Antioxidative Dietary Compounds Modulate Gene Expression Associated with Apoptosis, DNA Repair, Inhibition of Cell Proliferation and Migration
Abstract
:1. Introduction
2. Coix Polysaccharides Induce Cancer Cell Apoptosis
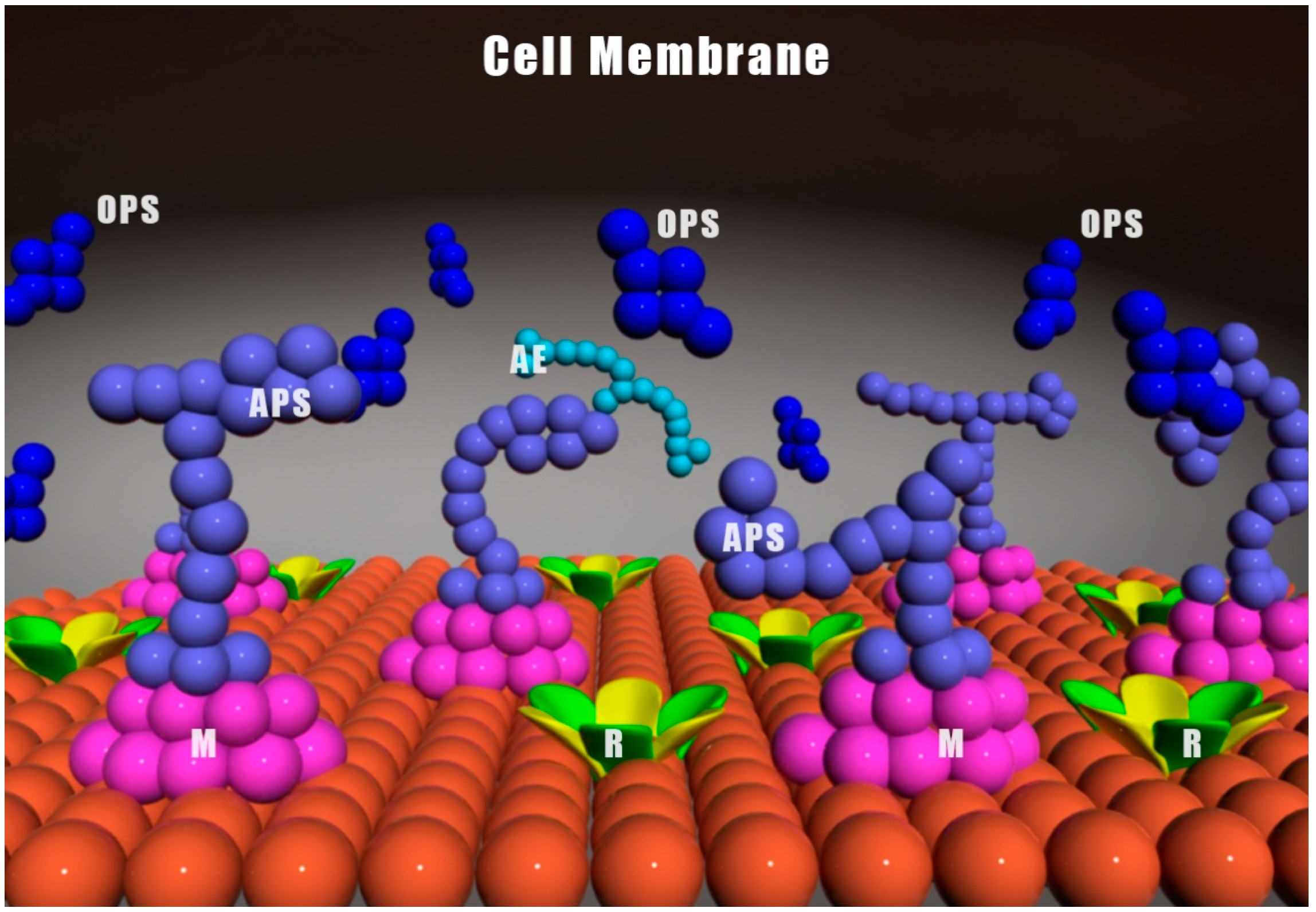
3. Inflammatory and Anti-Inflammatory Effects of Viili Polysaccharides
4. COX-2 (Cyclooxygenase-2) Is Involved in Anti-Inflammatory Processes via Ursolic Acid and Microbial Polysaccharides
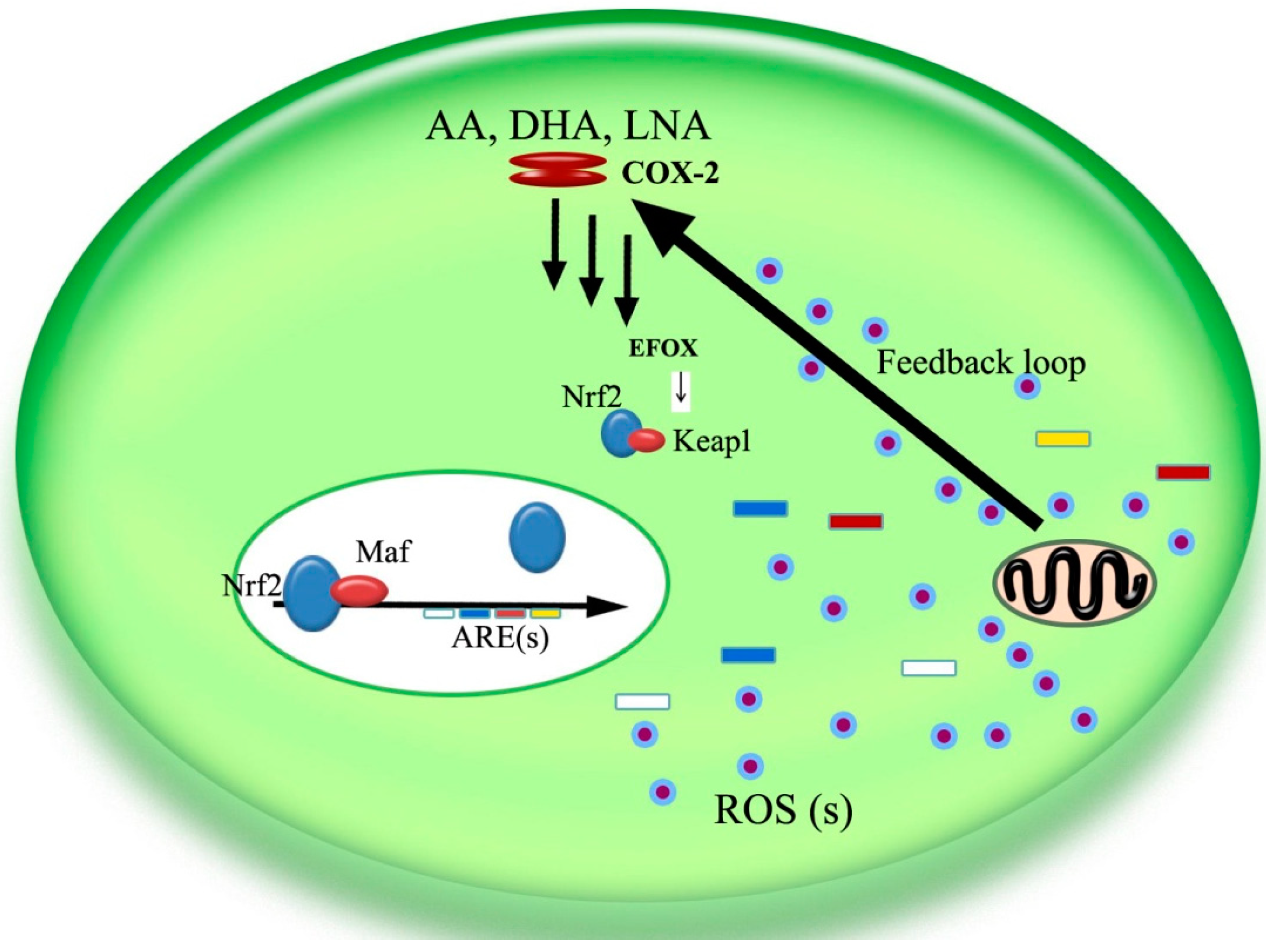
5. The COX-2/Nrf2/ARE Pathway
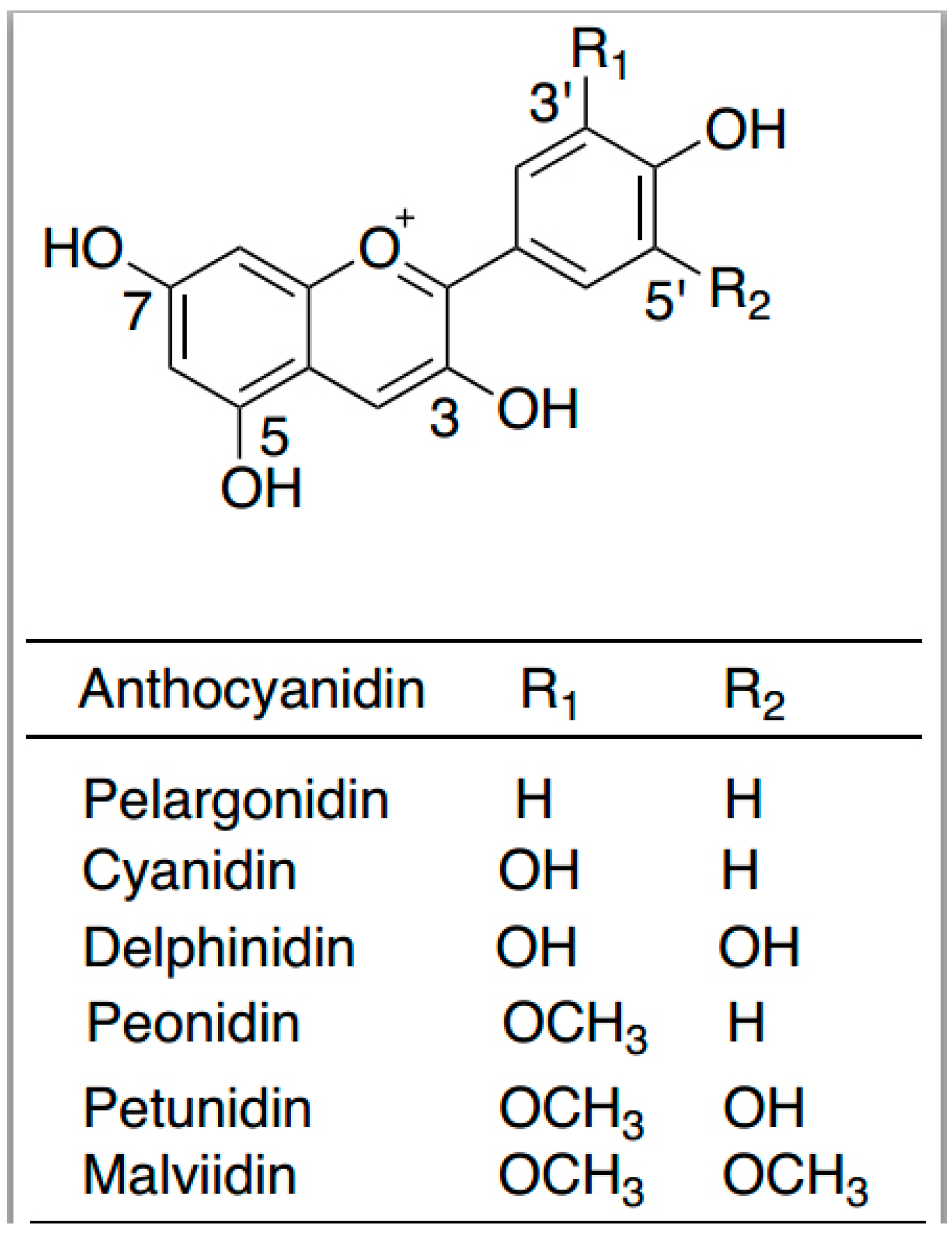
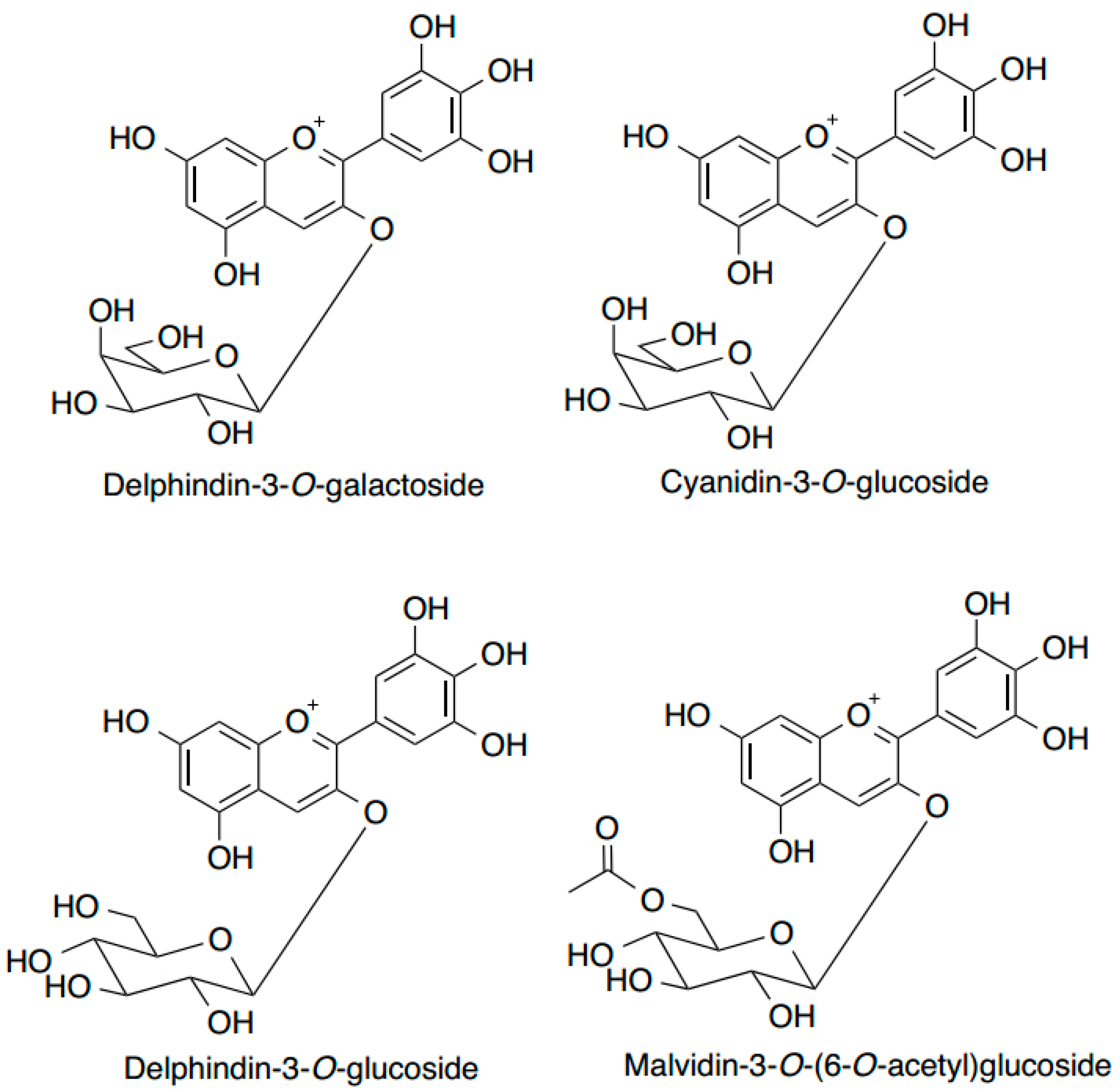
6. Anthocyanins
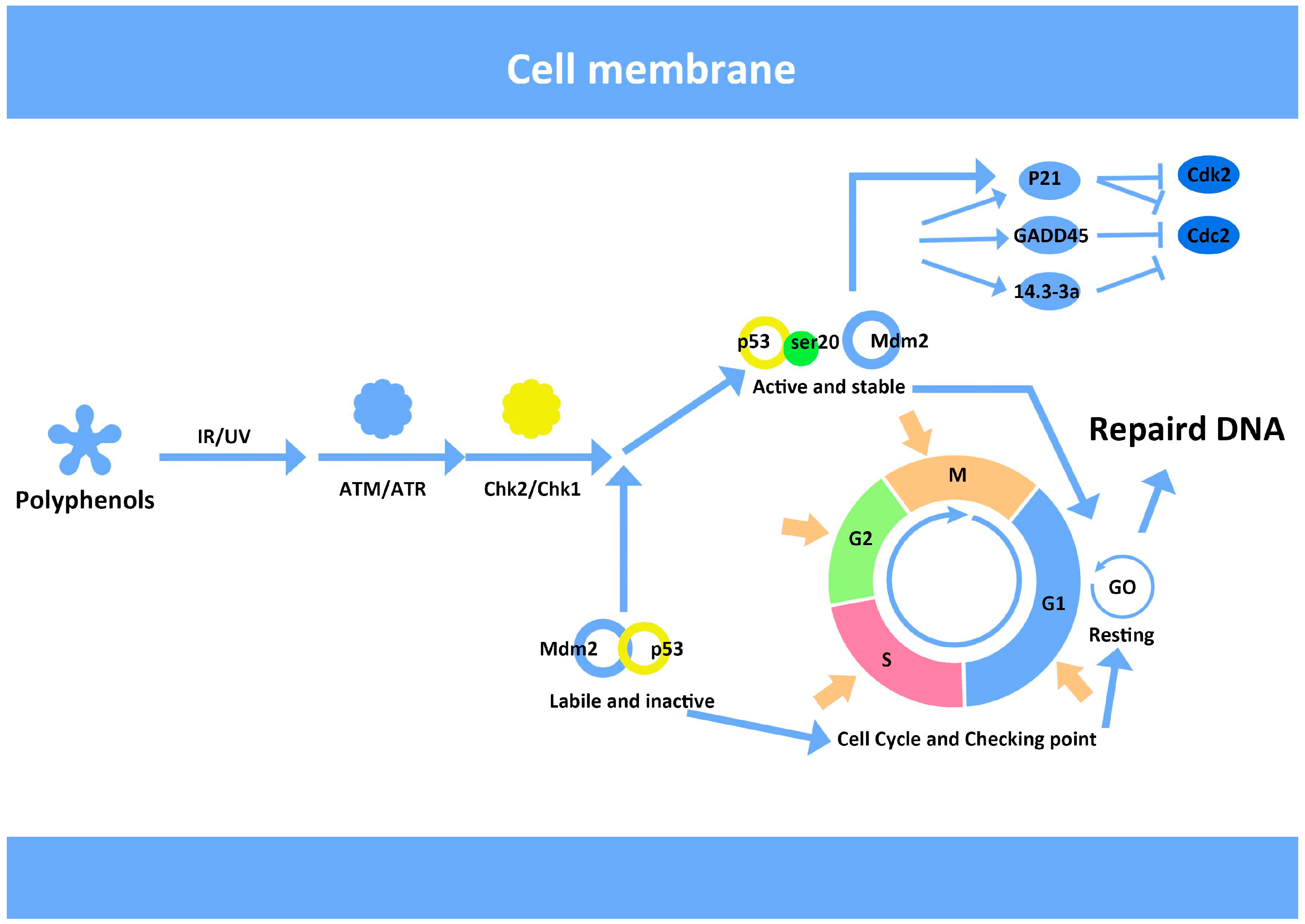
7. Anthocyanins and Modulation of DNA Repair
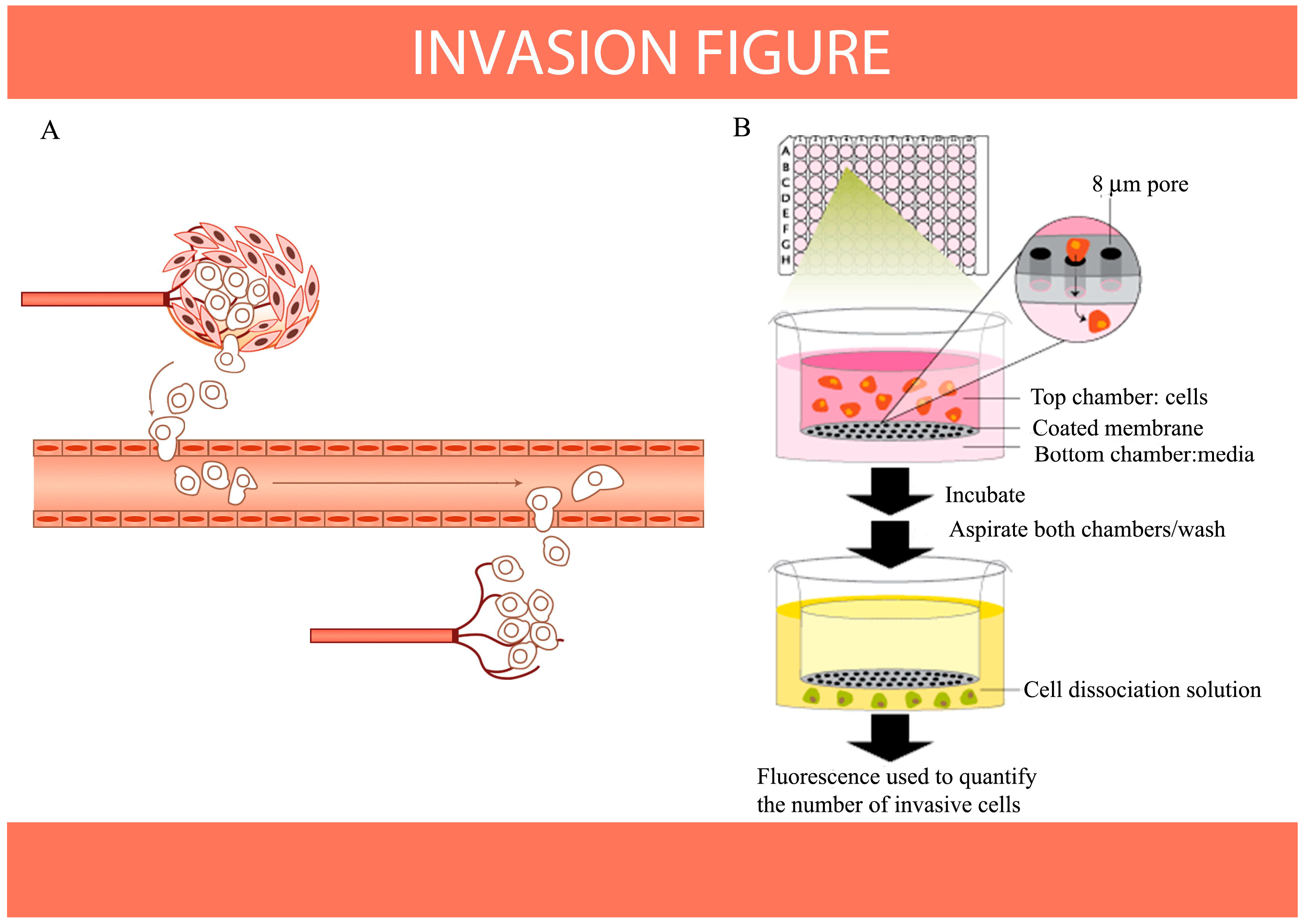
8. Antioxidants Inhibit Migration and Invasion of Cancer Cells as Indicated by HIF-1 and S100A4 Expression in Vitro
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, C.; Urgard, E.; Vooder, T.; Metspalu, A. The role of COX-2 and nrf2/are in anti-inflammation and antioxidative stress: Aging and anti-aging. Med. Hypotheses 2011, 77, 174–178. [Google Scholar]
- Jideani, I.A.; Jideani, V.A. Developments on the cereal grains Digitaria exilis (acha) and Digitaria iburua (iburu). J. Food Sci. Technol. 2011, 48, 251–259. [Google Scholar] [CrossRef]
- Hsia, S.M.; Chiang, W.; Kuo, Y.H.; Wang, P.S. Downregulation of progesterone biosynthesis in rat granulosa cells by adlay (Coix lachryma-jobi L. var. Ma-yuen Stapf.) bran extracts. Int. J. Impot. Res. 2006, 18, 264–274. [Google Scholar]
- Manosroi, J.; Khositsuntiwong, N.; Manosroi, A. Biological activities of fructooligosaccharide (fos)-containing Coix lachryma-jobi Linn. Extract. J. Food Sci. Technol. 2014, 51, 341–346. [Google Scholar] [CrossRef]
- Apirattananusorn, S.; Tongta, S.; Cui, S.W.; Wang, Q. Chemical, molecular, and structural characterization of alkali extractable nonstarch polysaccharides from Job’s tears. J. Agric. Food Chem. 2008, 56, 8459–8557. [Google Scholar] [CrossRef]
- Akhtar, M.; Cheng, Y.; Magno, R.M.; Ashktorab, H.; Smoot, D.T.; Meltzer, S.J.; Wilson, K.T. Promoter methylation regulates helicobacter pylori-stimulated cyclooxygenase-2 expression in gastric epithelial cells. Cancer Res. 2001, 61, 2399–2403. [Google Scholar]
