Triterpenoid Saponins from Stauntonia chinensis Ameliorate Insulin Resistance via the AMP-Activated Protein Kinase and IR/IRS-1/PI3K/Akt Pathways in Insulin-Resistant HepG2 Cells
Abstract
:1. Introduction
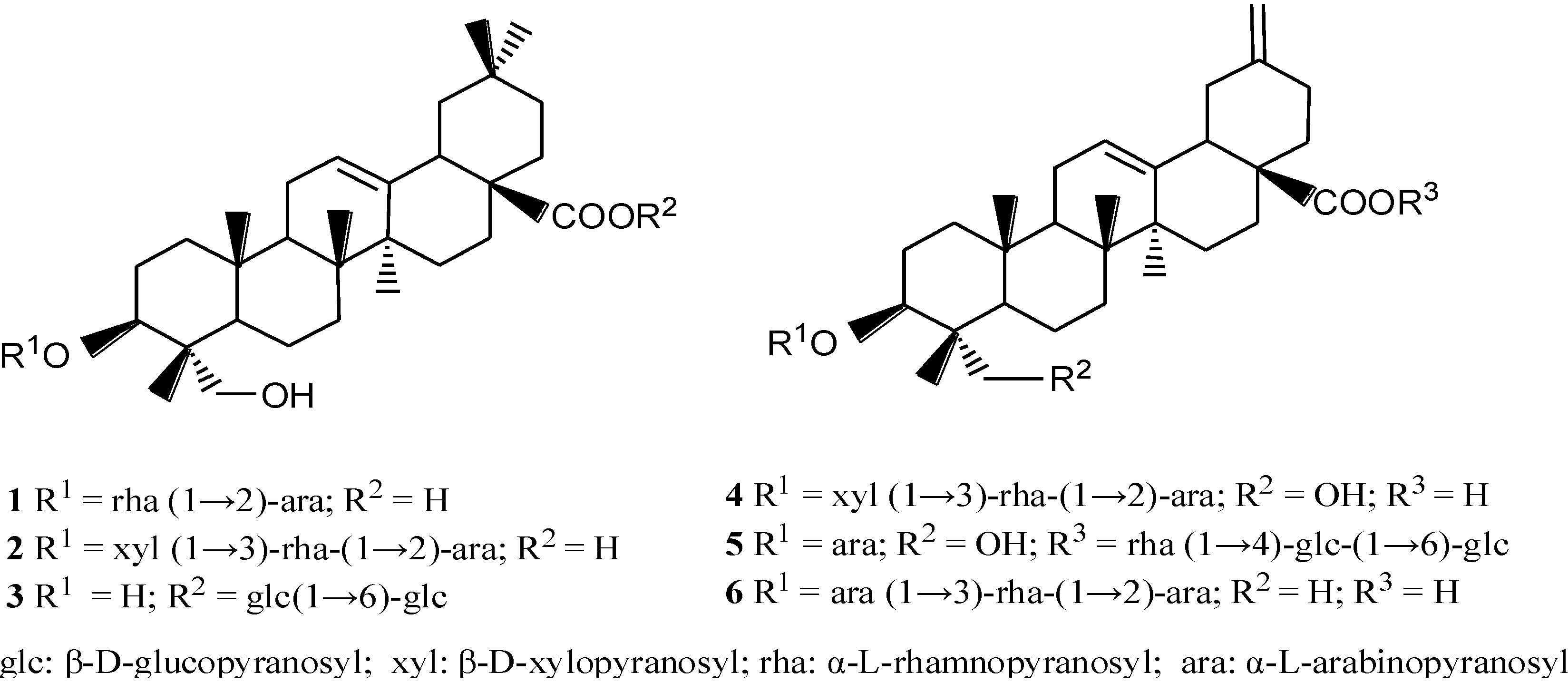
2. Results
2.1. Cytotoxicities of Compounds 1–6 and Their Effects on Glucose Uptake in Insulin-Resistant HepG2 Cells
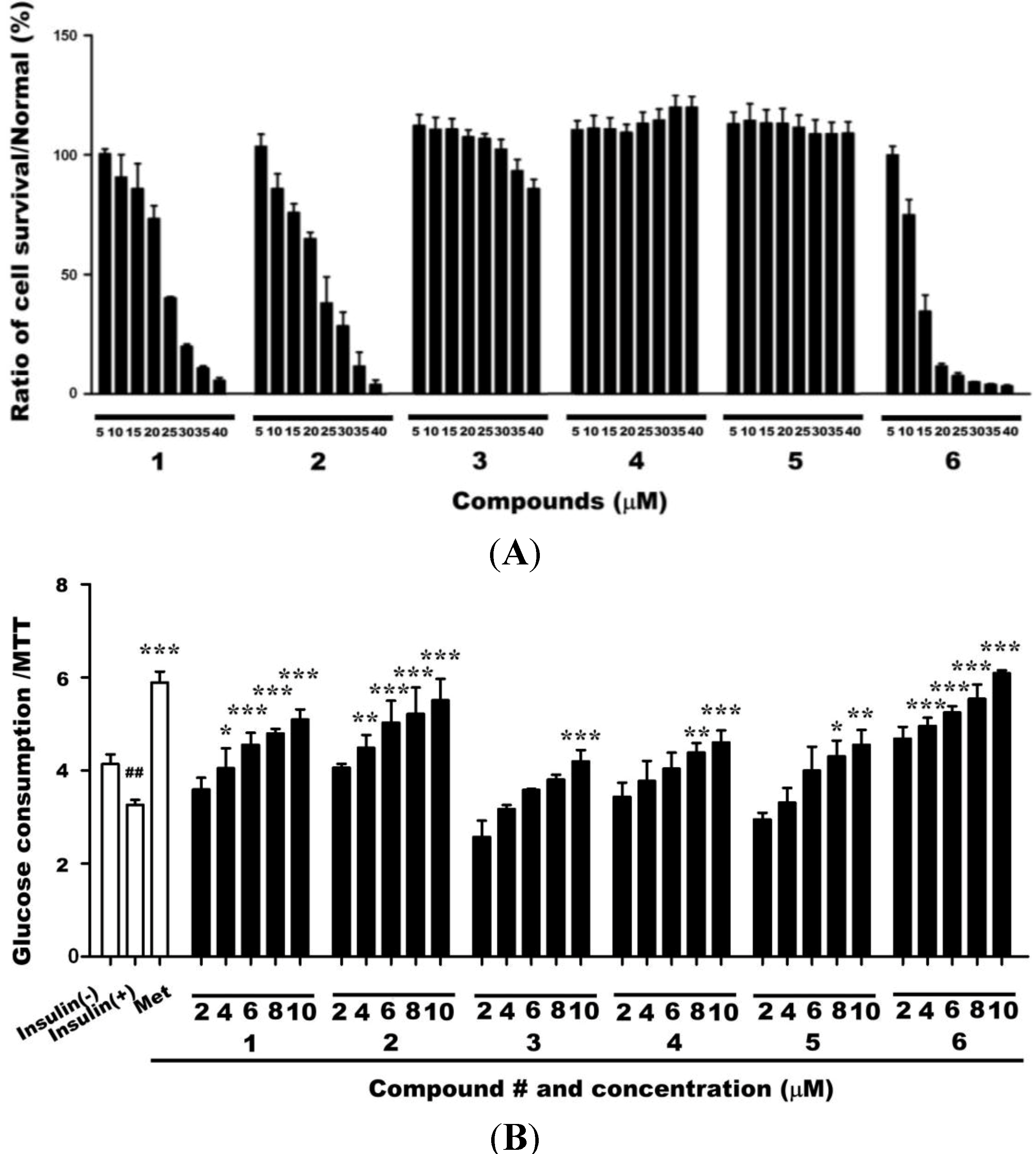
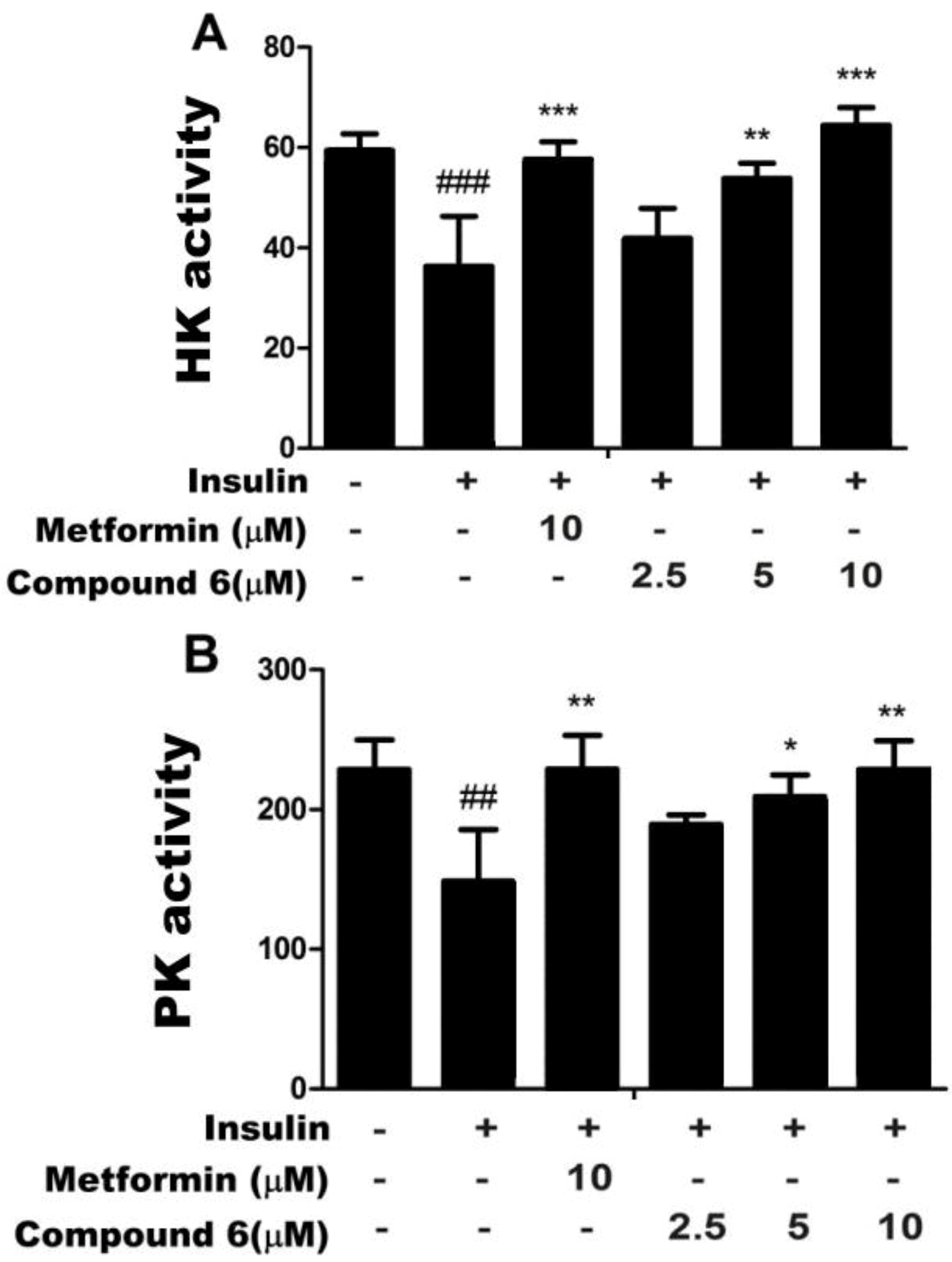
2.2. Effects of Compound 6 on Hexokinase (HK) and PK Activities in Insulin-Resistant HepG2
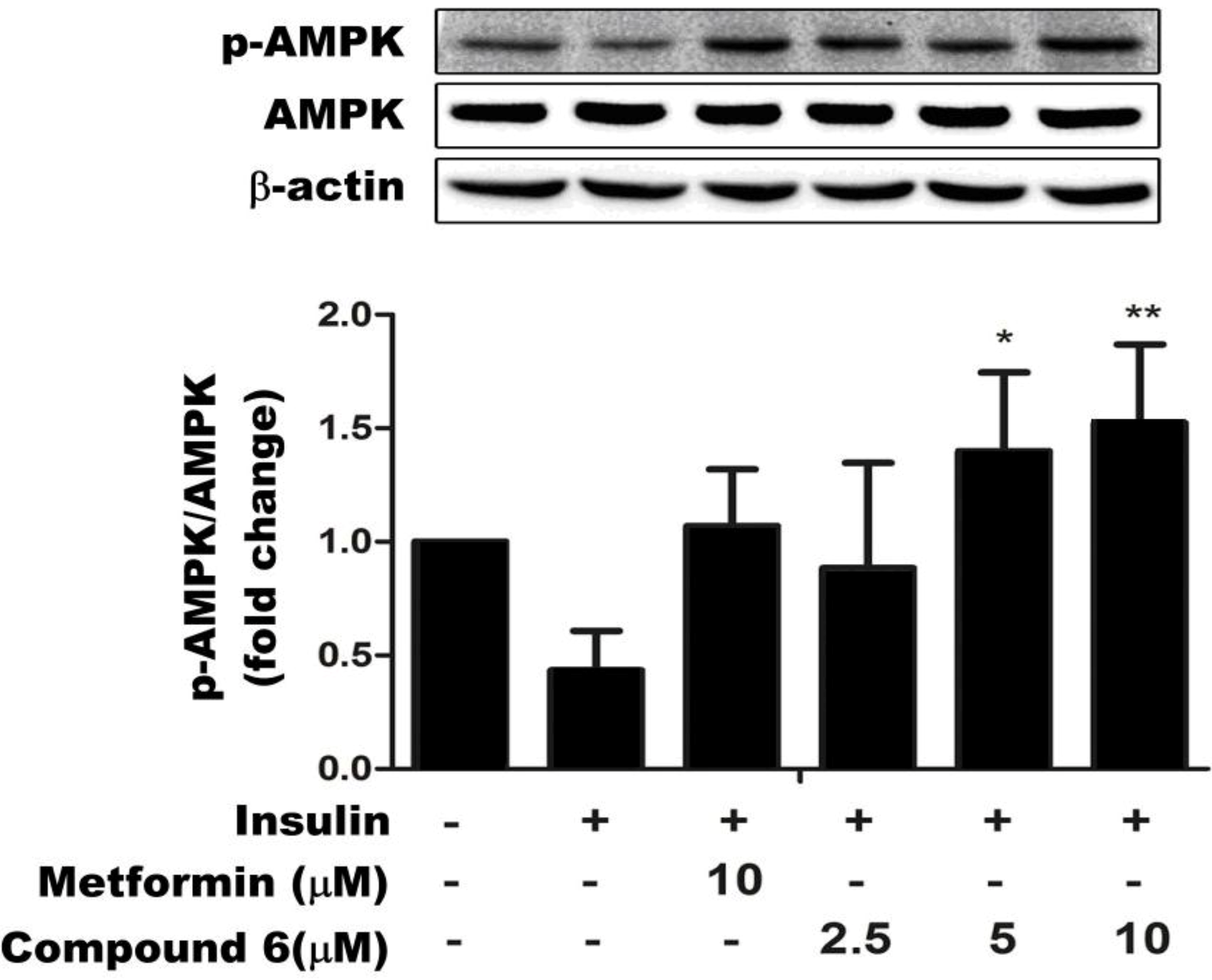
2.3. Compound 6 Activates AMPK Phosphorylation in Insulin-Resistant HepG Cells
2.4. Effects of Compound 6 on the IR/IRS-1/PI3K/Akt Signaling Pathway
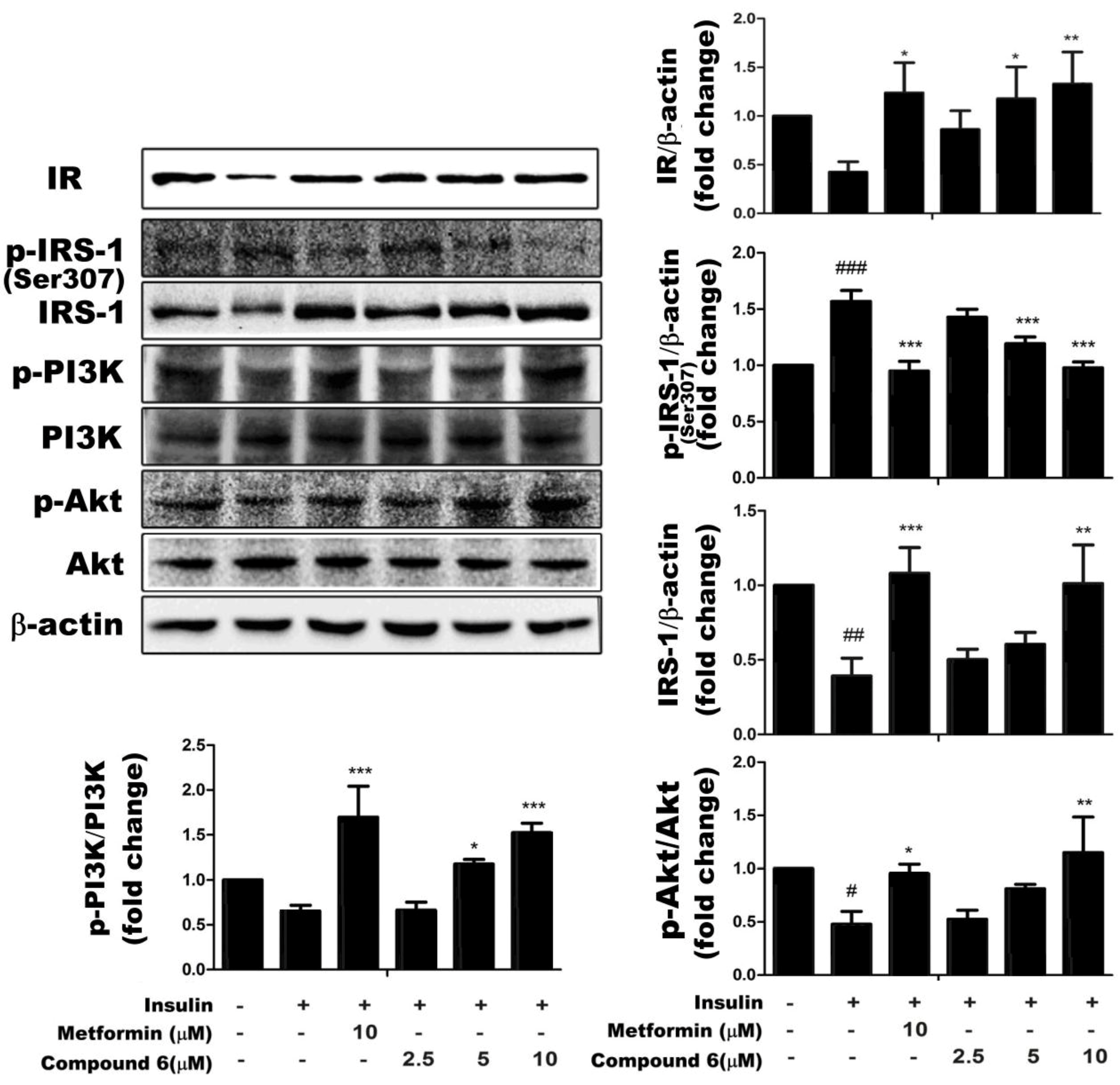
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Induction of Insulin-Resistant HepG2 Cells (HepG2/IR)
4.3. Cell Viability Assay
4.4. Glucose Uptake
4.5. Assays for HK and PK Activities
4.6. Western Blotting
4.7. Statistics and Graphics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Winder, W.W.; Hardie, D.G. AMP-activated protein kinase, ametabolic master switch: Possible roles in Type 2 diabetes. Am. J. Physiol. Endocrinol. MeTable 1999, 277, E1–E10. [Google Scholar]
