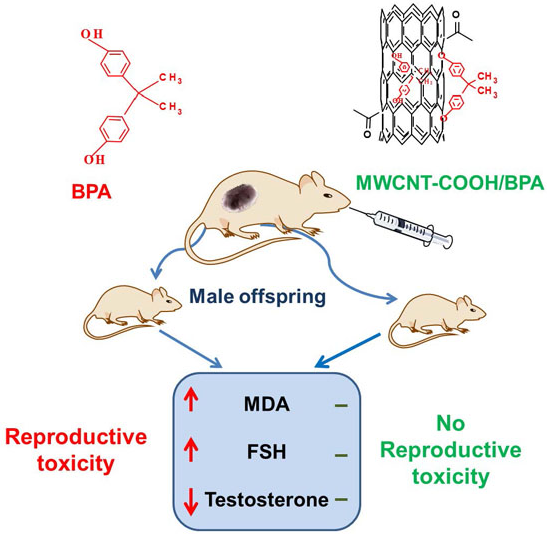
Adsorption of Bisphenol A to a Carbon Nanotube Reduced Its Endocrine Disrupting Effect in Mice Male Offspring
Abstract
:1. Introduction
2. Results
2.1. Properties of MWCNT–COOH and BPA/MWCNT–COOH Adduct
| TEM | MWCNT–COOH | BPA/MWCNT–COOH |
|---|---|---|
 |  | |
| Diameter (nm) inner/outer | ~20–30 | ~20–30 |
| Length distribution (μm) | ~0.5–2.0 | ~0.5–2.0 |
| Zeta potential (mV) in water | −37 ± 1.0 | −33.6 ± 0.46 |
| Adsorption (BPA μg/mg MWCNT–COOH) | 37 ± 7.5 | |
| Desorption/BPA after 24 h | 1% |
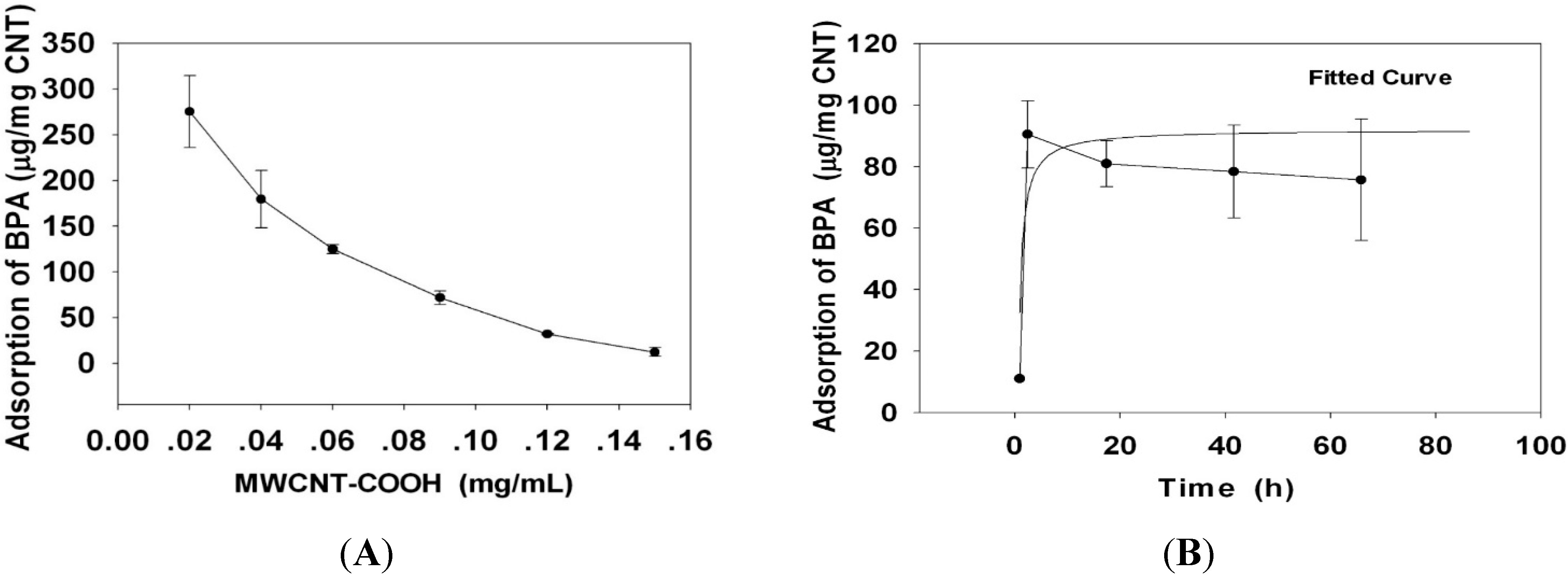
2.2. The Adsorption and Desorption of BPA to MWCNT–COOH
2.3. BPA/MWCNT–COOH Adduct Did not Affect the Maternal Reproduction during Pregnancy
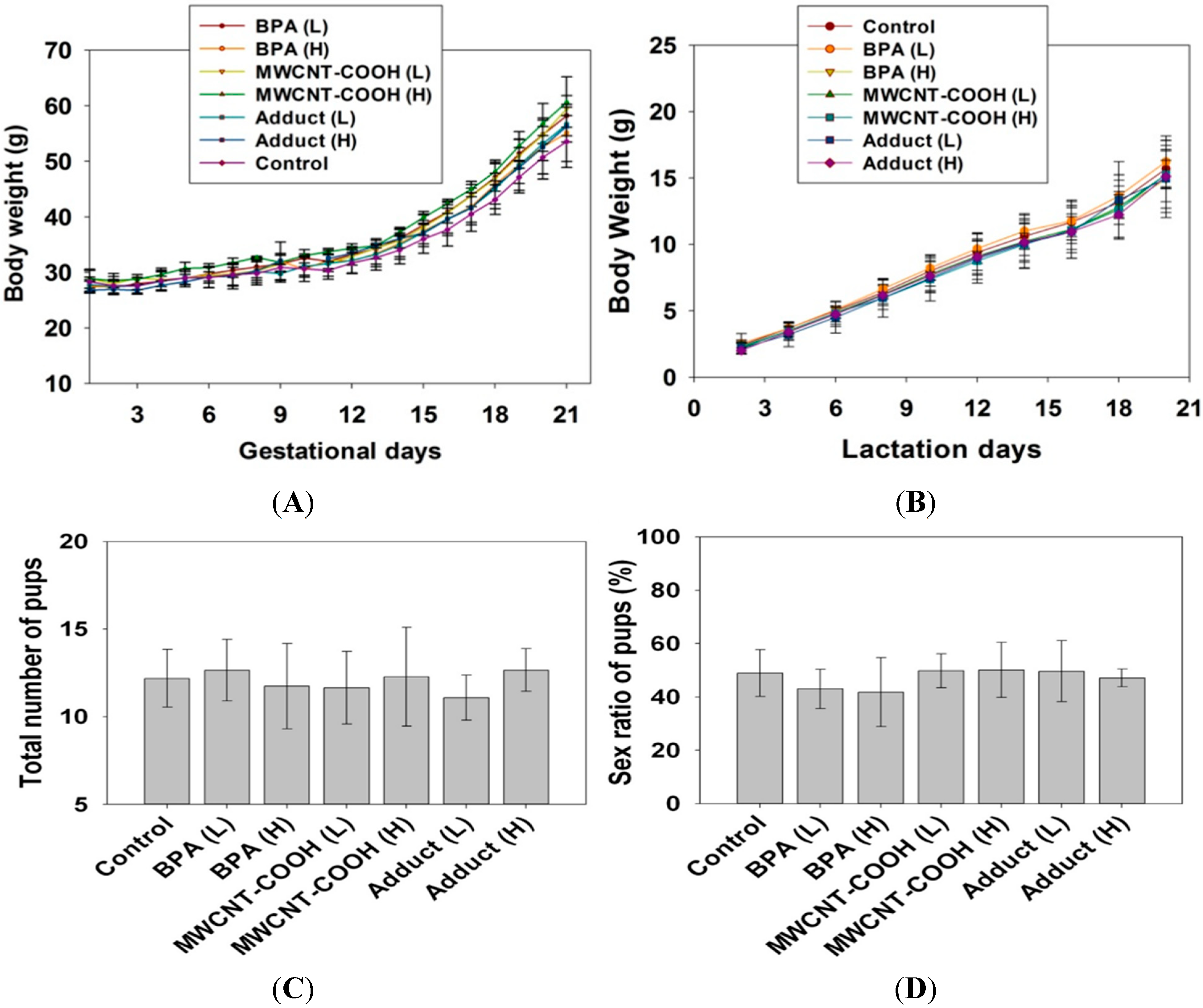
2.4. BPA/MWCNT–COOH Adduct Did not Affect the Body Weight and Organ Index of Male Offspring

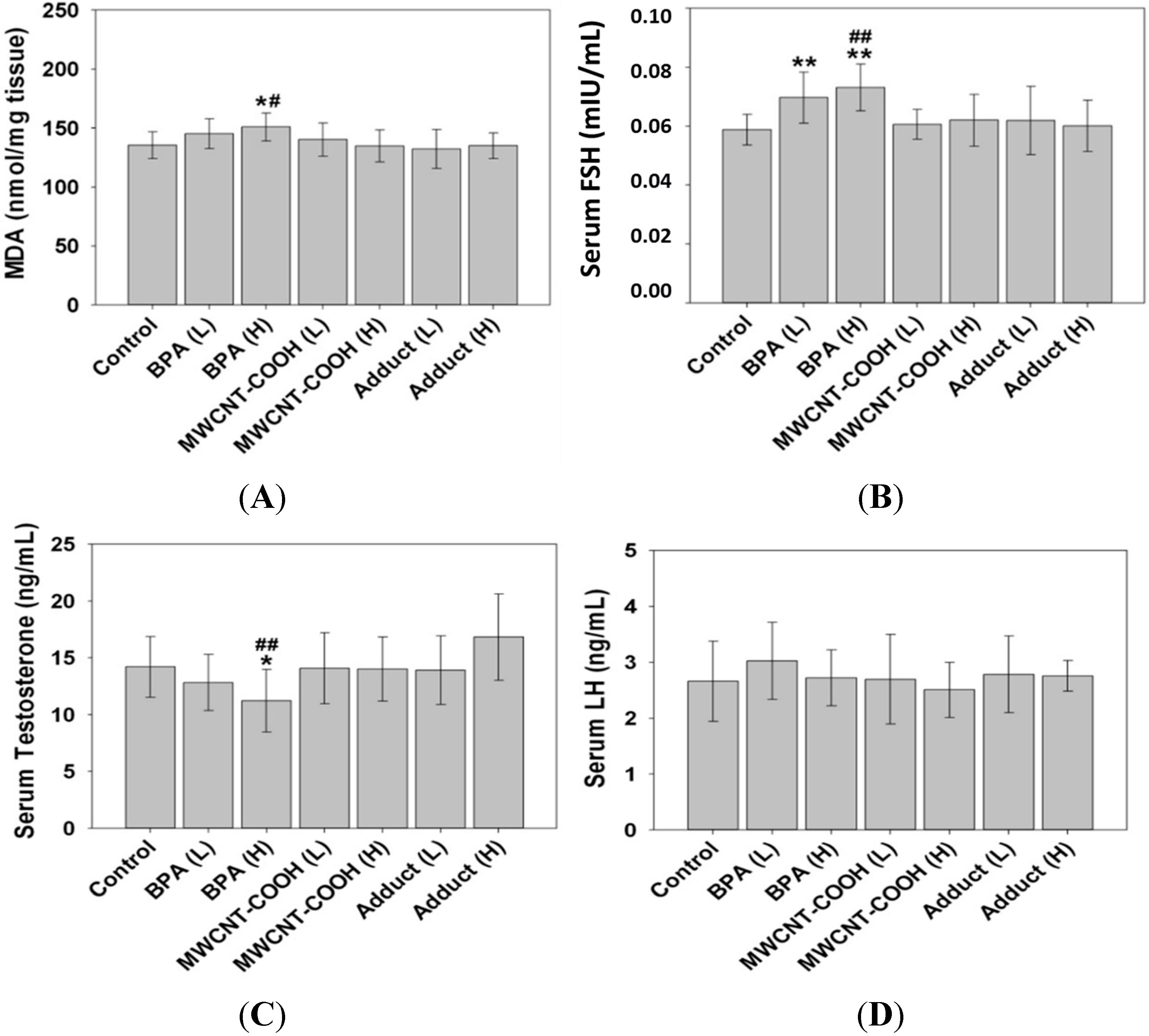
2.5. The BPA/MWCNT–COOH Adduct Reduced the Effect of BPA on the Male Offspring’s Reproduction System
3. Discussion
4. Experimental Section
4.1. Reagents
4.1.1. Preparation of MWCNT–COOH
4.1.2. Preparation of BPA/MWCNT–COOH Adduct
4.2. Quantification of BPA Adsorption on MWCNT–COOH

4.3. Desorption of BPA from BPA/MWCNT Adduct in Oral Exposure Solution

4.4. Preparation of the Oral Exposure Solutions for MWCNT and BPA
4.5. Animal Administration and Sampling

| Dose | Control | BPA | MWCNT–COOH | Adduct (BPA/MWCNT–COOH) |
|---|---|---|---|---|
| Low dose | Sterile water | 0.8 mg/kg/day | 22 mg/kg/day | 0.8 mg/22 mg/kg/day |
| High dose | Sterile water | 2.4 mg/kg/day | 65 mg/kg/day | 2.4 mg/65 mg/kg/day |
4.6. Reproductive Toxicity of Male Mice
