Aerobic Degradation of Trichloroethylene by Co-Metabolism Using Phenol and Gasoline as Growth Substrates
Abstract
:1. Introduction
2. Results and Discussion
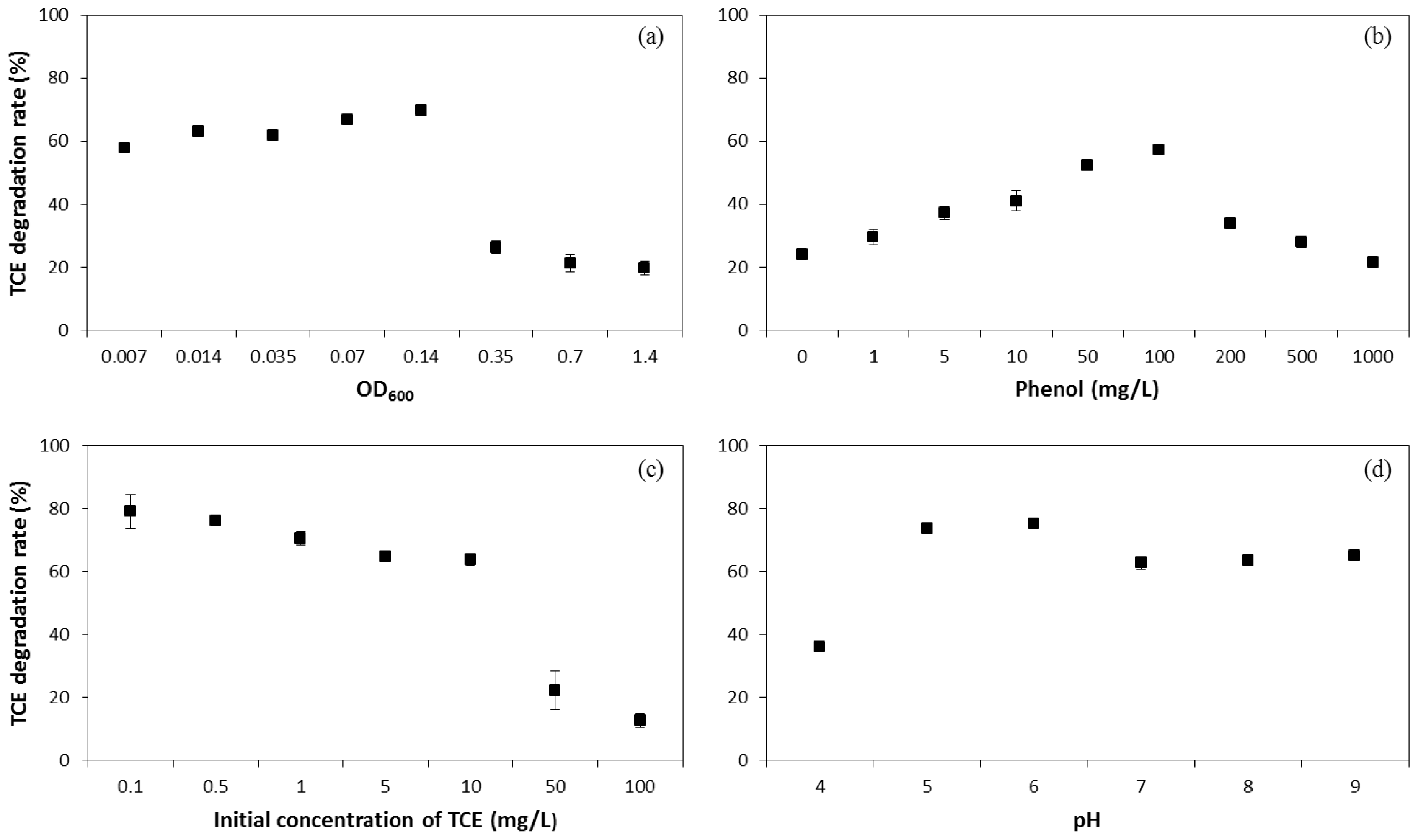
2.1. Influence of Operating Conditions on TCE Co-Metabolic Degradation Efficiency
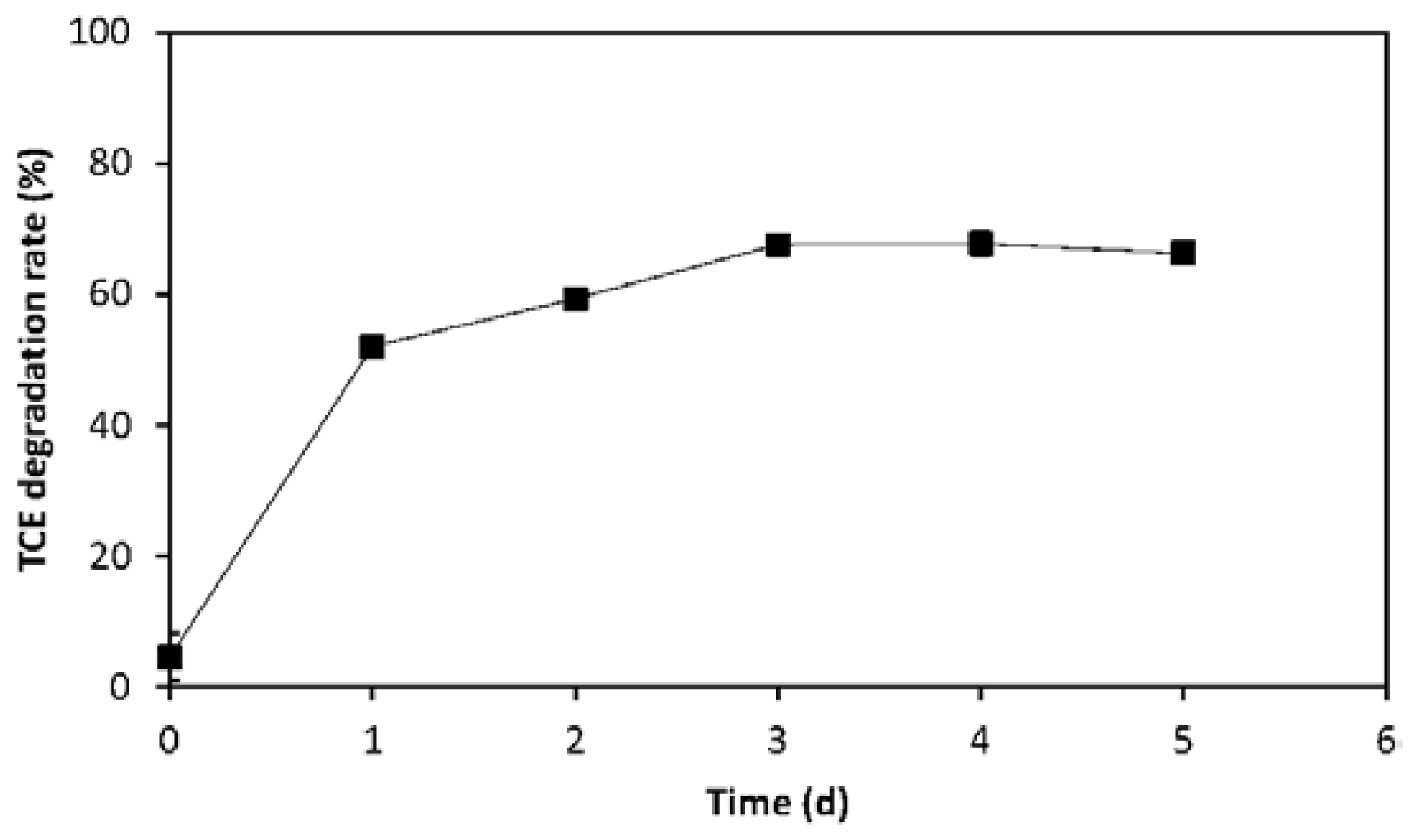
2.2. Evaluation of TCE Transformation Efficiency
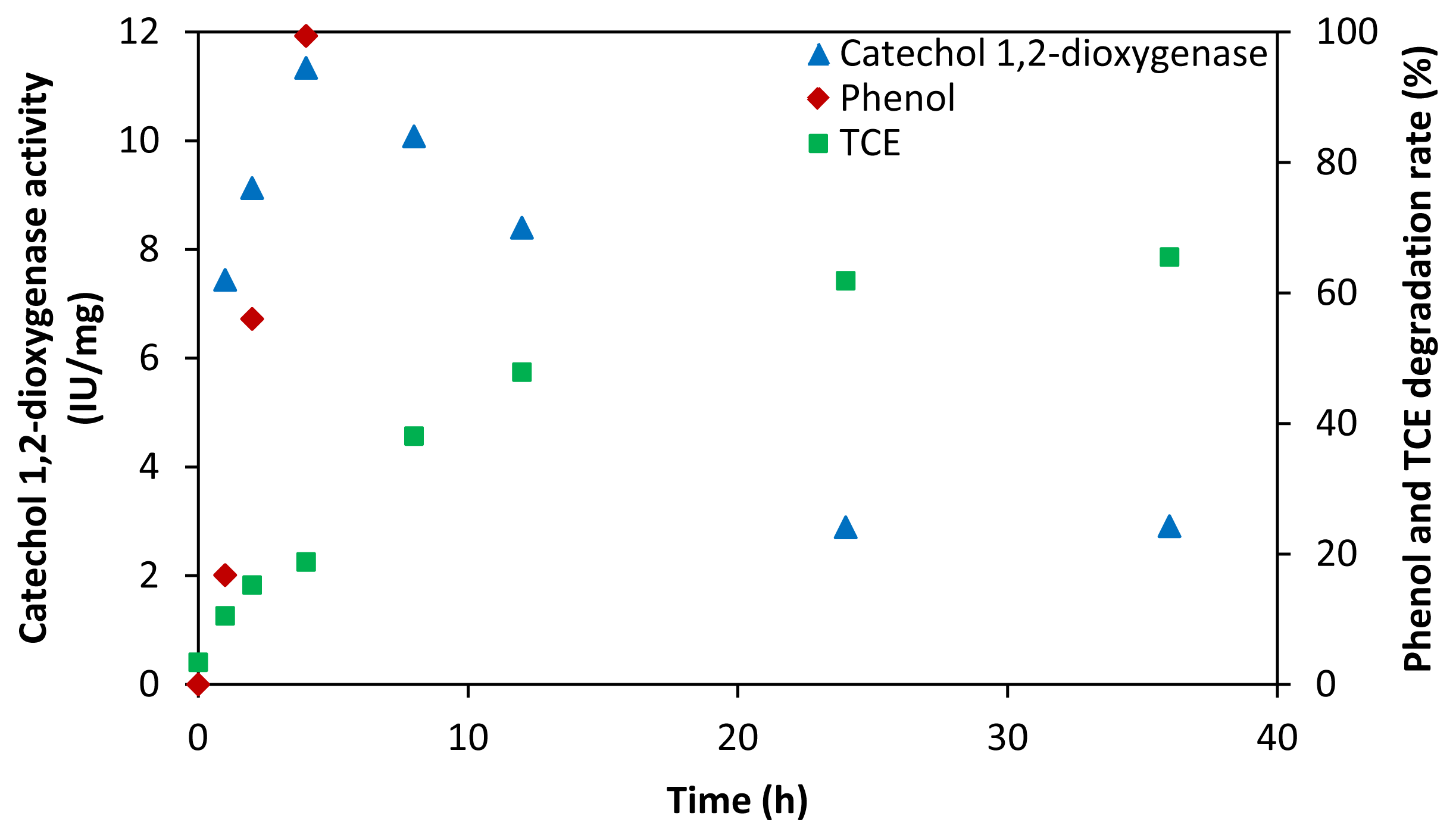
2.3. Key Enzymes and Products in TCE Co-Metabolic Degradation System
2.4. Ternary and Quaternary Co-Metabolic Degradation Systems of Phenol, TCE, Nutrient Broth and Gasoline
3. Experimental Section
3.1. Microorganism Culture
3.2. Culture Media
3.3. Experimental Procedure
3.4. Analytical Method
3.5. Assay of Enzyme Activities
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hopkins, G.D.; McCarty, P.L. Field evaluation of in-situ aerobic cometabolism of trichloroethylene and three dichloroethylene isomers using phenol and toluene as the primary substrates. Environ. Sci. Technol 1995, 29, 1628–1637. [Google Scholar]
- Popat, S.C.; Deshusses, M.A. Reductive Dehalogenation of trichloroethene vapors in an anaerobic biotrickling filter. Environ. Sci. Technol 2009, 43, 7856–7861. [Google Scholar]
- Yan, N. The Study of Groundwater Trichloroethylene Remediation in Siderite Catalyzed Hydrogen Peroxide and Persulfate System. Master’s Thesis, China University of Geosciences, Beijing, China, January 2013. [Google Scholar]
- Zhang, D.Z.; Chen, H.H.; Li, H.M. Halogenated hydrocarbon contaminants in shallow groundwater. Geol. China 2002, 29, 326–329. (In Chinese) [Google Scholar]
- Cao, Y.B. Experimental study on treatment of TCE-contaminated groundwater using potassium permanganate oxidation. Environ. Sci. Technol 2010, 23, 11–13. (In Chinese) [Google Scholar]
- Geng, C.N.; Luo, Q.S.; Chen, M.F.; Li, Z.Y.; Zhang, C.B. Quantitative risk assessment of trichloroethylene for a former chemical works in Shanghai, China. Hum. Ecol. Risk Assess 2010, 16, 429–443. [Google Scholar]