- Dinneen, S.; Gerich, J.; Rizza, R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1992, 327, 707–713. [Google Scholar] [CrossRef]
- Nelson, B.A.; Robinson, K.A.; Buse, M.G. High glucose and glucosamine induce insulin resistance via different mechanisms in 3T3-L1 adipocytes. Diabetes 2000, 49, 981–991. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Hung, H.Y.; Qian, K.; Morris-Natschke, S.L.; Hsu, C.S.; Lee, K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef]
- Hayashi, T.; Hirshman, M.F.; Kurth, E.J.; Winder, W.W.; Goodyear, L.J. Evidence for 5'AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 1998, 47, 1369–1373. [Google Scholar]
- Lochhead, P.A.; Salt, I.P.; Walker, K.S.; Hardie, D.G.; Sutherland, C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes 2000, 49, 896–903. [Google Scholar] [CrossRef]
- Foretz, M.; Carling, D.; Guichard, C.; Ferré, P.; Foufelle, F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J. Biol. Chem. 1998, 273, 14767–14771. [Google Scholar]
- Hardie, D.G. The AMP-activated protein kinase pathway—New players upstream and downstream. J. Cell Sci. 2004, 117, 5479–5487. [Google Scholar] [CrossRef]
- Kim, Y.D.; Park, K.G.; Lee, Y.S.; Park, Y.Y.; Kim, D.K.; Nedumaran, B.; Jang, W.G.; Cho, W.J.; Ha, J.; Lee, I.K.; et al. Metformin inhibits hepatic gluconeogenesis through AMP activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008, 57, 306–314. [Google Scholar]
- Pawson, T. Protein modules and signaling networks. Nature 1995, 373, 573–580. [Google Scholar] [CrossRef]
- Skolnik, E.Y.; Batzer, A.; Li, N.; Lee, C.H.; Lowenstein, E.; Mohammadi, M.; Schlessinger, J. The function of GRB2 in linking the insulin receptor to ras signaling pathways. Science 1993, 260, 1953–1955. [Google Scholar]
- Prada, P.O.; Coelho, M.S.; Zecchin, H.G.; Dolnikoff, M.S.; Gasparetti, A.L.; Furukawa, L.N.S.; Heimann, J.C. Low salt intake modulates insulin signaling, JNK activity and IRS-1ser307 phosphorylation in rat tissues. J. Endocrinol. 2005, 185, 429–437. [Google Scholar] [CrossRef]
- Jiangsu New Medical College. Zhong Yao Da Ci Dian; Shanghai Scientific and Technical Publishers: Shanghai, China, 1977; p. 2185. [Google Scholar]
- Wang, H.B.; Yu, D.Q.; Liang, X.T.; Watanabe, N.; Tamai, M.; Omura, S. Yemuoside YM7, YM11, YM13 and YM14: Four nortriterpenoid saponins from Stauntonia. chinensis. Planta Med. 1989, 55, 303–306. [Google Scholar] [CrossRef]
