Self-Assembled Polymeric Micelles Based on Hyaluronic Acid-g-Poly(d,l-lactide-co-glycolide) Copolymer for Tumor Targeting
Abstract
:1. Introduction
2. Results and Discussion
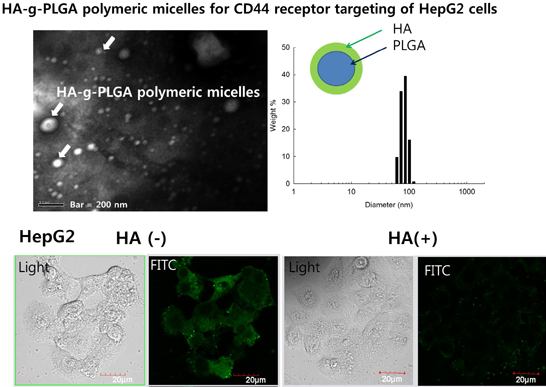
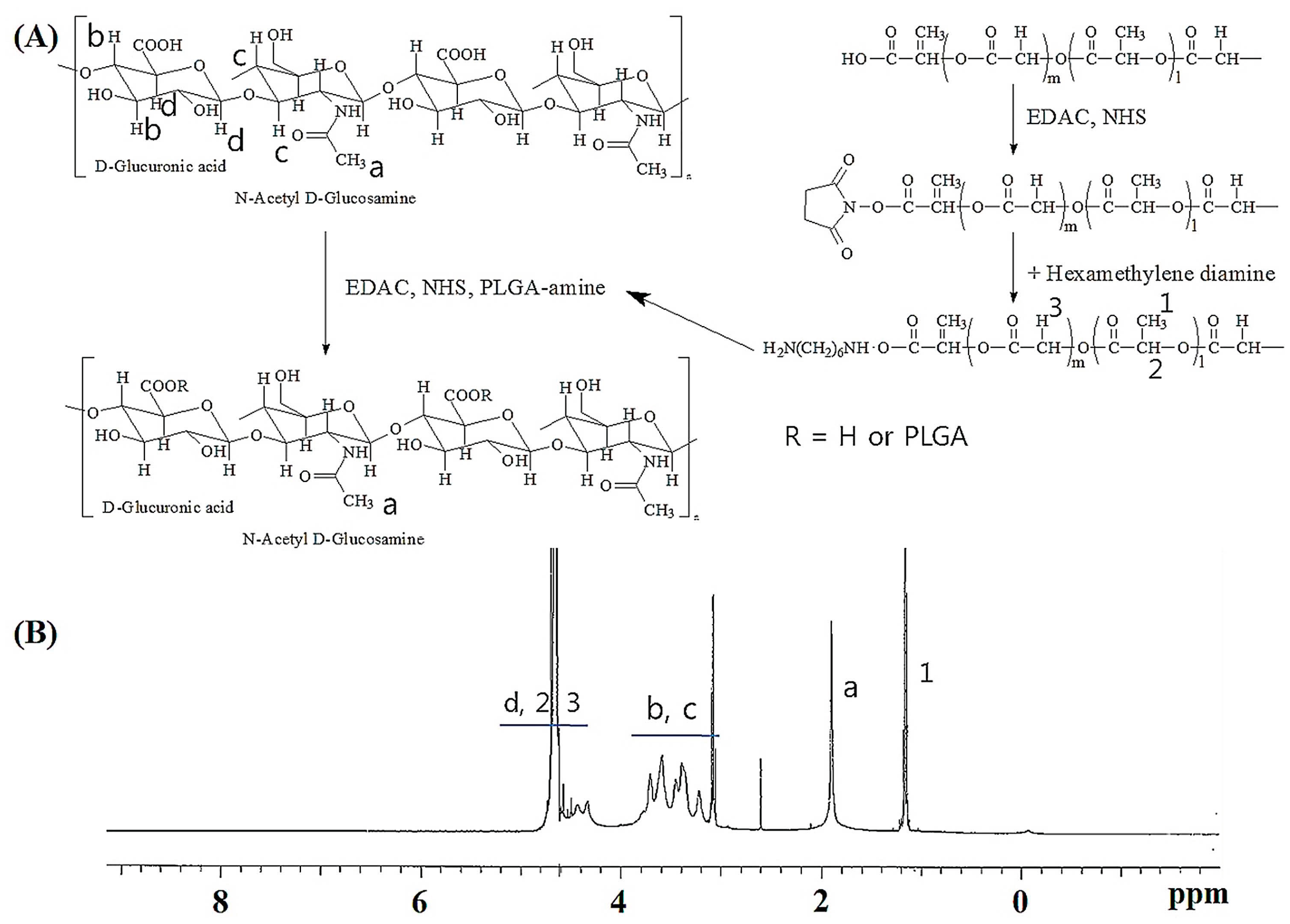
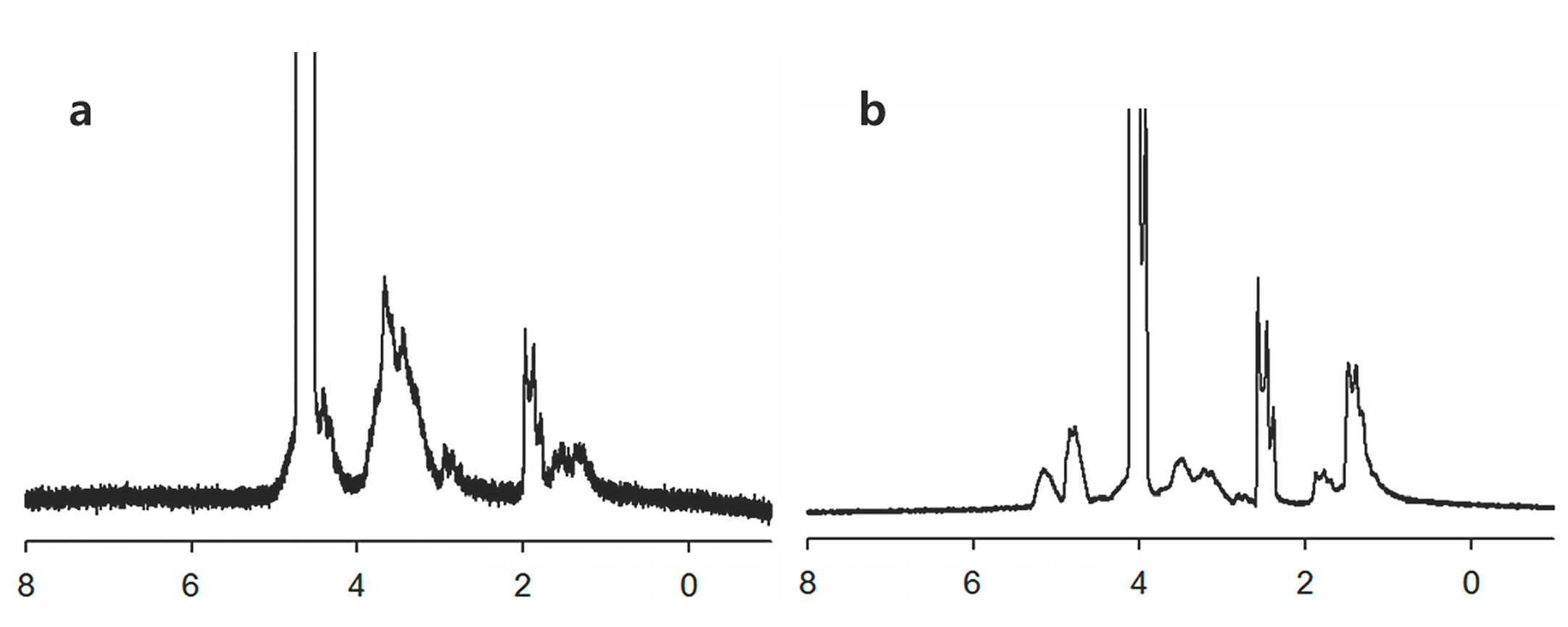
2.1. Characterization Hyaluronic Acid (HA) and Poly(d,l-lactide-co-glycolide) (PLGA) (HAgLG) Copolymer and HAgLG Polymeric Micelles
| HAgLG Copolymer | DS of PLGA * | Particle Size (nm) |
|---|---|---|
| HAgLG-1 | 4.9 | 49.5 ± 10.8 |
| HAgLG-2 | 9.4 | 81.3 ± 18.1 |
| HAgLG-3 | 18.6 | 102.6 ± 23.6 |

| HAgLG Copolymer | Drug/Polymer Weight (mg/mg) | Drug Contents (%, w/w) | Particle Size (nm) | |||
|---|---|---|---|---|---|---|
| Theoretical | Experimental | Before Lyophilization | After Lyophilization | Statistical Analysis * | ||
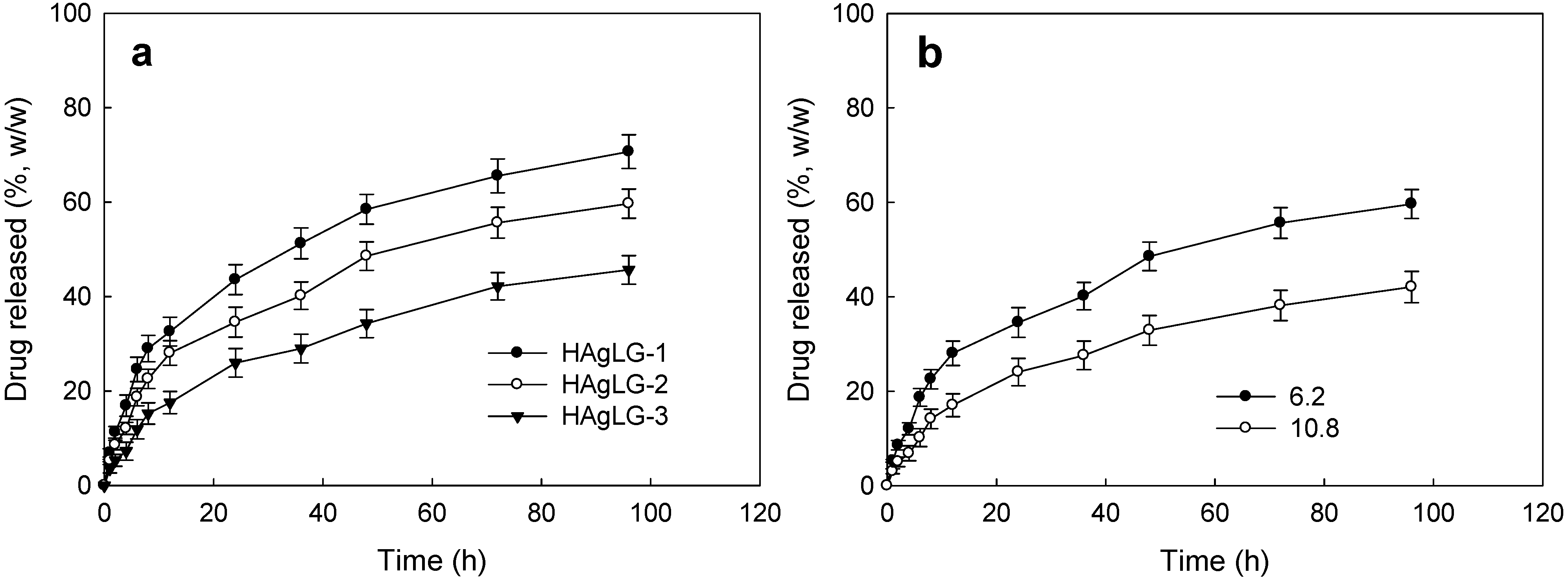
| HAgLG-1 | 5/40 | 11.1 | 5.4 | 89.6 ± 19.6 | 93.0 ± 21.0 | >0.001 |
| HAgLG-2 | 5/40 | 11.1 | 6.2 | 118.4 ± 23.1 | 124.3 ± 28.2 | >0.01 |