- He, J.T.; Li, Y.; Liu, S.; Chen, H.H. Chlorinate solvents natural biodegradation in shallow groundwater. Environ. Sci 2005, 26, 121–125. (In Chinese) [Google Scholar]
- Erwin, D.P.; Erickson, I.K.; Delwiche, M.E.; Colwell, F.S.; Strap, J.L.; Crawford, R.L. Diversity of oxygenase genes from methane- and ammonia-oxidizing bacteria in the eastern snake river plain aquifer. Appl. Environ. Microbiol 2005, 71, 2016–2025. [Google Scholar]
- Vancheeswaran, S.; Halden, R.U.; Williamson, K.J.; Ingle, J.D.; Semprini, L. Abiotic and biological transformation of tetraalkoxysilanes and trichloroethene/cis-1,2-Dichloroethene cometabolism driven by tetrabutoxysilane-degrading microorganisms. Environ. Sci. Technol 1999, 33, 1077–1085. [Google Scholar]
- Wilson, J.T.; Wilson, B.H. Biotransformation of trichloroethylene in soil. Appl. Environ. Microbiol 1985, 49, 242–243. [Google Scholar]
- Bradley, P.M. History and ecology of chloroethene biodegradation: A review. Bioremed. J 2003, 7, 81–109. [Google Scholar]
- Roberts, P.V.; Hopkins, G.D.; Mackay, D.M.; Semprini, L. A field evaluation of in-situ biodegradation of chlorinated ethenes: Part I, Methodology and field site characterization. Ground Water 1990, 28, 591–604. [Google Scholar]
- Semprini, L.; McCarty, P.L. Comparison between model simulations and field results for in-situ biorestoration of chlorinated aliphatics: Part 2. Cometabolic transformations. Ground Water 1992, 30, 37–44. [Google Scholar]
- Hopkins, G.D.; Semprini, L.; McCarty, P.L. Bioremediation of Hazardous Wastes; EPA Center for Environmental Research Information: Cincinnati, OH, USA, 1992; pp. 71–73. [Google Scholar]
- Hopkins, G.D.; Munakata, J.; Semprini, L.; McCarty, P.L. Trichloroethylene concentration effects on pilot field-scale in-situ groundwater bioremediation by phenol-oxidizing microorganisms. Environ. Sci. Technol 1993, 27, 2542–2547. [Google Scholar]
- Balasubramanian, P.; Philip, L.; Bhallamudi, S.M. Biodegradation of chlorinated and non-chlorinated VOCs from pharmaceutical industry. Appl. Biochem. Biotechnol 2011, 163, 497–518. [Google Scholar]