- Hung, W.C.; Chang, H.C. Methanolic extract of adlay seed suppresses COX-2 expression of human lung cancer cells via inhibition of gene transcription. J. Agric. Food Chem. 2003, 51, 7333–7337. [Google Scholar] [CrossRef]
- Yu, F.; Gao, J.; Zeng, Y.; Liu, C.X. Inhibition of Coix seed extract on fatty acid synthase, a novel target for anticancer activity. J. Ethnopharmacol. 2008, 119, 252–258. [Google Scholar] [CrossRef]
- Shih, C.K.; Chiang, W.; Kuo, M.L. Effects of adlay on azoxymethane-induced colon carcinogenesis in rats. Food Chem. Toxicol. 2004, 42, 1339–1347. [Google Scholar] [CrossRef]
- Takahashi, M.; Konno, C.; Hikino, H. Isolation and hypoglycemic activity of coixan-a, coixan-b and coixan-c, glycans of Coix lachryma-jobi var. ma-yuen seeds. Planta Med. 1986, 52, 64–65. [Google Scholar]
- Ooi, V.E.C.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef]
- Lu, X.Y.; Liu, W.; Wu, J.H.; Li, M.X.; Wang, J.C.; Wu, J.H.; Luo, C. A polysaccharide fraction of adlay seed (Coix lachryma-jobi L.) induces apoptosis in human non-small cell lung cancer a549 cells. Biochem. Biophys. Res. Commun. 2013, 430, 846–851. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Fairbairn, D.W.; Olive, P.L.; O’Neill, K.L. The comet assay: A comprehensive review. Mutat. Res. 1995, 339, 37–59. [Google Scholar] [CrossRef]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- McNeish, I.A.; Bell, S.; McKay, T.; Tenev, T.; Marani, M.; Lemoine, N.R. Expression of smac/diablo in ovarian carcinoma cells induces apoptosis via a caspase-9-mediated pathway. Exp. Cell Res. 2003, 286, 186–198. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Knoblach, S.M.; Fan, L.; Fox, G.B.; Goodnight, R.; Faden, A.I. Activation of cpp32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 1997, 17, 7415–7424. [Google Scholar]
- Wu, J.; Zhou, J.; Lang, Y.; Yao, L.; Xu, H.; Shi, H.; Xu, S. A polysaccharide from Armillaria mellea exhibits strong in vitro anticancer activity via apoptosis-involved mechanisms. Int. J. Biol. Macromol. 2012, 51, 663–667. [Google Scholar] [CrossRef]
- Nakajima, H.; Hirota, T.; Toba, T.; Itoh, T.; Adachi, S. Structure of the extracellular polysaccharide from slime-forming Lactococcus lactis subsp. Cremoris SBT 0495. Carbohydr. Res. 1992, 224, 245–253. [Google Scholar]
