TupA: A Tungstate Binding Protein in the Periplasm of Desulfovibrio alaskensis G20
Abstract
:1. Introduction
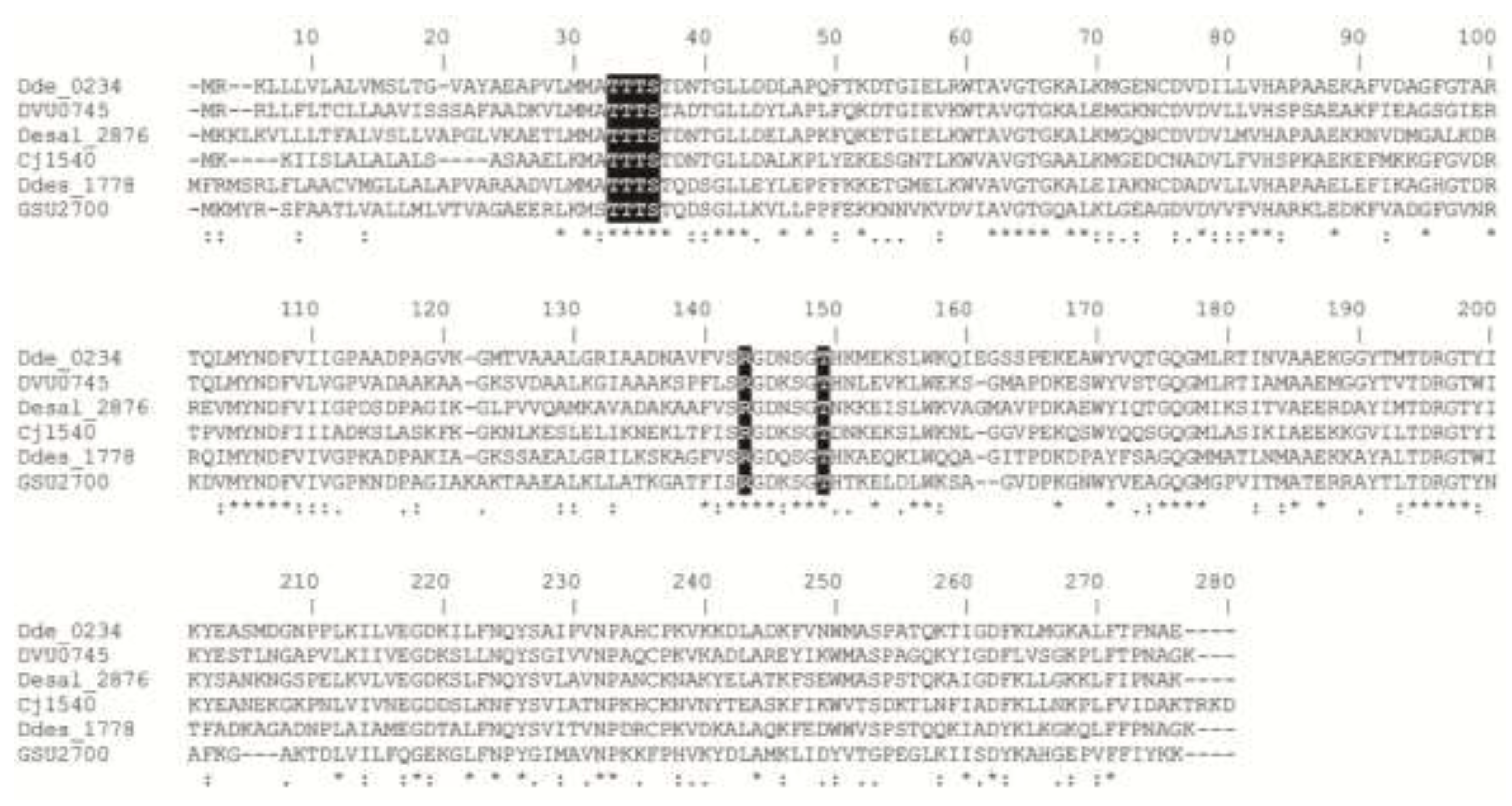
2. Results and Discussion
2.1. Cloning of tupA Gene and Purification of TupA Protein

2.2. UV-Visible Spectrum and Protein Sequence

2.3. Metal Binding Assays

2.4. Isothermal Titration Calorimetry (ITC)
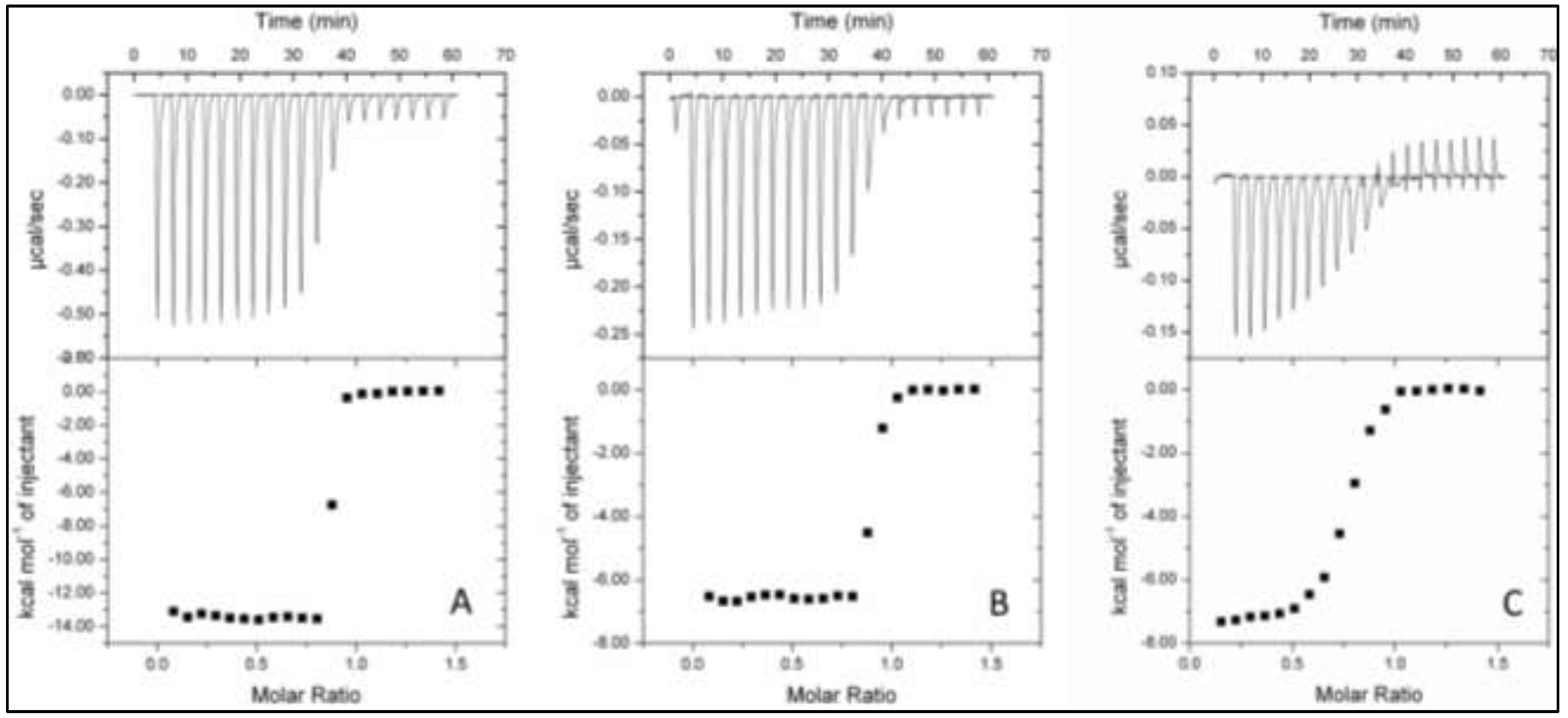
| Protein (+Oxyanion) | Ligand | n | KA (M−1) | KD (nM) | ∆H (kcal·mol−1) |
|---|---|---|---|---|---|
| TupA | WO42− | 0.842 ± 0.001 | 2 × 109 ± 2 × 109 | 0.5 ± 0.4 | −13.500 ± 0.005 |
| MoO42− | 0.868 ± 0.002 | 16 × 107 ± 2 × 107 | 6.1 ± 0.9 | −6.600 ± 0.003 | |
| TupA + 0.5 mM MoO42− | WO42− | 0.845 ± 0.003 | 1600 × 108 ± 6 × 108 | 6.30 × 10−3 ± 0.02 × 10−3 | −14.60 ± 0.04 |
| TupA + 0.5 mM WO42− | MoO42− | No displacement | |||
2.5. Crystallization and Data Processing
| Data Collection Parameters | |
|---|---|
| X-ray source | ID23-1 (ESRF, Grenoble) |
| Detector | PILATUS 6M-F |
| Wavelength (Å) | 0.954 |
| Processing Statistics | |
| Unit-cell parameters (Å, °) | a = 52.25; b = 42.50; c = 54.71; β = 95.43 |
| Space group | P1211 |
| Molecules per AU | 1 |
| Matthews coefficient (Å3, Da) | 2.09 |
| Mosaicity (°) | 0.22 |
| Resolution range (Å) | 42.50–1.43 (1.45–1.43) |
| <I/σI> | 10.3 (2.1) |
| Rmerge (%) * | 4.1 (33.5) |
| Rpim (%) + | 2.7 (23.4) |
| Rmeas (%) § | 5.0 (4.1) |
| Multiplicity | 3.0 (2.8) |
| No. of observed reflections | 132,115 (6040) |
| No. of unique reflections | 43,950 (2151) |
| Completeness (%) | 99.1 (98.8) |

2.6. Structure Determination
3. Experimental Section
3.1. Bacterial Strains and Plasmids
| Strain/Plasmid/Primer | Properties/Sequence | Source/Reference |
|---|---|---|
| DaG20 | Spontaneously nalidixic acid resistant derivative of G100A, isolated from the production fluids of offshore oil fields in Alaska | Feio, M.J. [21], Hauser, L.J. [34] and Wall, J.D. [35]. |
| pET-46 Ek/LIC vector | E. coli cloning vector plasmid | Novagen |
| NovaBlue GigaSingles cells | endA1 hsdR17 (rK12− mK12+) supE44 thi-1 recA1 gyrA96 relA1 lac [F'proA+B+ lacIqZΔM15::Tn10(TcR)] | Novagen |
| E coli BL21(DE3) | F− ompT gal dcm lon hsdSB(rB− mB−) λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) | Studier, F.W. [36] |
