Potential Therapeutic Roles of Tanshinone IIA in Human Bladder Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Tan-IIA Inhibited Cell Proliferation in Human Bladder Cancer Cells

2.2. Tan-IIA Induced Sub-G1 Population Accumulation in Human Bladder Cancer Cells
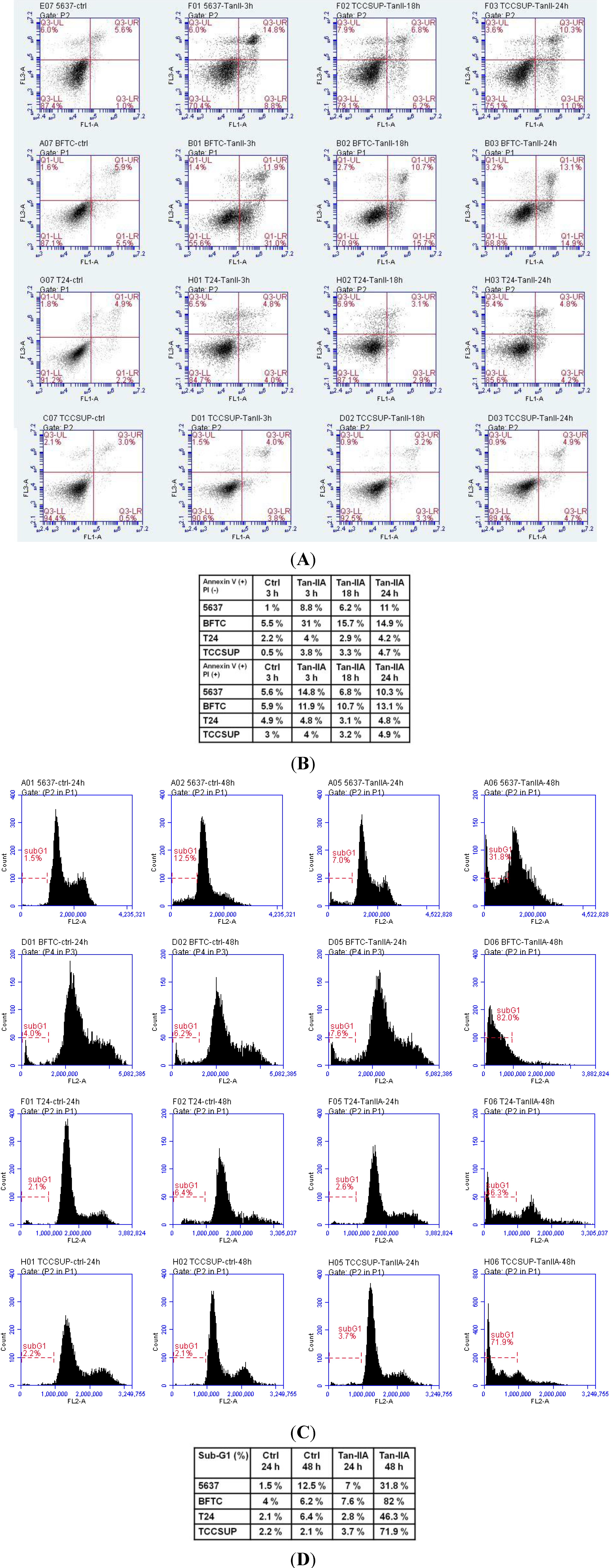
2.3. Tan-IIA Induced Mitochondria Dependent Apoptosis in Human Bladder Cancer Cells

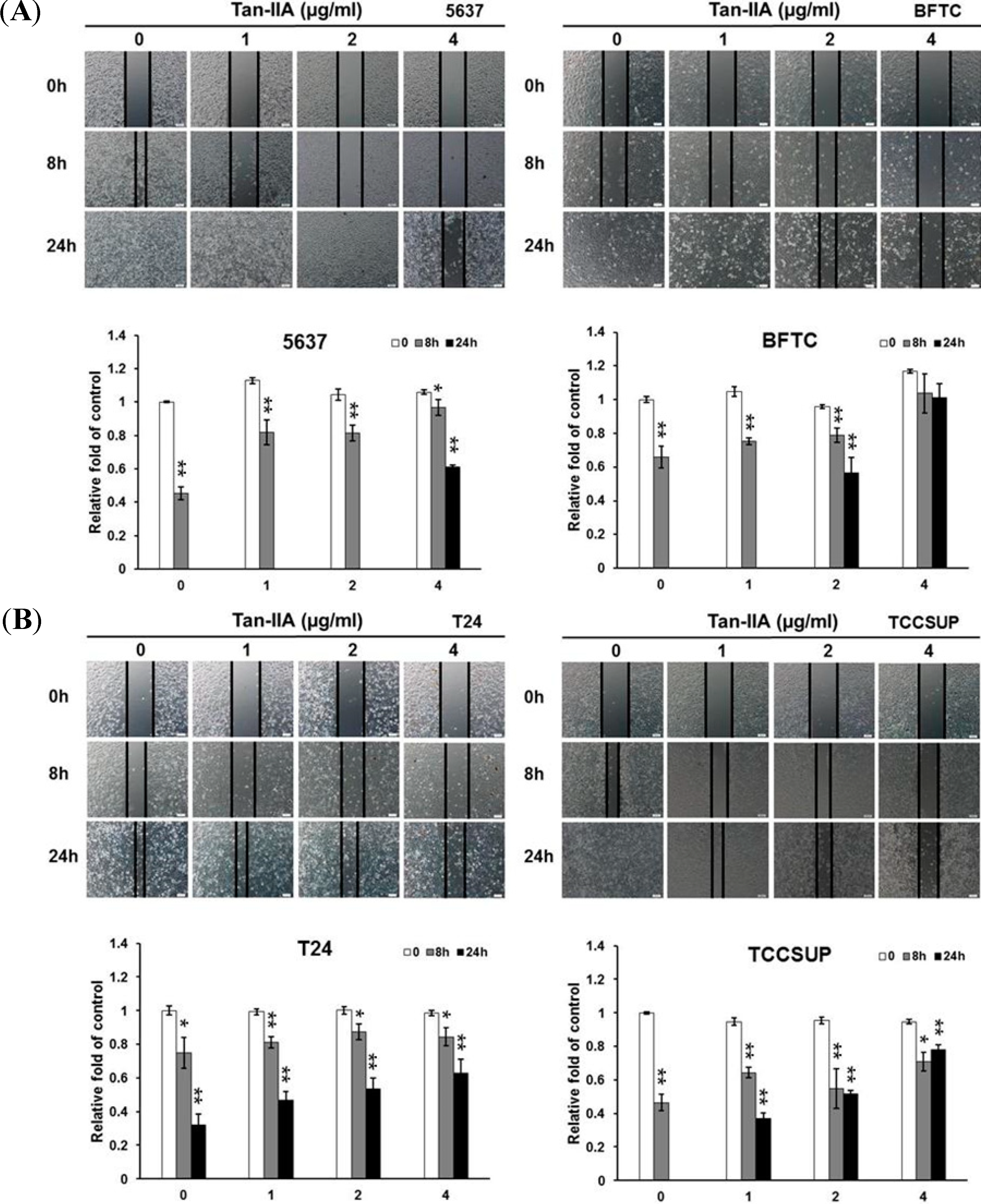
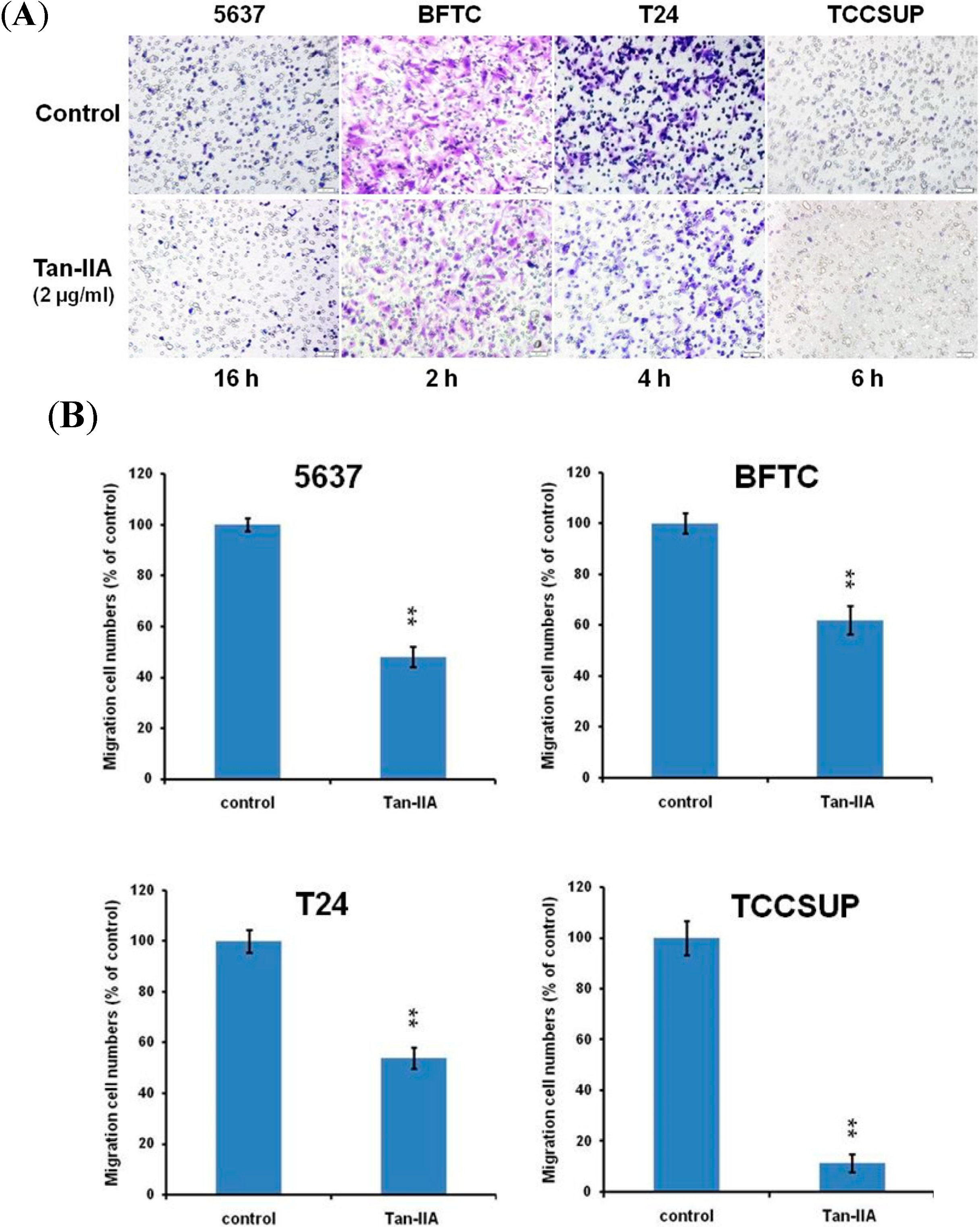
2.4. Effect of Tan-IIA on the Migration of Human Bladder Cancer Cells
2.5. Effect of Tan-IIA on the Combination Therapy with Cisplatin

3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Chemicals and Antibodies
4.3. Western Blot Analysis
4.4. MTT Assay
4.5. TUNEL Assay
4.6. Annexin V-FITC Staining
4.7. Flow Cytometric Analysis
4.8. Cell Migration Assay
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Lutzeyer, W.; Rubben, H.; Dahm, H. Prognostic parameters in superficial bladder-cancer—An analysis of 315 cases. J. Urol. 1982, 127, 250–252. [Google Scholar]
- Wu, X.R. Urothelial tumorigenesis: A tale of divergent pathways. Nat. Rev. Cancer 2005, 5, 713–725. [Google Scholar] [CrossRef]
- Botteman, M.F.; Pashos, C.L.; Redaelli, A.; Laskin, B.; Hauser, R. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics 2003, 21, 1315–1330. [Google Scholar] [CrossRef]
- Mariotto, A.B.; Yabroff, K.R.; Shao, Y.; Feuer, E.J.; Brown, M.L. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 2011, 103, 117–128. [Google Scholar] [CrossRef]
- Xu, D.; Lin, T.H.; Zhang, C.; Tsai, Y.C.; Li, S.; Zhang, J.; Yin, M.; Yeh, S.; Chang, C. The selective inhibitory effect of a synthetic tanshinone derivative on prostate cancer cells. Prostate 2012, 72, 803–816. [Google Scholar] [CrossRef]