- Wang, H.B.; Yu, D.Q.; Liang, X.T.; Watanabe, N.; Tamai, M.; Omura, S. The structures of yemuoside YM10 and YM12 from Stauntonia chinensis. Acta Pharm. Sin. 1989, 24, 444–451. [Google Scholar]
- Wang, H.B.; Yu, D.Q.; Liang, X.T.; Watanabe, N.; Tamai, M.; Omura, S. Structures of two nortriterpenoid saponins from Stauntonia chinensis. J. Nat. Prod. 1990, 53, 313–318. [Google Scholar] [CrossRef]
- Wang, H.B.; Yu, D.Q.; Liang, X.T. Yemuoside I, a new nortriterpenoid glycoside from Stauntonia chinensis. J. Nat. Prod. 1991, 54, 1097–1101. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, X.; Wang, N.L.; Liu, H.W.; Zhang, Q.H.; Song, S.S.; Yu, Y.; Yao, X.S. Triterpenoid saponins from Stauntonia chinensis. J. Asian Nat. Prod. Res. 2007, 9, 175–182. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Z.; Yao, Z.H.; Wu, N.; Dong, H.J.; Li, J.; Wang, N.L.; Ye, W.C.; Yao, X.S. Unusual nortriterpenoid saponins from Stauntonia chinensis. Helv. Chim. Acta 2008, 91, 451–458. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Z.; Yang, L.; Yu, Y.; Yao, Z.H.; Wang, N.L.; Zhou, G.X.; Ye, W.C.; Yao, X.S. Five new bidesmoside triterpenoid saponins from Stauntonia chinensis. Magn. Reson. Chem. 2008, 46, 630–637. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, F.; Chen, G.D.; Chen, S.D.; Yu, Y.; Yao, Z.H.; Lau, B.W.C.; Wang, Z.; Li, J.; Yao, X.S. Bidesmoside triterpenoid glycosides from Stauntonia chinensis and relationship to anti-inflammation. Phytochemistry 2009, 70, 795–806. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, G.P.; Yang, Y.S.; Su, Y.L.; Ji, T.F. A new bidesmoside triterpenoid saponin from Stauntonia chinensis. Chin. Chem. Lett. 2009, 20, 833–835. [Google Scholar] [CrossRef]
- Ha, D.T.; Tuan, D.T.; Thu, N.B.; Nhiem, N.X.; Ngoc, T.M.; Yim, N.; Bae, K. Palbinone and triterpenes from Moutan Cortex (Paeonia suffruticosa, Paeoniaceae) stimulate glucose uptake and glycogen synthesis via activation of AMPK in insulin-resistant human HepG2 Cells. Bioorg. Med. Chem. Lett. 2009, 19, 5556–5559. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Wang, S.; Wang, D.B.; Xu, J.; Yang, G.Z. Triterpenoid saponins from Stauntonia chinensis. Bull. Korean Chem. Soc. 2014, 35, 1212–1214. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.Á.; Goya, L.; Ramos, S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food Chem. Toxicol. 2014, 64, 10–19. [Google Scholar] [CrossRef]