4.7. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Niyogi, S.; Hamon, M.; Hu, H.; Zhao, B.; Bhowmik, P.; Sen, R.; Itkis, M.; Haddon, R. Chemistry of single-walled carbon nanotubes. Acc. Chem. Res. 2002, 35, 1105–1113. [Google Scholar]
- Petersen, E.J.; Pinto, R.A.; Landrum, P.F.; Weber, W.J., Jr. Influence of carbon nanotubes on pyrene bioaccumulation from contaminated soils by earthworms. Environ. Sci. Technol. 2009, 43, 4181–4187. [Google Scholar]
- Peng, X.; Li, Y.; Luan, Z.; Di, Z.; Wang, H.; Tian, B.; Jia, Z. Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. [Google Scholar]
- Yang, K.; Zhu, L.; Xing, B. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. [Google Scholar]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar]
- Li, Y.H.; Wang, S.; Luan, Z.; Ding, J.; Xu, C.; Wu, D. Adsorption of cadmium (II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 2003, 41, 1057–1062. [Google Scholar]
- Wang, X.; Chen, C.; Hu, W.; Ding, A.; Xu, D.; Zhou, X. Sorption of 243Am (III) to multiwall carbon nanotubes. Environ. Sci. Technol. 2005, 39, 2856–2860. [Google Scholar]
- Li, Y.H.; Wang, S.; Cao, A.; Zhao, D.; Zhang, X.; Xu, C.; Luan, Z.; Ruan, D.; Liang, J.; Wu, D. Adsorption of fluoride from water by amorphous alumina supported on carbon nanotubes. Chem. Phys. Lett. 2001, 350, 412–416. [Google Scholar]
- Lu, C.; Chung, Y.L.; Chang, K.F. Adsorption of trihalomethanes from water with carbon nanotubes. Water Res. 2005, 39, 1183–1189. [Google Scholar]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar]
- Pan, B.; Xing, B.S. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar]
- Cui, X.Y.; Jia, F.; Chen, Y.X.; Gan, J. Influence of single-walled carbon nanotubes on microbial availability of phenanthrene in sediment. Ecotoxicology 2011, 20, 1277–1285. [Google Scholar]