- Mahendra, S.; Grostern, A.; Alvarez-Cohen, L. The impact of chlorinated solvent co-contaminants on the biodegradation kinetics of 1,4-dioxane. Chemosphere 2013, 91, 88–92. [Google Scholar]
- Brown, R.A. Compound types in gasoline by mass spectrometer analysis. Anal. Chem 1951, 23, 430–437. [Google Scholar]
- Shim, H.; Ma, W.; Lin, A.J.; Chan, K. Bio-removal of mixture of benzene, toluene, ethylbenzene, and xylenes/total petroleum hydrocarbons/trichloroethylene from contaminated water. J. Environ. Sci 2009, 21, 758–763. [Google Scholar]
- Sui, H.; Li, X.G.; Huang, G.Q.; Jiang, B. A study on cometabolic bioventing for the in situ remediation of trichloroethylene. Environ. Geochem. Health 2006, 28, 147–152. [Google Scholar]
- Chang, W.K.; Criddle, C.S. Experimental evaluation of a model for cometabolism: Prediction of simultaneous degradation of trichloroethylene and methane by a methanotrophic mixed culture. Biotechnol. Bioeng 1997, 56, 492–501. [Google Scholar]
- Chang, H.L.; Alvarez-Cohen, L. Transformation capacities of chlorinated organics by mixed cultures enriched on methane, propane, toluene, or phenol. Biotechnol. Bioeng 1995, 45, 440–449. [Google Scholar]
- Landa, A.S.; Sipkema, E.M.; Weijma, J.; Beenackers, A.A.; Dolfing, J.; Janssen, D.B. Cometabolic degradation of trichloroethylene by Pseudomonas cepacia G4 in a chemostat with toluene as the primary substrate. Appl. Environ. Microbiol 1994, 60, 3368–3374. [Google Scholar]
- Chen, Y.M.; Lin, T.F.; Huang, C.; Lin, J.C. Cometabolic degradation kinetics of TCE and phenol by Pseudomonas putida. Chemosphere 2008, 72, 1671–1680. [Google Scholar]
- Liu, F.Y. The Screening of Endophytic Bacteria for Biological Control of Wheat Sharp Eyespot. Master’s Thesis, Henan University, Kaifeng, China, May 2008. [Google Scholar]