- Chen, J.; Shi, D.Y.; Liu, S.L.; Zhong, L. Tanshinone IIA induces growth inhibition and apoptosis in gastric cancer in vitro and in vivo. Oncol. Rep. 2012, 27, 523–528. [Google Scholar]
- Dong, Y.; Morris-Natschke, S.L.; Lee, K.H. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat. Prod. Rep. 2011, 28, 529–542. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef]
- Chiu, S.C.; Huang, S.Y.; Chen, S.P.; Su, C.C.; Chiu, T.L.; Pang, C.Y. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013, 16, 315–322. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Jiang, S.; Yuan, S.; Lin, P.; Zhang, J.; Lu, Y.; Wang, Q.; Xiong, Z.; Wu, Y.; et al. Growth inhibition and induction of apoptosis and differentiation of tanshinone IIA in human glioma cells. J. Neurooncol. 2007, 82, 11–21. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Yuan, S.; Liu, G.; Lu, Y.; Zhang, J.; Wang, W. Potential anticancer activity of tanshinone IIA against human breast cancer. Int. J. Cancer 2005, 116, 799–807. [Google Scholar] [CrossRef]
- Yuan, S.L.; Wei, Y.Q.; Wang, X.J.; Xiao, F.; Li, S.F.; Zhang, J. Growth inhibition and apoptosis induction of tanshinone II-A on human hepatocellular carcinoma cells. World J. Gastroenterol. 2004, 10, 2024–2028. [Google Scholar]
- Pan, T.L.; Wang, P.W.; Hung, Y.C.; Huang, C.H.; Rau, K.M. Proteomic analysis reveals tanshinone IIA enhances apoptosis of advanced cervix carcinoma CaSki cells through mitochondria intrinsic and endoplasmic reticulum stress pathways. Proteomics 2013, 13, 3411–3423. [Google Scholar] [CrossRef]
- Yan, M.Y.; Chien, S.Y.; Kuo, S.J.; Chen, D.R.; Su, C.C. Tanshinone IIA inhibits BT-20 human breast cancer cell proliferation through increasing caspase 12, GADD153 and phospho-p38 protein expression. Int. J. Mol. Med. 2012, 29, 855–863. [Google Scholar]