| 10/40 | 20 | 10.8 | 153.8 ± 31.2 | 170.6 ± 34.6 | >0.01 | |
| HAgLG-3 | 5/40 | 11.1 | 8.3 | 180.9 ± 36.8 | 193.8 ± 40.8 | >0.05 |
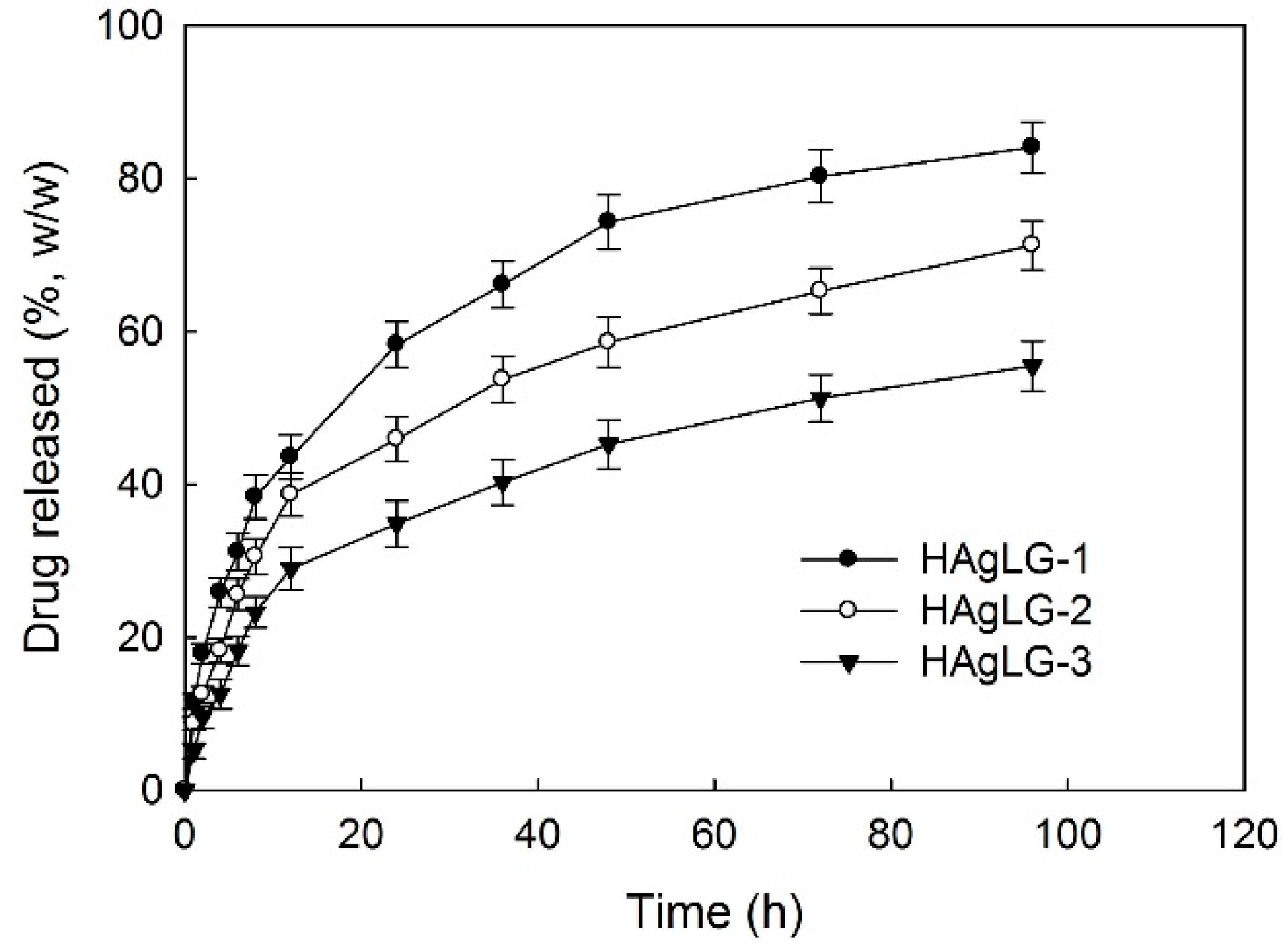
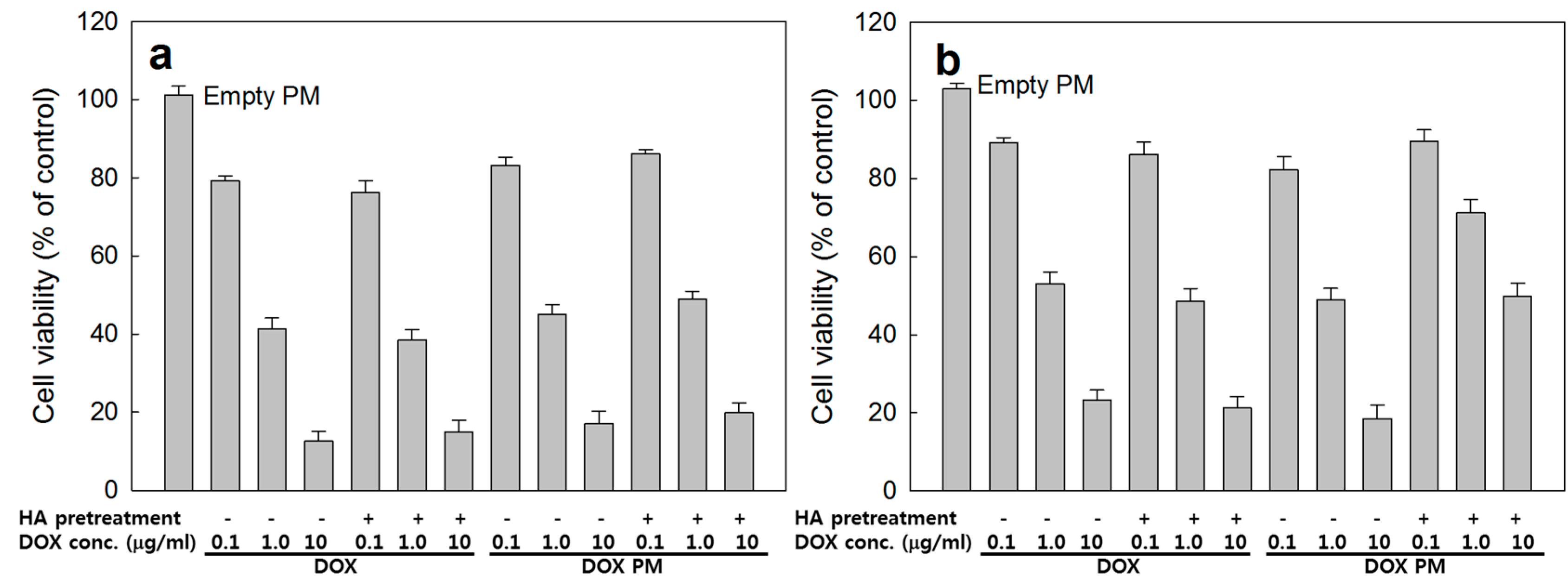
2.2. Receptor-Mediated Targeting of HAgLG Polymeric Micelles


3. Experimental Section
3.1. Materials
3.2. Synthesis of HAgLG Copolymer
3.3. Preparation of Polymeric Micelles
3.4. Analysis of HAgLG Polymeric Micelles
3.5. Fluorescein Isothiocyanate (FITC)-Labelling of HAgLG
3.6. Western Blot Analysis
3.7. Receptor-Mediated Endocytosis of Polymeric Micelles into HepG2 Cells
3.8. Cancer Cell Cytotoxicity
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, D.H.; Kim, M.D.; Choi, C.W.; Chung, C.W.; Ha, S.H.; Kim, C.H.; Shim, Y.H.; Jeong, Y.I.; Kang, D.H. Antitumor activity of sorafenib-incorporated nanoparticles of dextran/poly(d,l-lactide-co-glycolide) block copolymer. Nanoscale Res. Lett. 2012, 7, 91. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, G.Y.; Jeong, Y.I.; Kang, M.S.; Oh, J.S.; Song, C.E.; Lee, H.C. Paclitaxel-incorporated nanoparticles of hydrophobized polysaccharide and their antitumor activity. Int. J. Pharm. 2012, 433, 121–128. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Kim, D.H.; Chung, C.W.; Yoo, J.J.; Choi, K.H.; Kim, C.H.; Ha, S.H.; Kang, D.H. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly(d,l-lactide-co-glycolide) copolymer. Int. J. Nanomed. 2011, 6, 1415–1427. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Kim, S.T.; Jin, S.G.; Ryu, H.H.; Jin, Y.H.; Jung, T.Y.; Kim, I.Y.; Jung, S. Cisplatin-incorporated hyaluronic acid nanoparticles based on ion-complex formation. J. Pharm. Sci. 2008, 97, 1268–1276. [Google Scholar] [CrossRef]
- Luo, Y.; Prestwich, G.D. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug. Chem. 1999, 10, 755–763. [Google Scholar] [CrossRef]
- Entwistle, J.; Hall, C.L.; Turley, E.A. Hyaluronan receptors: Regulators of signaling to the cytoskeleton. J. Cell. Biochem. 1996, 61, 569–577. [Google Scholar] [CrossRef]
