Celastrol Attenuates Inflammatory and Neuropathic Pain Mediated by Cannabinoid Receptor Type 2
Abstract
:1. Introduction
2. Results and Discussion
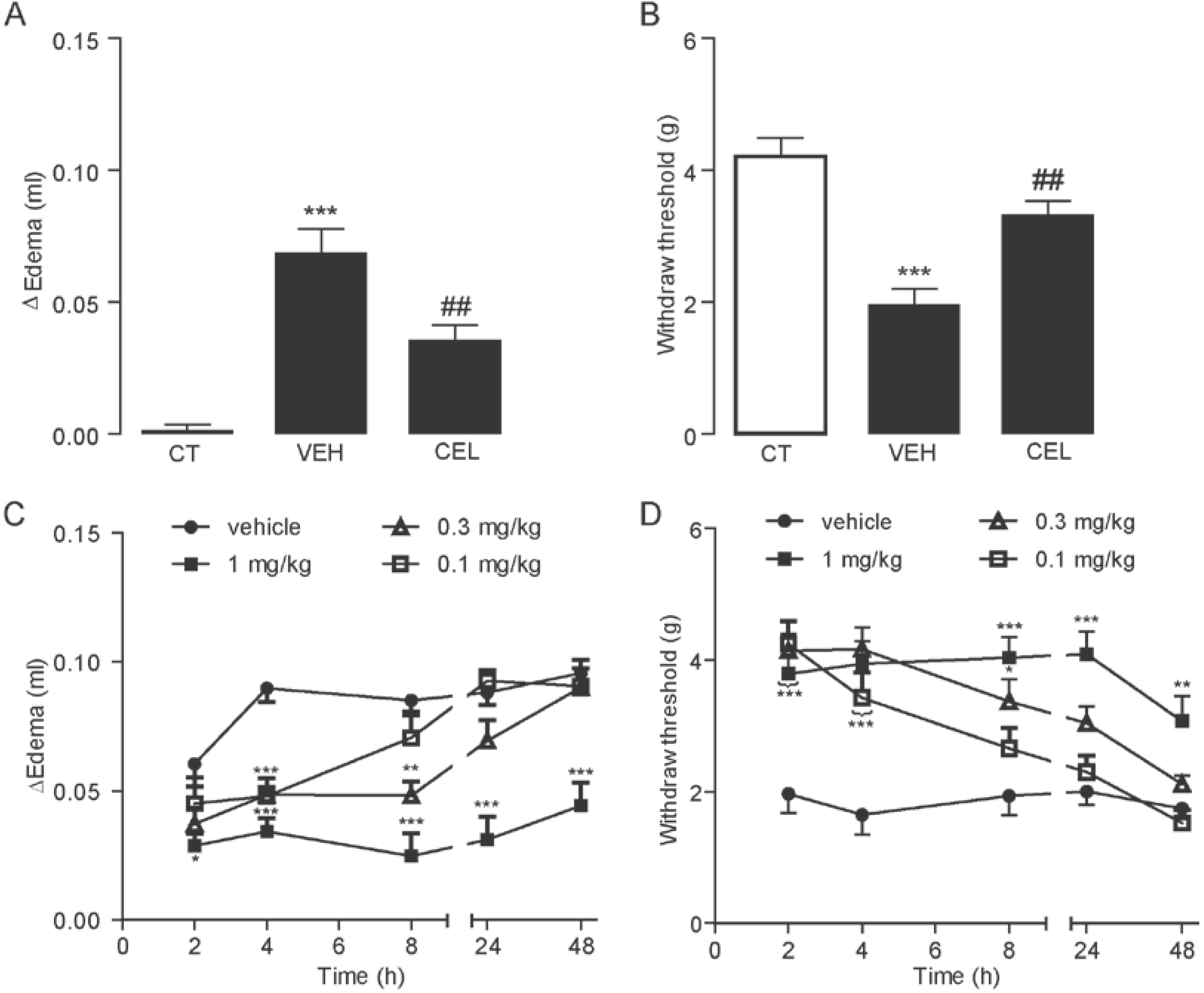
2.1. Celastrol Dose- and Time-Dependently Reduced Carrageenan-Induced Edema and Hyperalgesia
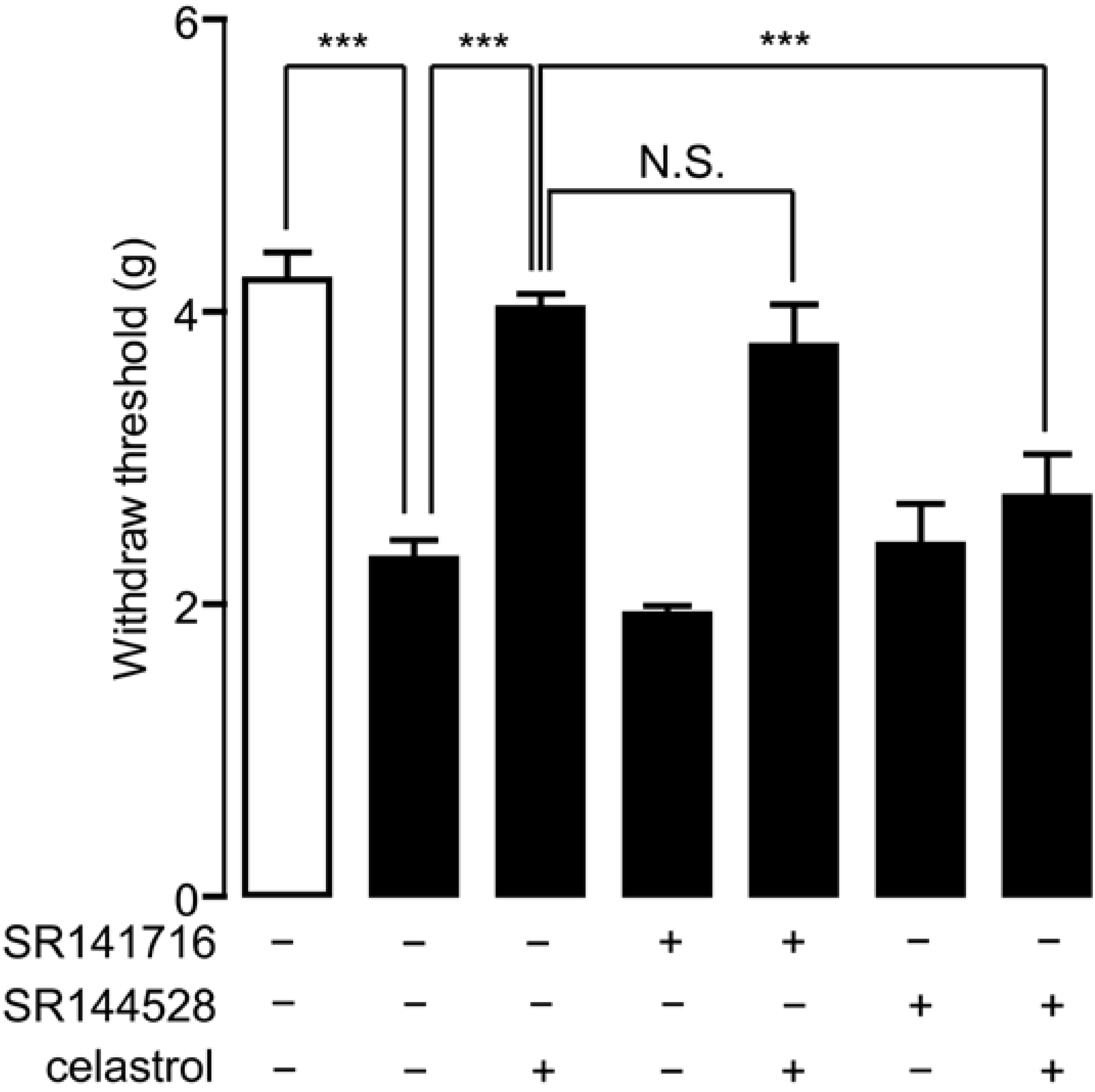
2.2. Celastrol Produced an Antinociceptive Effect through the Cannabinoid Receptor-2 (CB2) Signal in Carrageenan-Induced Inflammatory Pain
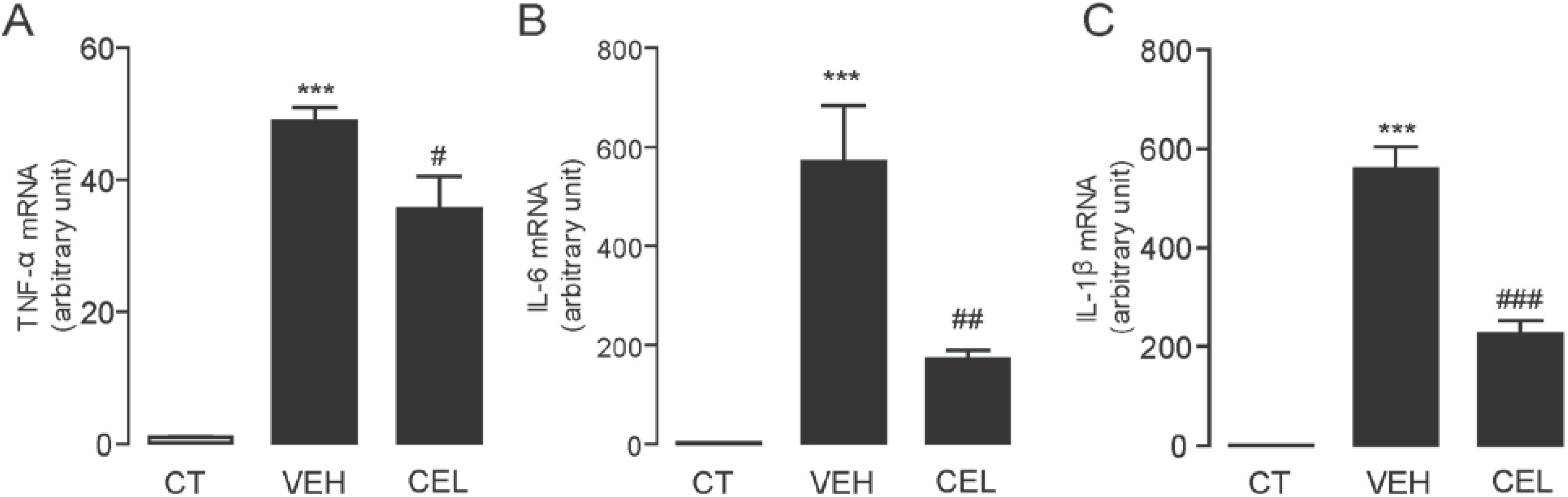
2.3. Celastrol Suppressed the mRNA Expression Levels of Inflammatory Cytokines Induced in Carrageenan-Injected Mice
2.4. Celastrol Alleviated Hyperalgesia in Neuropathic Pain Mice

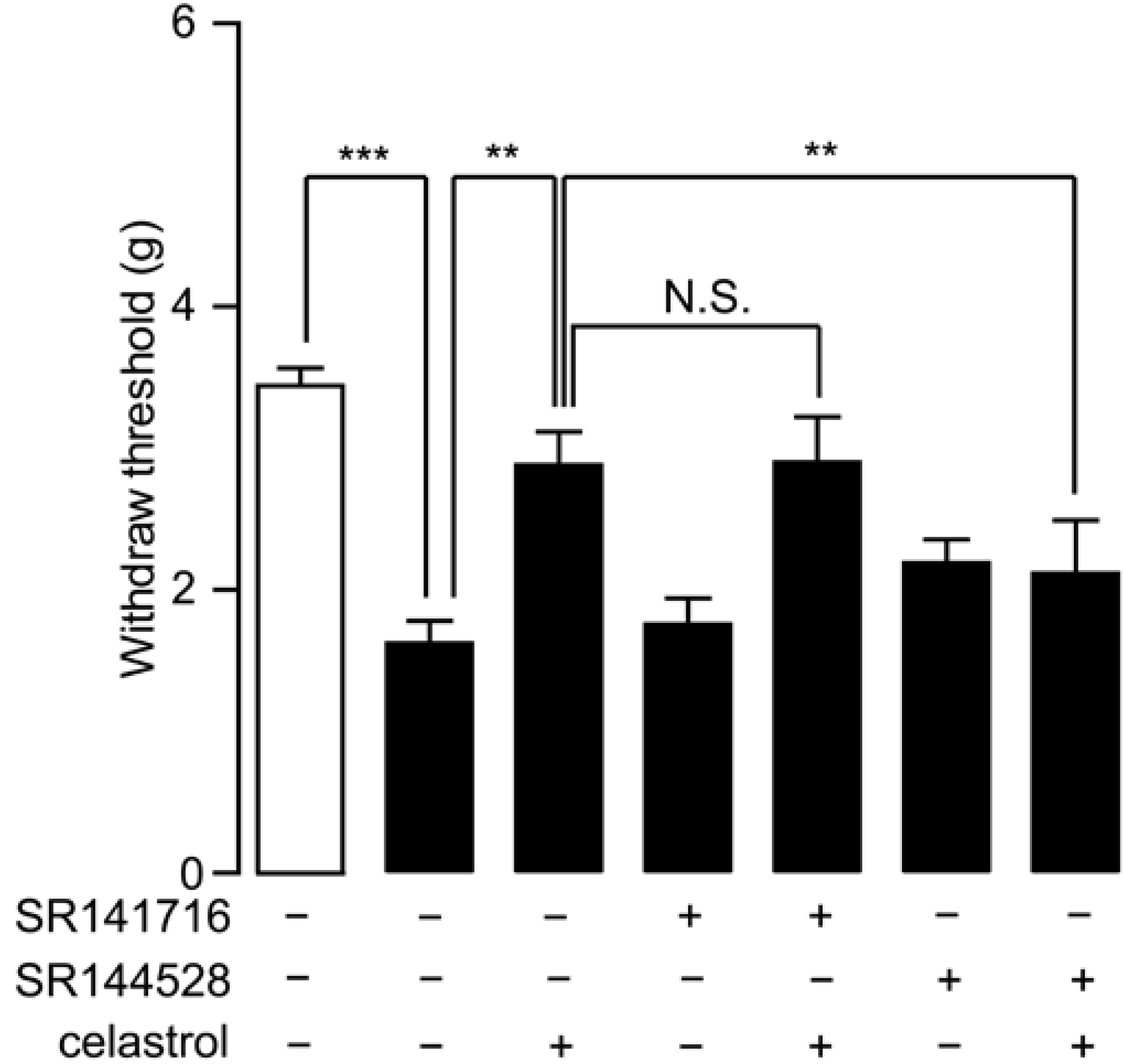
2.5. Analgesia Effect of Celastrol in Neuropathic Pain Was Mediated by the CB2 Signal
3. Experimental Section
3.1. Drug Administration
3.2. Carrageenan-Induced Inflammatory Pain
3.3. Spared Nerve Injury (SNI) Surgery
3.4. Allodynia Test
3.5. Edema Measurement
3.6. Quantitative Real-Time PCR
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, K.F.; Yuan, Y.; Luk, J.M. Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin. Exp. Pharmacol. Physiol. 2012, 39, 311–320. [Google Scholar]
- Kannaiyan, R.; Shanmugam, M.K.; Sethi, G. Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011, 303, 9–20. [Google Scholar]