| TupA_LIC_Fwd (sense) | GACGACGACAAGATGCTGGAAGTTCTGTTCCAGGGGCCCGAAGCACCGGTTCTTATG | This work |
| TupA_LIC_Rev (antisense) | GAGGAGAAGCCCGGTTATTCGGCGTTGGGGGT | This work |
3.2. Cloning of tupA Gene and Protein Expression Optimization
3.3. Protein Expression and Purification
3.4. Extinction Coefficient Determination
3.5. Protein Gel Shift Assay
3.6. Isothermal Titration Calorimetry

3.7. Crystallization
3.8. Data Collection and Processing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gonzalez, P.J.; Rivas, M.G.; Mota, C.S.; Brondino, C.D.; Moura, I.; Moura, J.J.G. Periplasmic nitrate reductases and formate dehydrogenases: Biological control of the chemical properties of Mo and W for fine tuning of reactivity, substrate specificity and metabolic role. Coord. Chem. Rev. 2013, 257, 315–331. [Google Scholar]
- Hagen, W.R. Cellular uptake of molybdenum and tungsten. Coord. Chem. Rev. 2011, 255, 1117–1128. [Google Scholar]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef]
- Grunden, A.M.; Shanmugam, K.T. Molybdate transport and regulation in bacteria. Arch. Microbiol. 1997, 168, 345–354. [Google Scholar]
- Maupin-Furlow, J.A.; Rosentel, J.K.; Lee, J.H.; Deppenmeier, U.; Gunsalus, R.P.; Shanmugam, K.T. Genetic analysis of the modABCD (molybdate transport) operon of Escherichia coli. J. Bacteriol. 1995, 177, 4851–4856. [Google Scholar]
- Rech, S.; Deppenmeier, U.; Gunsalus, R.P. Regulation of the molybdate transport operon, modABCD, of Escherichia coli in response to molybdate availability. J. Bacteriol. 1995, 177, 1023–1029. [Google Scholar]
- Anderson, L.A.; Palmer, T.; Price, N.C.; Bornemann, S.; Boxer, D.H.; Pau, R.N. Characterisation of the molybdenum-responsive ModE regulatory protein and its binding to the promoter region of the modABCD (molybdenum transport) operon of Escherichia coli. Eur. J. Biochem. 1997, 246, 119–126. [Google Scholar]
- Rech, S.; Wolin, C.; Gunsalus, R.P. Properties of the periplasmic ModA molybdate-binding protein of Escherichia coli. J. Biol. Chem. 1996, 271, 2557–2562. [Google Scholar]