- Hardie, D.G. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008, 582, 81–89. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.Z.; Ou, Z.L.; Yang, F.; Wang, D.B. Studying on anti-inflammatory activity of triterpenoid saponins from Stauntonia chinensis. J. Huazhong Norm. Univ. Nat. Sci. 2013, 52, 348–352. [Google Scholar]
- Yoshikawa, M.; Matsuda, H.; Harada, E.; Murakami, T.; Wariishi, N.; Yamahara, J.; Murakami, N.; Elatoside, E. A new hypoglycemic principle from the root cortex of Aralia elata Seem: Structure-related hypoglycemic activity of oleanolic acid glycosides. Chem. Pharm. Bull. 1994, 42, 1354–1356. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Y.; Wu, H.; Zhu, X.; Zheng, X.; Jiang, S.; Qiu, J. Protective effects of Astragalus saponin I on early stage of diabetic nephropathy in rats. J. Pharmacol. Sci. 2004, 95, 256–266. [Google Scholar] [CrossRef]
- Jeffrey, E.P.; Alan, R.S. Signaling pathways in insulin action: Molecular targets of insulin resistance. J. Clin. Investig. 2000, 106, 165–169. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Czech, M.P.; Corvera, S. Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 1999, 274, 1865–1868. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, X.; Wang, S.; Xu, J.; Wang, D.-B.; Chen, Y.; Yang, G.-Z. Triterpenoid Saponins from Stauntonia chinensis Ameliorate Insulin Resistance via the AMP-Activated Protein Kinase and IR/IRS-1/PI3K/Akt Pathways in Insulin-Resistant HepG2 Cells. Int. J. Mol. Sci. 2014, 15, 10446-10458. https://doi.org/10.3390/ijms150610446