- Xia, X.H.; Li, Y.R.; Zhou, Z.I.; Feng, C.L. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium. Chemosphere 2010, 78, 1329–1336. [Google Scholar] [CrossRef]
- Ferguson, P.L.; Chandler, G.T.; Templeton, R.C.; Demarco, A.; Scrivens, W.A.; Englehart, B.A. Influence of sediment-amendment with single-walled carbon nanotubes and diesel soot on bioaccumulation of hydrophobic organic contaminants by benthic invertebrates. Environ. Sci. Technol. 2008, 42, 3879–3885. [Google Scholar]
- Schwab, F.; Bucheli, T.D.; Camenzuli, L.; Magrez, A.; Knauer, K.; Sigg, L.; Nowack, B. Diuron sorbed to carbon nanotubes exhibits enhanced toxicity to chlorella vulgaris. Environ. Sci. Technol. 2013, 47, 7012–7019. [Google Scholar]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar]
- Taylor, J.A.; vom saal, F.S.; Welshons, W.V.; Drury, B.; Rottinghaus, G.; Hunt, P.A.; Toutain, P.L.; Laffont, C.M.; vandevoort, C.A. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: Relevance for human exposure. Environ. Health Perspect. 2011, 119, 422–430. [Google Scholar]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef]
- Savabieasfahani, M.; Kannan, K.; Astapova, O.; Evans, N.P.; Padmanabhan, V. Developmental programming: Differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology 2006, 147, 5956–5966. [Google Scholar]
- Akingbemi, B.T.; Sottas, C.M.; Koulova, A.I.; Klinefelter, G.R.; Hardy, M.P. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 2004, 145, 592–603. [Google Scholar]