- Nanjundaiah, S.M.; Venkatesha, S.H.; Yu, H.; Tong, L.; Stains, J.P.; Moudgil, K.D. Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthitis by modulating osteoimmune cross-talk. J. Biol. Chem. 2012, 287, 22216–22226. [Google Scholar]
- Venkatesha, S.H.; Yu, H.; Rajaiah, R.; Tong, L.; Moudgil, K.D. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J. Biol. Chem. 2011, 286, 15138–15146. [Google Scholar]
- Gu, L.; Bai, W.; Li, S.; Zhang, Y.; Han, Y.; Gu, Y.; Meng, G.; Xie, L.; Wang, J.; Xiao, Y.; et al. Celastrol prevents atherosclerosis via inhibiting lox-1 and oxidative stress. PLoS One 2013, 8, e65477. [Google Scholar]
- Paris, D.; Ganey, N.J.; Laporte, V.; Patel, N.S.; Beaulieu-Abdelahad, D.; Bachmeier, C.; March, A.; Ait-Ghezala, G.; Mullan, M.J. Reduction of β-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J. Neuroinflamm. 2010, 7, 17. [Google Scholar]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.; Alvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Jeoung, D.; Ro, J.Y. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur. J. Pharmacol. 2009, 612, 98–105. [Google Scholar]
- Xu, X.; Wu, Z.; Xu, C.; Ren, Y.; Ge, Y. Observation on serum anti-double stranded DNA antibodies of tripterine in systemic lupus erythematosus of (NZBxW)F1 mice. Ann. Rheum. Dis. 2003, 62, 377–378. [Google Scholar]