- Knudson, C.B.; Knudson, W. Hyaluronan binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993, 7, 1233–1241. [Google Scholar]
- Rooney, P.; Kumar, S.; Ponting, J.; Wang, M. The role of hyaluronan in tumor neovascularization. Int. J. Cancer 1995, 60, 632–636. [Google Scholar] [CrossRef]
- Ladeda, V.; Aguirre Ghiso, J.A.; Bal de Kier Joffé, E. Function and expression of CD44 during spreading, migration, and invasion of murine carcinoma cells. Exp. Cell Res. 1998, 242, 515–527. [Google Scholar] [CrossRef]
- Orian-Rousseau, V. CD44, a therapeutic target for metastasising tumours. Eur. J. Cancer 2010, 46, 1271–1277. [Google Scholar]
- Luo, Y.; Bernshaw, N.J.; Lu, Z.R.; Kopecek, J.; Prestwich, G.D. Targeted delivery of doxorubicin by HPMA copolymer–hyaluronan bioconjugates. Pharm. Res. 2002, 19, 396–402. [Google Scholar] [CrossRef]
- Auzenne, E.; Ghosh, S.C.; Khodadadian, M.; Rivera, B.; Farquhar, D.; Price, R.E.; Ravoori, M.; Kundra, V.; Freedman, R.S.; Klostergaard, J. Hyaluronic acid-paclitaxel: Antitumor efficacy against CD44+ human ovarian carcinoma xenografts. Neoplasia 2007, 9, 479–486. [Google Scholar] [CrossRef]