- Vom Saal, F.S.; Cooke, P.S.; Buchanan, D.L.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Parmigiani, S.; Welshons, W.V. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health 1998, 14, 239–260. [Google Scholar] [CrossRef]
- Wistuba, J.; Brinkworth, M.H.; Schlatt, S.; Chahoud, I.; Nieschlag, E. Intrauterine bisphenol A exposure leads to stimulatory effects on Sertoli cell number in rats. Environ. Res. 2003, 91, 95–103. [Google Scholar]
- Pan, B.; Lin, D.; Mashayekhi, H.; Xing, B. Adsorption and hysteresis of bisphenol A and 17α-ethinyl estradiol on carbon nanomaterials. Environ. Sci. Technol. 2008, 42, 5480–5485. [Google Scholar]
- Liu, Z.; Dong, X.; Song, L.; Zhang, H.; Liu, L.; Zhu, D.; Song, C.; Leng, X. Carboxylation of multiwalled carbon nanotube enhanced its biocompatibility with L02 cells through decreased activation of mitochondrial apoptotic pathway. J. Biomed. Mater. Res. A 2014, 102, 665–673. [Google Scholar]
- Lai, X.; Blazer-Yost, B.; Clack, J.; Fears, S.; Mitra, S.; Ntim, S.; Ringham, H.; Witzmann, F. Protein expression profiles of intestinal epithelial co-cultures: Effect of functionalised carbon nanotube exposure. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 1–36. [Google Scholar]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J., Jr. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar]
- Aydogan, M.; Korkmaz, A.; Barlas, N.; Kolankaya, D. Pro-oxidant effect of vitamin C coadministration with bisphenol A, nonylphenol, and octylphenol on the reproductive tract of male rats. Drug Chem. Toxicol. 2010, 33, 193–203. [Google Scholar] [CrossRef]
- Yu, J.-G.; Zhao, X.-H.; Yang, H.; Chen, X.-H.; Yang, Q.; Yu, L.-Y.; Jiang, J.-H.; Chen, X.-Q. Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci. Total Environ. 2014, 482–483, 241–251. [Google Scholar] [CrossRef]