- Higashimura, M.; Mulder-Bosman, B.W.; Reich, R.; Iwasaki, T.; Robijn, G.W. Solution properties of viilian, the exopolysaccharide from Lactococcus lactis subsp. Cremoris SBT 0495. Biopolymers 2000, 54, 143–158. [Google Scholar]
- Kitazawa, H.; Yamaguchi, T.; Miura, M.; Saito, T.; Itoh, T. B-Cell mitogen produced by slime-forming, encapsulated Lactococcus lactis ssp. Cremoris isolated from ropy sour milk, viili. J. Dairy Sci. 1993, 76, 1514–1519. [Google Scholar]
- Kekkonen, R.A.; Kajasto, E.; Miettinen, M.; Veckman, V.; Korpela, R.; Julkunen, I. Probiotic Leuconostoc mesenteroides ssp. Cremoris and Streptococcus thermophilus induce IL-12 and IFN-γ production. World J. Gastroenterol. 2008, 14, 1192–1203. [Google Scholar]
- Lee, M.Y.; Lee, J.A.; Seo, C.S.; Ha, H.; Lee, H.; Son, J.K.; Shin, H.K. Anti-inflammatory activity of Angelica dahurica ethanolic extract on raw 264.7 cells via upregulation of heme oxygenase-1. Food Chem. Toxicol. 2011, 49, 1047–1055. [Google Scholar]
- Shin, H.Y.; Shin, C.H.; Shin, T.Y.; Lee, E.J.; Kim, H.M. Effect of Bojungikki-tang on Lipopolysaccharide-Induced Cytokine Production from Peripheral Blood Mononuclear Cells of Chronic Fatigue Syndrome Patients. Immunopharmacol. Immunotoxicol. 2003, 25, 491–501. [Google Scholar] [CrossRef]
- Ohshima, H.; Bartsch, H. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1994, 305, 253–264. [Google Scholar] [CrossRef]
- Kroncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Liu, L.; An, Q.; Zhang, J.; Zhang, J.; Li, M.; Duan, W.; Liu, D.; Li, Z.; et al. Nitric oxide and interleukins are involved in cell proliferation of RAW264.7 macrophages activated by viili exopolysaccharides. Inflammation 2013, 36, 954–961. [Google Scholar]
- Tohno, M.; Kitazawa, H.; Shimosato, T.; Matsumoto, M.; Katoh, S.; Kawai, Y.; Saito, T. A swine toll-like receptor 2-expressing transfectant as a potential primary screening system for immunobiotic microorganisms. FEMS Immunol. Med. Microbiol. 2005, 44, 283–288. [Google Scholar] [CrossRef]
- Tohno, M.; Shimazu, T.; Ueda, W.; Anzawa, D.; Aso, H.; Nishimura, J.; Kawai, Y.; Saito, Y.; Saito, T.; Kitazawa, H. Molecular cloning of porcine rp105/md-1 involved in recognition of extracellular phosphopolysaccharides from Lactococcus lactis ssp. Cremoris. Mol. Immunol. 2007, 44, 2566–2577. [Google Scholar] [CrossRef]