- Zhang, Y.; Wei, R.X.; Zhu, X.B.; Cai, L.; Jin, W.; Hu, H. Tanshinone IIA induces apoptosis and inhibits the proliferation, migration, and invasion of the osteosarcoma MG-63 cell line in vitro. Anticancer Drugs 2012, 23, 212–219. [Google Scholar] [CrossRef]
- Wang, W.Q.; Liu, L.; Sun, H.C.; Fu, Y.L.; Xu, H.X.; Chai, Z.T.; Zhang, Q.B.; Kong, L.Q.; Zhu, X.D.; Lu, L.; et al. Tanshinone IIA inhibits metastasis after palliative resection of hepatocellular carcinoma and prolongs survival in part via vascular normalization. J. Hematol. Oncol. 2012, 5, 69. [Google Scholar] [CrossRef]
- Shan, Y.F.; Shen, X.; Xie, Y.K.; Chen, J.C.; Shi, H.Q.; Yu, Z.P.; Song, Q.T.; Zhou, M.T.; Zhang, Q.Y. Inhibitory effects of tanshinone II-A on invasion and metastasis of human colon carcinoma cells. Acta Pharmacol. Sin. 2009, 30, 1537–1542. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Lee, G.W.; Lee, J.S.; Cho, M.K.; Son, K.H.; Jeon, S.J.; Kang, S.S.; Kim, Y.S.; Lee, J.H.; Seo, H.G.; et al. Tanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules. Carcinogenesis 2008, 29, 1885–1892. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Chan, S.E.; Lai, H.W.; Su, C.C.; Kuo, S.J.; Chien, S.Y.; Lin, H.Y.; Chen, D.R. Effect of Supplementation of Tanshinone IIA and Sodium Tanshinone IIA Sulfonate on the Anticancer Effect of Epirubicin: An in Vitro Study. Evid.-Based Complement. Altern. Med.: eCAM 2011, 2011, 841564:1–841564:9. [Google Scholar]
- Cheng, C.Y.; Su, C.C. Tanshinone IIA may inhibit the growth of small cell lung cancer H146 cells by up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial membrane potential. Mol. Med. Rep. 2010, 3, 645–650. [Google Scholar]