- Hosseini, S.J.; Kokhdan, S.N.; Ghaedi, A.M.; Moosavian, S.S. Comparison of multiwalled carbon nanotubes and activated carbon for efficient removal of methyl orange: Kinetic and thermodynamic investigation. Fresenius Environ. Bull. 2011, 20, 219–234. [Google Scholar]
- Ghaedi, M.; Shokrollahi, A.; Hossainian, H.; Kokhdan, S.N. Comparison of activated carbon and multiwalled carbon nanotubes for efficient removal of eriochrome cyanine R (ECR): Kinetic, isotherm, and thermodynamic study of the removal process. J. Chem. Eng. Data 2011, 56, 3227–3235. [Google Scholar] [CrossRef]
- Baun, A.; Sorensen, S.N.; Rasmussen, R.F.; Hartmann, N.B.; Koch, C.B. Toxicity and bioaccumulation of xenobiotic organic compounds in the presence of aqueous suspensions of aggregates of nano-C-60. Aquat. Toxicol. 2008, 86, 379–387. [Google Scholar]
- Hougaard, K.S.; Jackson, P.; Kyjovska, Z.O.; Birkedal, R.K.; de Temmerman, P.-J.; Brunelli, A.; Verleysen, E.; Madsen, A.M.; Saber, A.T.; Pojana, G.; et al. Effects of lung exposure to carbon nanotubes on female fertility and pregnancy. A study in mice. Reprod. Toxicol. 2013, 41, 86–97. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.H.; Shin, I.S.; Park, N.H.; Moon, C.; Kang, S.S.; Park, S.C.; Kim, J.C. Maternal exposure to multiwall carbon nanotubes does not induce embryo-fetal developmental toxicity in rats. Birth Defects Res. B Dev. Repord. Toxicol. 2011, 92, 69–76. [Google Scholar]
- Shin, B.S.; Yoo, S.D.; Cho, C.Y.; Jung, J.H.; Lee, B.M.; Kim, J.H.; Lee, K.C.; Han, S.-Y.; Kim, H.S.; Park, K.L. Maternal-fetal disposition of bisphenol a in pregnant Sprague-Dawley rats. J. Toxicol. Environ. Health A 2002, 65, 395–406. [Google Scholar]
