Beuerlein, M.; Wilson, J.; Moreau, M.; Raso, V.J.; Mahood, J.; Wang, X.; Greenhill, B.; Bagnall, K.M. The critical stage of pinealectomy surgery after which scoliosis is produced in young chickens. Spine 2001, 26, 237–240. [Google Scholar]
- Inoh, H.; Kawakami, N.; Matsuyama, Y.; Aoki, T.; Kanemura, T.; Natsume, N.; Iwata, H. Correlation between the age of pinealectomy and the development of scoliosis in chickens. Spine 2001, 26, 1014–1021. [Google Scholar]
- Nette, F.; Dolynchuk, K.; Wang, X.; Daniel, A.; Demianczuk, C.; Moreau, M.; Raso, J.; Mahood, J.; Bagnall, K. The effects of exposure to intense, 24 h light on the development of scoliosis in young chickens. Stud. Health Technol. Inform. 2002, 91, 1–6. [Google Scholar]
- O'Kelly, C.; Wang, X.; Raso, J.; Moreau, M.; Mahood, J.; Zhao, J.; Bagnall, K. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine 1999, 24, 35–43. [Google Scholar]
- Turgut, M.; Yenisey, C.; Uysal, A.; Bozkurt, M.; Yurtseven, M.E. The effects of pineal gland transplantation on the production of spinal deformity and serum melatonin level following pinealectomy in the chicken. Eur. Spine J. 2003, 12, 487–494. [Google Scholar]
- Wang, X.; Jiang, H.; Raso, J.; Moreau, M.; Mahood, J.; Zhao, J.; Bagnall, K. Characterization of the scoliosis that develops after pinealectomy in the chicken and comparison with adolescent idiopathic scoliosis in humans. Spine 1997, 22, 2626–2635. [Google Scholar]
- Wang, X.; Moreau, M.; Raso, V.J.; Zhao, J.; Jiang, H.; Mahood, J.; Bagnall, K. Changes in serum melatonin levels in response to pinealectomy in the chicken and its correlation with development of scoliosis. Spine 1998, 23, 2377–2381. [Google Scholar]
- Cheung, K.M.; Lu, D.S.; Poon, A.M.; Wang, T.; Luk, K.D.; Leong, J.C. Effect of melatonin suppression on scoliosis development in chickens by either constant light or surgical pinealectomy. Spine 2003, 28, 1941–1944. [Google Scholar]
- Cheung, K.M.; Wang, T.; Hu, Y.G.; Leong, J.C. Primary thoracolumbar scoliosis in pinealectomized chickens. Spine 2003, 28, 2499–2504. [Google Scholar]
- Poon, A.M.; Cheung, K.M.; Lu, D.S.; Leong, J.C. Changes in melatonin receptors in relation to the development of scoliosis in pinealectomized chickens. Spine 2006, 31, 2043–2047. [Google Scholar]
- Fagan, A.B.; Kennaway, D.J.; Oakley, A.P. Pinealectomy in the chicken: A good model of scoliosis? Eur. Spine J. 2009, 18, 1154–1159. [Google Scholar]
- Akel, I.; Kocak, O.; Bozkurt, G.; Alanay, A.; Marcucio, R.; Acaroglu, E. The effect of calmodulin antagonists on experimental scoliosis: A pinealectomized chicken model. Spine 2009, 34, 533–538. [Google Scholar]
- Turhan, E.; Acaroglu, E.; Bozkurt, G.; Alanay, A.; Yazici, M.; Surat, A. Unilateral enucleation affectsthe laterality but not the incidence of scoliosis in pinealectomized chicken. Spine 2006, 31, 133–138. [Google Scholar]
- Fu, G.; Yoshihara, H.; Kawakami, N.; Goto, M.; Tsuji, T.; Ohara, T.; Imagama, S. Microcomputed tomographic evaluation of vertebral microarchitecture in pinealectomized scoliosis chickens. J. Pediatr. Orthop. B 2011, 20, 382–388. [Google Scholar]
- Yoshihara, H.; Kawakami, N.; Matsuyama, Y.; Inoh, H.; Imagama, S.; Ishiguro, N. A histomorphologic study of scoliosis in pinealectomized chickens. Spine 2005, 30, 2244–2251. [Google Scholar]
- Fjelldal, P.G.; Grotmol, S.; Kryvi, H.; Gjerdet, N.R.; Taranger, G.L.; Hansen, T.; Porter, M.J.; Totland, G.K. Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon, Salmo salar. J. Pineal Res. 2004, 36, 132–139. [Google Scholar]