- Piscitelli, F.; di Marzo, V. “Redundancy” of endocannabinoid inactivation: New challenges and opportunities for pain control. ACS Chem. Neurosci. 2012, 3, 356–363. [Google Scholar] [CrossRef]
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar]
- Alvarez-Jaimes, L.J.; Palmer, J.A. The role of endocannabinoids in pain modulation and the therapeutic potential of inhibiting their enzymatic degradation. Curr. Pharm. Biotechnol. 2011, 12, 1644–1659. [Google Scholar]
- Iversen, L. Cannabis and the brain. Brain 2003, 126, 1252–1270. [Google Scholar]
- Pertwee, R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar]
- Moreira, F.A.; Grieb, M.; Lutz, B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 133–144. [Google Scholar]
- Rice, A.S.; Farquhar-Smith, W.P.; Nagy, I. Endocannabinoids and pain: Spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot. Essent. Fat.Acids 2002, 66, 243–256. [Google Scholar]
- Valenzano, K.J.; Tafesse, L.; Lee, G.; Harrison, J.E.; Boulet, J.M.; Gottshall, S.L.; Mark, L.; Pearson, M.S.; Miller, W.; Shan, S.; et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 2005, 48, 658–672. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Deng, H.; Zvonok, A.; Cockayne, D.A.; Kwan, J.; Mata, H.P.; Vanderah, T.W.; Lai, J.; Porreca, F.; Makriyannis, A.; et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. USA 2003, 100, 10529–10533. [Google Scholar] [CrossRef]