- Brivio, M.; Schlosrich, J.; Ahmad, M.; Tolond, C.; Bugg, T.D.H. Investigation of acid–base catalysis in the extradiol and intradiol catechol dioxygenase reactions using a broad specificity mutant enzyme and model chemistry. Org. Biomol. Chem 2009, 7, 1368–1373. [Google Scholar]
- Silva, A.S.; Jacques, R.J.S.; Andreazza, R.; Bento, F.M.; Roesch, L.F.W.; Camargo, F.A.O. Properties of catechol 1,2-dioxygenase in the cell free extract and immobilized extract of Mycobacterium fortuitum. Braz. J. Microbiol 2013, 44, 291–297. [Google Scholar]
- Wang, S.; Loh, K. New cell growth pattern on mixed substrates and substrate utilization in co-metabolic transformation of 4-chlorophenol. Water Res 2000, 34, 3786–3794. [Google Scholar]
- Arp, D.J.; Yeager, C.M.; Hyman, M.R. Molecular and cellular fundamentals of aerobic cometabolism of trichloroethylene. Biodegradation 2001, 12, 81–103. [Google Scholar]
- Elango, V.; Kurtz, H.D.; Freedman, D.L. Aerobic cometabolism of trichloroethene and cis-dichloroethene with benzene and chlorinated benzenes as growth substrates. Chemosphere 2011, 84, 247–253. [Google Scholar]
- Suttinun, O.; Mueller, R.; Luepromchai, E. Trichloroethylene cometabolic degradation by Rhodococcus sp. L4 induced with plant essential oils. Biodegradation 2009, 20, 281–291. [Google Scholar]
- Liang, S.H.; Liu, J.K.; Lee, K.H.; Kuo, Y.C.; Kao, C.M. Use of specific gene analysis to assess the effectiveness of surfactant-enhanced trichloroethylene. J. Hazard. Mater 2011, 198, 323–330. [Google Scholar]
- Ensign, S.A.; Hyman, M.R.; Arp, D.J. Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene-grown Xanthobacter strain. Appl. Environ. Microbiol 1992, 58, 3038–3046. [Google Scholar]
- Futamata, H.; Harayama, S.; Watanabe, K. Group-specific monitoring of phenol hydroxylase genes for a functional assessment of phenol-stimulated trichloroethylene bioremediation. Appl. Environ. Microbiol 2001, 67, 4671–4677. [Google Scholar]
- Lontoh, S.; Semrau, J.D. Methane and trichloroethylene degradation by Methylosinus trichosporium OB3b expressing particulate methane monooxygenase. Appl. Environ. Microbiol 1998, 64, 1106–1114. [Google Scholar]