- Cheng, C.Y.; Su, C.C. Tanshinone IIA inhibits Hep-J5 cells by increasing calreticulin, caspase 12 and GADD153 protein expression. Int. J. Mol. Med. 2010, 26, 379–385. [Google Scholar]
- Chien, S.Y.; Kuo, S.J.; Chen, Y.L.; Chen, D.R.; Cheng, C.Y.; Su, C.C. Tanshinone IIA inhibits human hepatocellular carcinoma J5 cell growth by increasing Bax and caspase 3 and decreasing CD31 expression in vivo. Mol. Med. Rep. 2012, 5, 282–286. [Google Scholar]
- Chiu, T.L.; Su, C.C. Tanshinone IIA induces apoptosis in human lung cancer A549 cells through the induction of reactive oxygen species and decreasing the mitochondrial membrane potential. Int. J. Mol. Med. 2010, 25, 231–236. [Google Scholar]
- Su, C.C.; Chien, S.Y.; Kuo, S.J.; Chen, Y.L.; Cheng, C.Y.; Chen, D.R. Tanshinone IIA inhibits human breast cancer MDA-MB-231 cells by decreasing LC3-II, Erb-B2 and NF-kappaBp65. Mol. Med. Rep. 2012, 5, 1019–1022. [Google Scholar]
- Chang, L.C.; Wu, C.L.; Liu, C.W.; Chuo, W.H.; Li, P.C.; Tsai, T.R. Preparation, characterization and cytotoxicity evaluation of tanshinone IIA nanoemulsions. J. Biomed. Nanotechnol. 2011, 7, 558–567. [Google Scholar] [CrossRef]
- Mothana, R.A.; Jansen, R.; Gruenert, R.; Bednarski, P.J.; Lindequist, U. Antimicrobial and cytotoxic abietane diterpenoids from the roots of Meriandera benghalensis (Roxb.) Benth. Die Pharm. 2009, 64, 613–615. [Google Scholar]
- Su, C.C.; Chen, G.W.; Lin, J.G. Growth inhibition and apoptosis induction by tanshinone I in human colon cancer Colo 205 cells. Int. J. Mol. Med. 2008, 22, 613–618. [Google Scholar]
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Szarvas, T.; vom Dorp, F.; Ergun, S.; Rubben, H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat. Rev. Urol. 2011, 8, 241–254. [Google Scholar] [CrossRef]