- Lyons, C.R.; Orloff, G.J.; Cunningham, J.M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J. Boil. Chem. 1992, 267, 6370–6374. [Google Scholar]
- Manucha, W.; Oliveros, L.; Carrizo, L.; Seltzer, A.; Valles, P. Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney Int. 2004, 65, 2091–2107. [Google Scholar] [CrossRef]
- Jin, M.; Suh, S.-J.; Yang, J.H.; Lu, Y.; Kim, S.J.; Kwon, S.; Jo, T.H.; Kim, J.W.; Park, Y.I.; Ahn, G.W. Anti-inflammatory activity of bark of Dioscorea batatas DECNE through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-κB and ERK1/2 inactivation. Food Chem. Toxicol. 2010, 48, 3073–3079. [Google Scholar]
- Lau, F.C.; Joseph, J.A.; McDonald, J.E.; Kalt, W. Attenuation of iNOS and COX2 by blueberry polyphenols is mediated through the suppression of NF-κB activation. J. Funct. Foods 2009, 1, 274–283. [Google Scholar] [CrossRef]
- Luo, C.; Kallajoki, M.; Gross, R.; Mulari, M.; Teros, T.; Ylinen, L.; Makinen, M.; Laine, J.; Simell, O. Cellular distribution and contribution of cyclooxygenase (COX)-2 to diabetogenesis in NOD mouse. Cell Tissue Res. 2002, 310, 169–175. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Schmidt, A.; Caron, E.; Hall, A. Lipopolysaccharide-Induced Activation of β2-Integrin Function in Macrophages Requires Irak Kinase Activity, p38 Mitogen- Activated Protein Kinase, and the Rap1 GTPase. Mol. Cell. Biol. 2001, 21, 438–448. [Google Scholar] [CrossRef]
- Begum, R.; Nur-E-Kamal, M.S.A.; Zaman, M.A. The role of Rho GTPases in the regulation of the rearrangement of actin cytoskeleton and cell movement. Exp. Mol. Med. 2004, 36, 358–366. [Google Scholar] [CrossRef]
- Lodato, F.; Mazzella, G.; Festi, D.; Azzaroli, F.; Colecchia, A.; Roda, E. Hepatocellular carcinoma prevention: A worldwide emergence between the opulence of developed countries and the economic constraints of developing nations. World J. Gastroenterol. 2006, 12, 7239–7249. [Google Scholar]
- El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Clin. Liver Dis. 2001, 5, 87–107. [Google Scholar]
- Goodgame, B.; Shaheen, N.J.; Galanko, J.; el-Serag, H.B. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: Publication bias? Am. J. Gastroenterol. 2003, 98, 2535–2542. [Google Scholar]