- Kerr, D.M.; Harhen, B.; Okine, B.N.; Egan, L.J.; Finn, D.P.; Roche, M. The monoacylglycerol lipase inhibitor JZL184 attenuates LPS-induced increases in cytokine expression in the rat frontal cortex and plasma: Differential mechanisms of action. Br. J. Pharmacol. 2013, 169, 808–819. [Google Scholar]
- Ghosh, S.; Wise, L.E.; Chen, Y.; Gujjar, R.; Mahadevan, A.; Cravatt, B.F.; Lichtman, A.H. The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci. 2013, 92, 498–505. [Google Scholar]
- Hohmann, A.G. Inhibitors of monoacylglycerol lipase as novel analgesics. Br. J. Pharmacol. 2007, 150, 673–675. [Google Scholar]
- Dossou, K.S.; Devkota, K.P.; Morton, C.; Egan, J.M.; Lu, G.; Beutler, J.A.; Moaddel, R. Identification of CB1/CB2ligands from Zanthoxylum bungeanum. J. Nat. Prod. 2013, 76, 2060–2064. [Google Scholar]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. β-Caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- King, A.R.; Dotsey, E.Y.; Lodola, A.; Jung, K.M.; Ghomian, A.; Qiu, Y.; Fu, J.; Mor, M.; Piomelli, D. Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem. Biol. 2009, 6, 1045–1052. [Google Scholar]