Hu X, Wang S, Xu J, Wang D-B, Chen Y, Yang G-Z. Triterpenoid Saponins from Stauntonia chinensis Ameliorate Insulin Resistance via the AMP-Activated Protein Kinase and IR/IRS-1/PI3K/Akt Pathways in Insulin-Resistant HepG2 Cells. International Journal of Molecular Sciences. 2014; 15(6):10446-10458. https://doi.org/10.3390/ijms150610446
Chicago/Turabian StyleHu, Xin, Sha Wang, Jing Xu, De-Bing Wang, Yu Chen, and Guang-Zhong Yang. 2014. "Triterpenoid Saponins from Stauntonia chinensis Ameliorate Insulin Resistance via the AMP-Activated Protein Kinase and IR/IRS-1/PI3K/Akt Pathways in Insulin-Resistant HepG2 Cells" International Journal of Molecular Sciences 15, no. 6: 10446-10458. https://doi.org/10.3390/ijms150610446
APA StyleHu, X., Wang, S., Xu, J., Wang, D.-B., Chen, Y., & Yang, G.-Z. (2014). Triterpenoid Saponins from Stauntonia chinensis Ameliorate Insulin Resistance via the AMP-Activated Protein Kinase and IR/IRS-1/PI3K/Akt Pathways in Insulin-Resistant HepG2 Cells. International Journal of Molecular Sciences, 15(6), 10446-10458. https://doi.org/10.3390/ijms150610446