- Ossipov, D.A. Nanostructured hyaluronic acid-based materials for active delivery to cancer. Exp. Opin. Drug Deliv. 2010, 7, 681–703. [Google Scholar] [CrossRef]
- Hyung, W.; Ko, H.; Park, J.; Lim, E.; Park, S.B.; Park, Y.J.; Yoon, H.G.; Suh, J.S.; Haam, S.; Huh, Y.M. Novel hyaluronic acid (HA) coated drug carriers (HCDCs) for human breast cancer treatment. Biotechnol. Bioeng. 2008, 99, 442–454. [Google Scholar] [CrossRef]
- Rivkin, I.; Cohen, K.; Koffler, J.; Melikhov, D.; Peer, D.; Margalit, R. Paclitaxel-clusters coated with hyaluronan as selective tumor-targeted nanovectors. Biomaterials 2010, 31, 7106–7114. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Son, G.M.; Kim, H.Y.; Ryu, J.H.; Chu, C.W.; Kang, D.H.; Park, S.B.; Jeong, Y.-I. Self-Assembled Polymeric Micelles Based on Hyaluronic Acid-g-Poly(d,l-lactide-co-glycolide) Copolymer for Tumor Targeting. Int. J. Mol. Sci. 2014, 15, 16057-16068. https://doi.org/10.3390/ijms150916057