- Makdessi, K.; Andreesen, J.R.; Pich, A. Tungstate uptake by a highly specific ABC transporter in Eubacterium acidaminophilum. J. Biol. Chem. 2001, 276, 24557–24564. [Google Scholar] [CrossRef]
- Bevers, L.E.; Hagedoorn, P.L.; Krijger, G.C.; Hagen, W.R. Tungsten transport protein A (WtpA) in Pyrococcus furiosus: The first member of a new class of tungstate and molybdate transporters. J. Bacteriol. 2006, 188, 6498–64505. [Google Scholar] [CrossRef]
- Imperial, J.; Hadi, M.; Amy, N.K. Molybdate binding by ModA, the periplasmic component of the Escherichia coli mod molybdate transport system. Biochim. Biophys. Acta 1998, 1370, 337–346. [Google Scholar] [CrossRef]
- Smart, J.P.; Cliff, M.J.; Kelly, D.J. A role for tungsten in the biology of Campylobacter jejuni: Tungstate stimulates formate dehydrogenase activity and is transported via an ultra-high affinity ABC system distinct from the molybdate transporter. Mol. Microbiol. 2009, 74, 742–757. [Google Scholar]
- Balan, A.; Santacruz, C.P.; Moutran, A.; Ferreira, R.C.; Medrano, F.J.; Perez, C.A.; Ramos, C.H.; Ferreira, L.C. The molybdate-binding protein (ModA) of the plant pathogen Xanthomonas axonopodis pv. citri. Protein Expr. Purif. 2006, 50, 215–222. [Google Scholar] [CrossRef]
- Taveirne, M.E.; Sikes, M.L.; Olson, J.W. Molybdenum and tungsten in Campylobacter jejuni: Their physiological role and identification of separate transporters regulated by a single ModE-like protein. Mol. Microbiol. 2009, 74, 758–771. [Google Scholar] [CrossRef]
- Bevers, L.E.; Schwarz, G.; Hagen, W.R. A molecular basis for tungstate selectivity in prokaryotic ABC transport systems. J. Bacteriol. 2011, 193, 4999–5001. [Google Scholar]
- Andreesen, J.R.; Makdessi, K. Tungsten, the surprisingly positively acting heavy metal element for prokaryotes. Ann. N. Y. Acad. Sci. 2008, 1125, 215–229. [Google Scholar]
- Hu, Y.; Rech, S.; Gunsalus, R.P.; Rees, D.C. Crystal structure of the molybdate binding protein ModA. Nat. Struct. Biol. 1997, 4, 703–707. [Google Scholar]
- Lawson, D.M.; Williams, C.E.; Mitchenall, L.A.; Pau, R.N. Ligand size is a major determinant of specificity in periplasmic oxyanion-binding proteins: The 1.2 Å resolution crystal structure of Azotobacter vinelandii ModA. Structure 1998, 6, 1529–1539. [Google Scholar] [CrossRef]