- Cheung, K.M.; Wang, T.; Poon, A.M.; Carl, A.; Tranmer, B.; Hu, Y.; Luk, K.D.; Leong, J.C. The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine 2005, 30, 2009–2013. [Google Scholar]
- Machida, M.; Murai, I.; Miyashita, Y.; Dubousset, J.; Yamada, T.; Kimura, J. Pathogenesis of idiopathic scoliosis—Experimental study in rats. Spine 1999, 24, 1985–1989. [Google Scholar]
- Oyama, J.; Murai, I.; Kanazawa, K.; Machida, M. Bipedal ambulation induces experimental scoliosis in C57BL/6J mice with reduced plasma and pineal melatonin levels. J. Pineal Res. 2006, 40, 219–224. [Google Scholar]
- Ebihara, S.; Marks, T.; Hudson, D.J.; Menaker, M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 1986, 231, 491–493. [Google Scholar]
- Sheng, M.H.; Baylink, D.J.; Beamer, W.G.; Donahue, L.R.; Rosen, C.J.; Lau, K.H.; Wergedal, J.E. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone 1999, 25, 421–429. [Google Scholar]
- Turner, C.H.; Hsieh, Y.F.; Muller, R.; Bouxsein, M.L.; Rosen, C.J.; McCrann, M.E.; Donahue, L.R.; Beamer, W.G. Variation in bone biomechanical properties, microstructure, and density in BXH recombinant inbred mice. J. Bone Miner. Res. 2001, 16, 206–213. [Google Scholar]
- Chen, C.; Kalu, D.N. Strain differences in bone density and calcium metabolism between C3H/HeJ and C57BL/6J mice. Bone 1999, 25, 413–420. [Google Scholar]
- Dimai, H.P.; Linkhart, T.A.; Linkhart, S.G.; Donahue, L.R.; Beamer, W.G.; Rosen, C.J.; Farley, J.R.; Baylink, D.J. Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone 1998, 22, 211–216. [Google Scholar]
- Richman, C.; Kutilek, S.; Miyakoshi, N.; Srivastava, A.K.; Beamer, W.G.; Donahue, L.R.; Rosen, C.J.; Wergedal, J.E.; Baylink, D.J.; Mohan, S. Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J. Bone Miner. Res. 2001, 16, 386–397. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Pilar Terron, M.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar]
- Illes, T.; Horvath, G.; Bagnall, K.M.; Raso, J.; Moreau, M.; Mahood, J.; Wang, X.; Zhao, J. Pinealectomy and scoliosis. J. Bone Jt. Surg. Am. 2000, 82, 1197–1198. [Google Scholar]
- Janssen, M.M.; de Wilde, R.F.; Kouwenhoven, J.W.; Castelein, R.M. Experimental animal models in scoliosis research: A review of the literature. Spine J. 2011, 11, 347–358. [Google Scholar]
- Hilibrand, A.S.; Blakemore, L.C.; Loder, R.T.; Greenfield, M.L.; Farley, F.A.; Hensinger, R.N.; Hariharan, M. The role of melatonin in the pathogenesis of adolescent idiopathic scoliosis. Spine 1996, 21, 1140–1146. [Google Scholar]
- Bagnall, K.M.; Raso, V.J.; Hill, D.L.; Moreau, M.; Mahood, J.K.; Jiang, H.; Russell, G.; Bering, M.; Buzzell, G.R. Melatonin levels in idiopathic scoliosis—Diurnal and nocturnal serum melatonin levels in girls with adolescent idiopathic scoliosis. Spine 1996, 21, 1974–1978. [Google Scholar]
- Burwell, R.G.; Dangerfield, P.H.; Freeman, B.J. Concepts on the pathogenesis of adolescent idiopathic scoliosis—Bone growth and mass, vertebral column, spinal cord, brain, skull, extra-spinal left-right skeletal length asymmetries, disproportions and molecular pathogenesis. Stud. Health Technol. Inform. 2008, 135, 3–52. [Google Scholar]
- Cheng, J.C.; Qin, L.; Cheung, C.S.; Sher, A.H.; Lee, K.M.; Ng, S.W.; Guo, X. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J. Bone Miner. Res. 2000, 15, 1587–1595. [Google Scholar]
- Cook, S.D.; Harding, A.F.; Morgan, E.L.; Nicholson, R.J.; Thomas, K.A.; Whitecloud, T.S.; Ratner, E.S. Trabecular bone mineral density in idiopathic scoliosis. J. Pediatr. Orthop. 1987, 7, 168–174. [Google Scholar]