- Soga, N.; Arima, K.; Sugimura, Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int. J. Urol. 2008, 15, 800–803. [Google Scholar] [CrossRef]
- Von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Drayton, R.M.; Catto, J.W. Molecular mechanisms of cisplatin resistance in bladder cancer. Expert Rev. Anticancer Ther. 2012, 12, 271–281. [Google Scholar] [CrossRef]
- Jiao, J.W.; Wen, F. Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol. Rep. 2011, 25, 781–788. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chiu, S.-C.; Huang, S.-Y.; Chang, S.-F.; Chen, S.-P.; Chen, C.-C.; Lin, T.-H.; Liu, H.-H.; Tsai, T.-H.; Lee, S.-S.; Pang, C.-Y.; et al. Potential Therapeutic Roles of Tanshinone IIA in Human Bladder Cancer Cells. Int. J. Mol. Sci. 2014, 15, 15622-15637. https://doi.org/10.3390/ijms150915622
Chiu S-C, Huang S-Y, Chang S-F, Chen S-P, Chen C-C, Lin T-H, Liu H-H, Tsai T-H, Lee S-S, Pang C-Y, et al. Potential Therapeutic Roles of Tanshinone IIA in Human Bladder Cancer Cells. International Journal of Molecular Sciences. 2014; 15(9):15622-15637. https://doi.org/10.3390/ijms150915622
Chicago/Turabian StyleChiu, Sheng-Chun, Sung-Ying Huang, Shu-Fang Chang, Shee-Ping Chen, Chi-Cheng Chen, Tien-Huang Lin, Hsin-Ho Liu, Tsung-Hsun Tsai, Shang-Sen Lee, Cheng-Yoong Pang, and et al. 2014. "Potential Therapeutic Roles of Tanshinone IIA in Human Bladder Cancer Cells" International Journal of Molecular Sciences 15, no. 9: 15622-15637. https://doi.org/10.3390/ijms150915622
APA StyleChiu, S.-C., Huang, S.-Y., Chang, S.-F., Chen, S.-P., Chen, C.-C., Lin, T.-H., Liu, H.-H., Tsai, T.-H., Lee, S.-S., Pang, C.-Y., & Hsieh, T.-F. (2014). Potential Therapeutic Roles of Tanshinone IIA in Human Bladder Cancer Cells. International Journal of Molecular Sciences, 15(9), 15622-15637. https://doi.org/10.3390/ijms150915622