- Brusseau, G.A.; Tsien, H.C.; Hanson, R.S.; Wackett, L.P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble methane monooxygenase activity. Biodegradation 1990, 1, 19–29. [Google Scholar]
- Wackett, L.P.; Gibson, D.T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl. Environ. Microbiol 1988, 54, 1703–1708. [Google Scholar]
- Newman, L.M.; Wackett, L.P. Trichloroethylene oxidation by purified toluene 2-monooxygenase: Products, kinetics, and turnover-dependent inactivation. J. Bacteriol 1997, 179, 90–96. [Google Scholar]
- Bugg, T.D.H.; Winfield, C.J. Enzymatic cleavage of aromatic rings: mechanistic aspects of the catechol dioxygenases and later enzymes of bacterial oxidative cleavage pathways. Nat. Prod. Rep 1998, 15, 513–530. [Google Scholar]
- Dagley, S. A biochemical approach to some problems of environmental pollution. Essays Biochem 1975, 11, 81–138. [Google Scholar]
- Hayaishi, O.; Katagiri, M.; Rothberg, S. Mechanism of the pyrocatechase reaction. J. Am. Chem. Soc 1955, 77, 5450–5451. [Google Scholar]
- Kojima, Y.; Itada, N.; Hayaishi, O. Metapyrocatachase: A new catechol-cleaving enzyme. J. Biol. Chem 1961, 236, 2223–2228. [Google Scholar]
- Hayaishi, O. Crystalline oxygenases of pseudomonads. Bacteriol. Rev 1966, 30, 720–731. [Google Scholar]
- Kneidel, A.L.; Yang, S.T. Kinetic study of trichloroethylene biodegradation by Methylosinus trichosporium OB3b PP358 immobilized in a fibrous-bed bioreactor. J. Chin. Inst. Chem. Eng 2003, 34, 65–73. [Google Scholar]
- Gossett, J.M. Sustained aerobic oxidation of vinyl chloride at low oxygen concentrations. Environ. Sci. Technol 2010, 44, 1405–1411. [Google Scholar]
- Frascari, D.; Fraraccio, S.; Nocentini, M.; Pinelli, D. Aerobic/anaerobic/aerobic sequenced biodegradation of a mixture of chlorinated ethenes, ethanes and methanes in batch bioreactors. Bioresour. Technol 2013, 128, 479–486. [Google Scholar]