Son GM, Kim HY, Ryu JH, Chu CW, Kang DH, Park SB, Jeong Y-I. Self-Assembled Polymeric Micelles Based on Hyaluronic Acid-g-Poly(d,l-lactide-co-glycolide) Copolymer for Tumor Targeting. International Journal of Molecular Sciences. 2014; 15(9):16057-16068. https://doi.org/10.3390/ijms150916057
Chicago/Turabian StyleSon, Gyung Mo, Hyun Yul Kim, Je Ho Ryu, Chong Woo Chu, Dae Hwan Kang, Su Bum Park, and Young-IL Jeong. 2014. "Self-Assembled Polymeric Micelles Based on Hyaluronic Acid-g-Poly(d,l-lactide-co-glycolide) Copolymer for Tumor Targeting" International Journal of Molecular Sciences 15, no. 9: 16057-16068. https://doi.org/10.3390/ijms150916057
APA StyleSon, G. M., Kim, H. Y., Ryu, J. H., Chu, C. W., Kang, D. H., Park, S. B., & Jeong, Y.-I. (2014). Self-Assembled Polymeric Micelles Based on Hyaluronic Acid-g-Poly(d,l-lactide-co-glycolide) Copolymer for Tumor Targeting. International Journal of Molecular Sciences, 15(9), 16057-16068. https://doi.org/10.3390/ijms150916057