- Simonetti, R.G.; Camma, C.; Fiorello, F.; Politi, F.; D’Amico, G.; Pagliaro, L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig. Dis. Sci. 1991, 36, 962–972. [Google Scholar]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer a549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Choi, Y.H.; Baek, J.H.; Yoo, M.A.; Chung, H.Y.; Kim, N.D.; Kim, K.W. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int. J. Oncol. 2000, 17, 565–571. [Google Scholar]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar]
- Nishino, H.; Nishino, A.; Takayasu, J.; Hasegawa, T.; Iwashima, A.; Hirabayashi, K.; Iwata, S.; Shibata, S. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988, 48, 5210–5215. [Google Scholar]
- Cha, H.J.; Bae, S.K.; Lee, H.Y.; Lee, O.H.; Sato, H.; Seiki, M.; Park, B.C.; Kim, K.W. Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 (MMP-9) in HT1080 human fibrosarcoma cells. Cancer Res. 1996, 56, 2281–2284. [Google Scholar]
- Saxelin, M.-L.; Nurmiaho-Lassila, E.-L.; Meriläinen, V.T.; Forsén, R.I. Ultrastructure and Host Specificity of Bacteriophages of Streptococcus cremoris, Streptococcus lactis subsp. diacetylactis, and Leuconostoc cremoris from Finnish Fermented Milk “Viili”. Appl. Environ. Microb. 1986, 52, 771–777. [Google Scholar]
- Liu, L.; Wu, J.; Zhang, J.; Li, Z.; Wang, C.; Chen, M.; Wang, Y.; Sun, Y.; Wang, L.; Luo, C. A compatibility assay of ursolic acid and foodborne microbial exopolysaccharides by antioxidant power and anti-proliferative properties in hepatocarcinoma cells. J. Food Agric. Environ. 2012, 10, 111–114. [Google Scholar]
- Kitazawa, H.; Yamaguchi, T.; Itoh, T. B-cell mitogenic activity of slime products produced from slime-forming, encapsulated Lactococcus lactis ssp. cremoris. J. Dairy Sci. 1992, 75, 2946–2951. [Google Scholar] [CrossRef]
- Shao, B.-M.; Xu, W.; Dai, H.; Tu, P.; Li, Z.; Gao, X.-M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004, 320, 1103–1111. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Shariat, S.F.; Kim, J.-H.; Ayala, G.E.; Kho, K.; Wheeler, T.M.; Lerner, S.P. Cyclooxygenase-2 is highly expressed in carcinoma in situ and T1 transitional cell carcinoma of the bladder. J. Urol. 2003, 169, 938–942. [Google Scholar] [CrossRef]
- Leng, J.; Han, C.; Demetris, A.J.; Michalopoulos, G.K.; Wu, T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through AKT activation: Evidence for AKT inhibition in celecoxib-induced apoptosis. Hepatology 2003, 38, 756–768. [Google Scholar] [CrossRef]