- Dutra, R.C.; Simao da Silva, K.A.; Bento, A.F.; Marcon, R.; Paszcuk, A.F.; Meotti, F.C.; Pianowski, L.F.; Calixto, J.B. Euphol, a tetracyclic triterpene produces antinociceptive effects in inflammatory and neuropathic pain: The involvement of cannabinoid system. Neuropharmacology 2012, 63, 593–605. [Google Scholar]
- Rani Sagar, D.; Burston, J.J.; Woodhams, S.G.; Chapman, V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos. Trans. R.Soc. Lond. B 2012, 367, 3300–3311. [Google Scholar]
- Shields, S.D.; Eckert, W.A., III; Basbaum, A.I. Spared nerve injury model of neuropathic pain in the mouse: A behavioral and anatomic analysis. J. Pain 2003, 4, 465–470. [Google Scholar] [CrossRef]
- Thaler, A.; Gupta, A.; Cohen, S.P. Cannabinoids for pain management. Adv. Psychosom. Med. 2011, 30, 125–138. [Google Scholar]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar]
- Ashton, J.C.; Milligan, E.D. Cannabinoids for the treatment of neuropathic pain: Clinical evidence. Curr. Opin. Investig. Drugs 2008, 9, 65–75. [Google Scholar]
- Campbell, F.A.; Tramer, M.R.; Carroll, D.; Reynolds, D.J.; Moore, R.A.; McQuay, H.J. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 2001, 323, 13–16. [Google Scholar]