- Takahashi, O.; Oishi, S. Disposition of orally administered 2,2-bis (4-hydroxyphenyl) propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ. Health Perspect. 2000, 108, 931–935. [Google Scholar]
- Takai, Y.; Tsutsumi, O.; Ikezuki, Y.; Kamei, Y.; Osuga, Y.; Yano, T.; Taketan, Y. Preimplantation exposure to bisphenol A advances postnatal development. Reprod. Toxicol. 2000, 15, 71–74. [Google Scholar]
- Mu, Q.; Broughton, D.L.; Yan, B. Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: Developing a model for cell uptake. Nano Lett. 2009, 9, 4370–4375. [Google Scholar]
- Systat Software Inc. Available online: http://www.sigmaplot.com/ (accessed on 30 April 2011).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, W.; Jiang, C.; Zhu, L.; Liang, N.; Liu, X.; Jia, J.; Zhang, C.; Zhai, S.; Zhang, B. Adsorption of Bisphenol A to a Carbon Nanotube Reduced Its Endocrine Disrupting Effect in Mice Male Offspring. Int. J. Mol. Sci. 2014, 15, 15981-15993. https://doi.org/10.3390/ijms150915981
Wang W, Jiang C, Zhu L, Liang N, Liu X, Jia J, Zhang C, Zhai S, Zhang B. Adsorption of Bisphenol A to a Carbon Nanotube Reduced Its Endocrine Disrupting Effect in Mice Male Offspring. International Journal of Molecular Sciences. 2014; 15(9):15981-15993. https://doi.org/10.3390/ijms150915981
Chicago/Turabian StyleWang, Wenwei, Cuijuan Jiang, Ledong Zhu, Nana Liang, Xuejiao Liu, Jianbo Jia, Chengke Zhang, Shumei Zhai, and Bin Zhang. 2014. "Adsorption of Bisphenol A to a Carbon Nanotube Reduced Its Endocrine Disrupting Effect in Mice Male Offspring" International Journal of Molecular Sciences 15, no. 9: 15981-15993. https://doi.org/10.3390/ijms150915981