- Balan, A.; Santacruz-Perez, C.; Moutran, A.; Ferreira, L.C.; Neshich, G.; Goncalves Barbosa, J.A. Crystallographic structure and substrate-binding interactions of the molybdate-binding protein of the phytopathogen Xanthomonas axonopodis pv. citri. Biochim. Biophys. Acta 2008, 1784, 393–399. [Google Scholar] [CrossRef]
- Hollenstein, K.; Comellas-Bigler, M.; Bevers, L.E.; Feiters, M.C.; Meyer-Klaucke, W.; Hagedoorn, P.L.; Locher, K.P. Distorted octahedral coordination of tungstate in a subfamily of specific binding proteins. J. Biol. Inorg. Chem. 2009, 14, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Feio, M.J.; Zinkevich, V.; Beech, I.B.; Llobet-Brossa, E.; Eaton, P.; Schmitt, J.; Guezennec, J. Desulfovibrio alaskensis sp. nov., a sulphate-reducing bacterium from a soured oil reservoir. Int. J. Syst. Evol. Microbiol. 2004, 54, 1747–1752. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Lewandowski, Z.; Nielsen, P.H.; Hamilton, W.A. Role of sulfate-reducing bacteria in corrosion of mild-steel—A review. Biofouling 1995, 8, 165–168. [Google Scholar] [CrossRef]
- Hamilton, W.A. Microbially influenced corrosion as a model system for the study of metal microbe interactions: A unifying electron transfer hypothesis. Biofouling 2003, 19, 65–76. [Google Scholar] [CrossRef]
- Sanders, P.F.; Sturman, P.J. Biofouling in the oil industry. In Petroleum Microbiology; Olivier, B., Magot, M., Eds.; American Society for Microbiology: Washington, DC, USA, 2005; pp. 171–198. [Google Scholar]
- Peck, H.D. The ATP-dependent reduction of sulfate with hydrogen in extracts of Desulfovibrio desulfuricans. Proc. Natl. Acad. Sci. USA 1959, 45, 701–708. [Google Scholar] [CrossRef]
- Peck, H.D.J. Enzymatic basis for assimilatory and dissimilatory sulfate reduction. J. Bacteriol. 1961, 82, 933–939. [Google Scholar]
- Biswas, K.C.; Woodards, N.A.; Xu, H.; Barton, L.L. Reduction of molybdate by sulfate-reducing bacteria. Biometals 2009, 22, 131–139. [Google Scholar] [CrossRef]
- Nair, R.R.; Silveira, C.M.; Dinis, M.S.; Almeida, M.G.; Moura, J.J.G.; Rivas, M.G.; REQUIMTE/CQFB, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal. Unpublished work. 2014.