- Cunha, T.M.; Verri, W.A., Jr.; Silva, J.S.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Bourquin, A.F.; Suveges, M.; Pertin, M.; Gilliard, N.; Sardy, S.; Davison, A.C.; Spahn, D.R.; Decosterd, I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 2006, 122, 14.e11–14.e14. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, L.; Li, Y.; Ren, J.; Zhu, C.; Fu, J.; Lin, D.; Qiu, Y. Celastrol Attenuates Inflammatory and Neuropathic Pain Mediated by Cannabinoid Receptor Type 2. Int. J. Mol. Sci. 2014, 15, 13637-13648. https://doi.org/10.3390/ijms150813637
Yang L, Li Y, Ren J, Zhu C, Fu J, Lin D, Qiu Y. Celastrol Attenuates Inflammatory and Neuropathic Pain Mediated by Cannabinoid Receptor Type 2. International Journal of Molecular Sciences. 2014; 15(8):13637-13648. https://doi.org/10.3390/ijms150813637
Chicago/Turabian StyleYang, Longhe, Yanting Li, Jie Ren, Chenggang Zhu, Jin Fu, Donghai Lin, and Yan Qiu. 2014. "Celastrol Attenuates Inflammatory and Neuropathic Pain Mediated by Cannabinoid Receptor Type 2" International Journal of Molecular Sciences 15, no. 8: 13637-13648. https://doi.org/10.3390/ijms150813637
APA StyleYang, L., Li, Y., Ren, J., Zhu, C., Fu, J., Lin, D., & Qiu, Y. (2014). Celastrol Attenuates Inflammatory and Neuropathic Pain Mediated by Cannabinoid Receptor Type 2. International Journal of Molecular Sciences, 15(8), 13637-13648. https://doi.org/10.3390/ijms150813637