- Hung, V.W.; Qin, L.; Cheung, C.S.; Lam, T.P.; Ng, B.K.; Tse, Y.K.; Guo, X.; Lee, K.M.; Cheng, J.C. Osteopenia: A new prognostic factor of curve progression in adolescent idiopathic scoliosis. J. Bone Jt. Surg. Am. 2005, 87, 2709–2016. [Google Scholar]
- Nakade, O.; Koyama, H.; Ariji, H.; Yajima, A.; Kaku, T. Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J. Pineal Res. 1999, 27, 106–110. [Google Scholar]
- Satomura, K.; Tobiume, S.; Tokuyama, R.; Yamasaki, Y.; Kudoh, K.; Maeda, E.; Nagayama, M. Melatonin at pharmacological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. J. Pineal Res. 2007, 42, 231–239. [Google Scholar]
- Roth, J.A.; Kim, B.G.; Lin, W.L.; Cho, M.I. Melatonin promotes osteoblast differentiation and bone formation. J. Biol. Chem. 1999, 274, 22041–22047. [Google Scholar]
- Cardinali, D.P.; Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mautalen, C. Melatonin effects on bone: Experimental facts and clinical perspectives. J. Pineal Res. 2003, 34, 81–87. [Google Scholar]
- Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mondelo, N.; Mastaglia, S.; Somoza, J.; Cardinali, D.P. Melatonin effect on bone metabolism in rats treated with methylprednisolone. J. Pineal Res. 2006, 40, 297–304. [Google Scholar]
- Ladizesky, M.G.; Cutrera, R.A.; Boggio, V.; Somoza, J.; Centrella, J.M.; Mautalen, C.; Cardinali, D.P. Effect of melatonin on bone metabolism in ovariectomized rats. Life Sci. 2001, 70, 557–565. [Google Scholar]
- Man, G.C.; Wang, W.W.; Yeung, B.H.; Lee, S.K.; Ng, B.K.; Hung, W.Y.; Wong, J.H.; Ng, T.B.; Qiu, Y.; Cheng, J.C. Abnormal proliferation and differentiation of osteoblasts from girls with adolescent idiopathic scoliosis to melatonin. J. Pineal Res. 2010, 49, 69–77. [Google Scholar]
- Man, G.C.; Wong, J.H.; Wang, W.W.; Sun, G.Q.; Yeung, B.H.; Ng, T.B.; Lee, S.K.; Ng, B.K.; Qiu, Y.; Cheng, J.C. Abnormal melatonin receptor 1B expression in osteoblasts from girls with adolescent idiopathic scoliosis. J. Pineal Res. 2011, 50, 395–402. [Google Scholar]
- Yim, A.P.; Yeung, H.Y.; Sun, G.; Lee, K.M.; Ng, T.B.; Lam, T.P.; Ng, B.K.; Qiu, Y.; Moreau, A.; Cheng, J.C. Abnormal skeletal growth in adolescent idiopathic scoliosis is associated with abnormal quantitative expression of melatonin receptor, MT2. Int. J. Mol. Sci. 2013, 14, 6345–6358. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wai, M.G.C.; Jun, W.W.W.; Yee, Y.A.P.; Ho, W.J.; Bun, N.T.; Ping, L.T.; Man, L.S.K.; Wah, N.B.K.; Chiu, W.C.; Yong, Q.; et al. A Review of Pinealectomy-Induced Melatonin-Deficient Animal Models for the Study of Etiopathogenesis of Adolescent Idiopathic Scoliosis. Int. J. Mol. Sci. 2014, 15, 16484-16499. https://doi.org/10.3390/ijms150916484
Wai MGC, Jun WWW, Yee YAP, Ho WJ, Bun NT, Ping LT, Man LSK, Wah NBK, Chiu WC, Yong Q, et al. A Review of Pinealectomy-Induced Melatonin-Deficient Animal Models for the Study of Etiopathogenesis of Adolescent Idiopathic Scoliosis. International Journal of Molecular Sciences. 2014; 15(9):16484-16499. https://doi.org/10.3390/ijms150916484
Chicago/Turabian StyleWai, Man Gene Chi, Wang William Wei Jun, Yim Annie Po Yee, Wong Jack Ho, Ng Tzi Bun, Lam Tsz Ping, Lee Simon Kwong Man, Ng Bobby Kin Wah, Wang Chi Chiu, Qiu Yong, and et al. 2014. "A Review of Pinealectomy-Induced Melatonin-Deficient Animal Models for the Study of Etiopathogenesis of Adolescent Idiopathic Scoliosis" International Journal of Molecular Sciences 15, no. 9: 16484-16499. https://doi.org/10.3390/ijms150916484