- Baek, J.Y.; Hur, W.; Wang, J.S.; Bae, S.H.; Yoon, S.K. Selective COX-2 inhibitor, NS-398, suppresses cellular proliferation in human hepatocellular carcinoma cell lines via cell cycle arrest. World J. Gastroenterol. 2007, 13, 1175–1181. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Li, M.; Zhang, X.; Zhang, J.; Li, Z.; Wang, L.; Wu, J.; Luo, C. Inhibition of hepg2 cell proliferation by ursolic acid and polysaccharides via the downregulation of cyclooxygenase-2. Mol. Med. Rep. 2014, 9, 2505–2511. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Eapen, C.; Madesh, M.; Balasubramanian, K.; Pulimood, A.; Mathan, M.; Ramakrishna, B. Mucosal mitochondrial function and antioxidant defences in patients with gastric carcinoma. Scand. J. Gastroenterol. 1998, 33, 975–981. [Google Scholar] [CrossRef]
- Ozturk, H.S.; Karaayvaz, M.; Kacmaz, M.; Kavutcu, M.; Akgul, H.; Durak, I. Activities of the enzymes participating in purine and free-radical metabolism in cancerous human colorectal tissues. Cancer Biochem. Biophys. 1998, 16, 157–168. [Google Scholar]
- Chen, C. Cox-2’s new role in inflammation. Nat. Chem. Biol. 2010, 6, 401–402. [Google Scholar] [CrossRef]
- Groeger, A.L.; Cipollina, C.; Cole, M.P.; Woodcock, S.R.; Bonacci, G.; Rudolph, T.K.; Rudolph, V.; Freeman, B.A.; Schopfer, F.J. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat. Chem. Biol. 2010, 6, 433–441. [Google Scholar] [CrossRef]
- Elein, K.; Zucker, R. Comparison of cellular and nuclear flow cytometric techniques for diseriminating apoptotic subpopulation. Exp. Cell Res. 1994, 211, 332–331. [Google Scholar] [CrossRef]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar]
- Lebeer, S.; Claes, I.J.; Verhoeven, T.L.; Vanderleyden, J.; de Keersmaecker, S.C. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb. Biotechnol. 2011, 4, 368–374. [Google Scholar] [CrossRef]
- Goodman, M.T.; Wu, A.H.; Tung, K.H.; McDuffie, K.; Kolonel, L.N.; Nomura, A.M.; Terada, K.; Wilkens, L.R.; Murphy, S.; Hankin, J.H. Association of dairy products, lactose, and calcium with the risk of ovarian cancer. Am. J. Epidemiol. 2002, 156, 148–157. [Google Scholar] [CrossRef]
- Satué-Gracia, M.T.; Heinonen, M.; Frankel, E.N. Anthocyanins as antioxidants on human low-density lipoprotein and lecithin-liposome systems. J. Agric. Food Chem. 1997, 45, 3362–3367. [Google Scholar]