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using clustalW and clustalX. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2005; pp. 571–607. [Google Scholar]
- Padilla, J.E.; Yeates, T.O. A statistic for local intensity differences: Robustness to anisotropy and pseudo-centering and utility for detecting twinning. Acta Crystallogr. D 2003, 59, 1124–1130. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef]
- Postgate, J. Cultivation and Gowth. In The Sulphate-Reducing Bacteria, 2nd ed.; Cambridge University Press: London, UK, 1984; pp. 24–40. [Google Scholar]
- Hauser, L.J.; Land, M.L.; Brown, S.D.; Larimer, F.; Keller, K.L.; Rapp-Giles, B.J.; Price, M.N.; Lin, M.; Bruce, D.C.; Detter, J.C.; et al. Complete genome sequence and updated annotation of Desulfovibrio alaskensis G20. J. Bacteriol. 2011, 193, 4268–4269. [Google Scholar] [CrossRef]
- Wall, J.D.; Rapp-Giles, B.J.; Rousset, M. Characterization of a small plasmid from Desulfovibrio desulfuricans and its use for shuttle vector construction. J. Bacteriol. 1993, 175, 4121–4128. [Google Scholar]
- Studier, F.W.; Moffatt, B.A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986, 189, 113–130. [Google Scholar] [CrossRef]
- Altschul, S.F.; Lipman, D.J. Protein database searches for multiple alignments. Proc. Nat. Acad. Sci. USA 1990, 87, 5509–5513. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. ClustalW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Velazquez-Campoy, A.; Freire, E. Isothermal titration calorimetry to determine association constants for high-affinity ligands. Nat. Protoc. 2006, 1, 186–191. [Google Scholar] [CrossRef]
- Jancarik, J.; Kim, S.-H. Sparse matrix sampling: A screening method for crystallization of proteins. J. Appl. Cryst. 1991, 24, 409–411. [Google Scholar] [CrossRef]
- Matthews, B.W. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
- Kabsch, W. Xds. Acta Crystallogr. D 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D 2006, 62, 72–82. [Google Scholar] [CrossRef]
- The CCP4 Suite. Programs for protein crystallography. Acta Crystallogr. D 1994, 50, 760–763. [Google Scholar] [CrossRef]
- Bevers, L.E.; Hagedoorn, P.-L.; Hagen, W.R. The bioinorganic chemistry of tungsten. Coord. Chem. Rev. 2009, 253, 269–290. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Otrelo-Cardoso, A.R.; Nair, R.R.; Correia, M.A.S.; Rivas, M.G.; Santos-Silva, T. TupA: A Tungstate Binding Protein in the Periplasm of Desulfovibrio alaskensis G20. Int. J. Mol. Sci. 2014, 15, 11783-11798. https://doi.org/10.3390/ijms150711783
Otrelo-Cardoso AR, Nair RR, Correia MAS, Rivas MG, Santos-Silva T. TupA: A Tungstate Binding Protein in the Periplasm of Desulfovibrio alaskensis G20. International Journal of Molecular Sciences. 2014; 15(7):11783-11798. https://doi.org/10.3390/ijms150711783
Chicago/Turabian StyleOtrelo-Cardoso, Ana Rita, Rashmi R. Nair, Márcia A. S. Correia, Maria G. Rivas, and Teresa Santos-Silva. 2014. "TupA: A Tungstate Binding Protein in the Periplasm of Desulfovibrio alaskensis G20" International Journal of Molecular Sciences 15, no. 7: 11783-11798. https://doi.org/10.3390/ijms150711783