- Dangles, O.; Dufour, C. Flavonoid-protein interactions. In Flavonoids: Chemistry, Biochemistey and Applications; Andersen, Ø.M., Markham, K.R., Eds.; 2006; pp. 443–469. [Google Scholar]
- Serafini, M.; Testa, M.F.; Villaño, D.; Pecorari, M.; van Wieren, K.; Azzini, E.; Brambilla, A.; Maiani, G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radic. Biol. Med. 2009, 46, 769–774. [Google Scholar] [CrossRef]
- Hou, D.X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 2003, 3, 149–159. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Andres-Lacueva, C. Polyphenols and health: Current state and progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography-electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Gao, L.; Mazza, G. Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Nyberg, K.A.; Michelson, R.J.; Putnam, C.W.; Weinert, T.A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002, 36, 617–656. [Google Scholar] [CrossRef]
- Kim, S.H.; Hur, Y.J.; Lee, S.J.; Kim, S.J.; Park, C.G.; Oh, Y.K.; Jung, W.W.; Seo, J.B.; Nam, M.H.; Choi, I.; et al. E6 and E7 fusion immunoglobulin from human papilloma virus 16 induces dendritic cell maturation and antigen specific activation of T helper 1 response. Biotechnol. Lett. 2011, 33, 663–671. [Google Scholar] [CrossRef]
- Khosravi, R.; Maya, R.; Gottlieb, T.; Oren, M.; Shiloh, Y.; Shkedy, D. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl. Acad. Sci. USA 1999, 96, 14973–14977. [Google Scholar] [CrossRef]
- Iliakis, G.; Wang, Y.; Guan, J.; Wang, H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 2003, 22, 5834–5847. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Y.; Hamrick, H.E.; Duerksen-Hughes, P.J. ATM, ATR and DNA-PK: Initiators of the cellular genotoxic stress responses. Carcinogenesis 2003, 24, 1571–1580. [Google Scholar] [CrossRef]
- Liu, W.; Lu, X.; He, G.; Gao, X.; Li, M.; Wu, J.; Li, Z.; Wu, J.; Wang, J.; Luo, C. Cytosolic protection against ultraviolet induced DNA damage by blueberry anthocyanins and anthocyanidins in hepatocarcinoma HepG2 cells. Biotechnol. Lett. 2013, 35, 491–498. [Google Scholar] [CrossRef]
- DNA Repair. Available online: http://www.web-books.com/ MoBio/Free/Ch4Hp53.htm (accessed on 5 September 2014).
- Serrano, M.C.; Pagani, R.; Manzano, M.; Comas, J.V.; Portoles, M.T. Mitochondrial membrane potential and reactive oxygen species content of endothelial and smooth muscle cells cultured on poly(epsilon-caprolactone) films. Biomaterials 2006, 27, 4706–4714. [Google Scholar] [CrossRef]
- Long, X.; Goldenthal, M.J.; Marin-Garcia, J. Oxidative stress enhances phosphorylation of p53 in neonatal rat cardiomyocytes. Mol. Cell. Biochem. 2007, 303, 167–174. [Google Scholar] [CrossRef]
- Gadbois, D.M.; Crissman, H.A.; Nastasi, A.; Habbersett, R.; Wang, S.-K.; Chen, D.; Lehnert, B.E. Alterations in the progression of cells through the cell cycle after exposure to alpha particles or gamma rays. Radiat. Res. 1996, 146, 414–424. [Google Scholar] [CrossRef]
- Bae, I.; Fan, S.; Bhatia, K.; Kohn, K.W.; Fornace, A.J.; O’Connor, P.M. Relationships between G1 arrest and stability of the p53 and p21Cip1/Waf1 proteins following γ-irradiation of human lymphoma cells. Cancer Res. 1995, 55, 2387–2393. [Google Scholar]
- Chen, C.-Y.; Oliner, J.D.; Zhan, Q.; Fornace, A.J.; Vogelstein, B.; Kastan, M.B. Interactions between p53 and mdm2 in a mammalian cell cycle checkpoint pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 2684–2688. [Google Scholar] [CrossRef]
- Cory, G. Scratch-wound assay. Methods Mol. Biol. 2011, 769, 25–30. [Google Scholar]
- Dai, J.; Fishback, J.A.; Zhou, Y.-D.; Nagle, D.G. Sodwanone and yardenone triterpenes from a south african species of the marine sponge Axinella inhibit hypoxia-inducible factor-1 (HIF-1) activation in both breast and prostate tumor cells. J. Nat. Prod. 2006, 69, 1715–1720. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Han, S.; Wang, D.; Mo, S.; Yu, L.; Huang, H.; Tsui, K.; Shen, J.; Chen, J. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One 2013, 8, e68566. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef]
- b>Li, M.L.; Zhang, J.K.; Li, Z.J.; Zhang, X.Q.; Luo, C. Influences of S100A4 gene expression, and migration and invasion of A549 Lung Cancer Cells by Coix Polysaccharides in vitro. Priv. Commun. 2014. submitted. [Google Scholar]
- Liu, F.-T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, L.; Gao, S.; Jiang, W.; Luo, C.; Xu, M.; Bohlin, L.; Rosendahl, M.; Huang, W. Antioxidative Dietary Compounds Modulate Gene Expression Associated with Apoptosis, DNA Repair, Inhibition of Cell Proliferation and Migration. Int. J. Mol. Sci. 2014, 15, 16226-16245. https://doi.org/10.3390/ijms150916226
Wang L, Gao S, Jiang W, Luo C, Xu M, Bohlin L, Rosendahl M, Huang W. Antioxidative Dietary Compounds Modulate Gene Expression Associated with Apoptosis, DNA Repair, Inhibition of Cell Proliferation and Migration. International Journal of Molecular Sciences. 2014; 15(9):16226-16245. https://doi.org/10.3390/ijms150916226
Chicago/Turabian StyleWang, Likui, Shijuan Gao, Wei Jiang, Cheng Luo, Maonian Xu, Lars Bohlin, Markus Rosendahl, and Wenlin Huang. 2014. "Antioxidative Dietary Compounds Modulate Gene Expression Associated with Apoptosis, DNA Repair, Inhibition of Cell Proliferation and Migration" International Journal of Molecular Sciences 15, no. 9: 16226-16245. https://doi.org/10.3390/ijms150916226
APA StyleWang, L., Gao, S., Jiang, W., Luo, C., Xu, M., Bohlin, L., Rosendahl, M., & Huang, W. (2014). Antioxidative Dietary Compounds Modulate Gene Expression Associated with Apoptosis, DNA Repair, Inhibition of Cell Proliferation and Migration. International Journal of Molecular Sciences, 15(9), 16226-16245. https://doi.org/10.3390/ijms150916226