- Frascari, D.; Zanaroli, G.; Bucchi, G.; Rosato, A.; Tavanaie, N.; Fraraccio, S.; Pinelli, D.; Fava, F. Trichloroethylene aerobic cometabolism by suspended and immobilized butane-growing microbial consortia: A kinetic study. Bioresour. Technol 2013, 144, 529–538. [Google Scholar]
- Chang, M.; Voice, T.C.; Criddle, C.S. Kinetics of competitive inhibition and cometabolism in the biodegradation of benzene, toluene, and p-xylene by two Pseudomonas isolates. Biotechnol. Bioeng 1993, 41, 1057–1065. [Google Scholar]
- Tobajas, M.; Monsalvo, V.M.; Mohedano, A.F.; Rodriguez, J.J. Enhancement of cometabolic biodegradation of 4-chlorophenol induced with phenol and glucose as carbon sources by C. testosteroni. J. Environ. Manag 2012, 95, 116–121. [Google Scholar]
- Tao, X.Q.; Lu, G.N.; Dang, Z.; Yang, C.; Yi, X.Y. A phenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Process Biochem 2007, 42, 401–408. [Google Scholar]
- Liu, Z.; Yang, H.; Huang, Z.; Zhou, P.; Liu, S.J. Degradation of aniline by newly isolated, extremely aniline-tolerant Delftia sp. AN3. Appl. Microbiol. Biotechnol 2002, 58, 679–682. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]

| Substrates | G. 10 mg/L | G. 50 mg/L | G. 100 mg/L | |||
|---|---|---|---|---|---|---|
| TCE | G. | TCE | G. | TCE | G. | |
| TCE + G. | 23.9 ± 0.8 | 81.3 ± 1.6 | 24.9 ± 0.5 | 28.5 ± 0.8 | 28.5 ± 0.4 | 20.7 ± 0.4 |
| TCE + G. + P. | 58.8 ± 1.1 | 68.8 ± 1.2 | 44.9 ± 0.8 | 18.0 ± 0.3 | 47.0 ± 0.8 | 21.6 ± 0.5 |
| TCE + G. + NB | 23.7 ± 0.5 | 31.3 ± 0.9 | 25.1 ± 0.5 | 21.1 ± 0.5 | 25.1 ± 0.6 | 15.2 ± 0.5 |
| TCE + G. + P. + NB | 45.0 ± 0.9 | 33.3 ± 1.1 | 44.5 ± 0.9 | 8.8 ± 0.1 | 32.5 ± 0.9 | 5.5 ± 0.1 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Li, B.; Wang, C.-P.; Fan, J.-Z.; Sun, H.-W. Aerobic Degradation of Trichloroethylene by Co-Metabolism Using Phenol and Gasoline as Growth Substrates. Int. J. Mol. Sci. 2014, 15, 9134-9148. https://doi.org/10.3390/ijms15059134
Li Y, Li B, Wang C-P, Fan J-Z, Sun H-W. Aerobic Degradation of Trichloroethylene by Co-Metabolism Using Phenol and Gasoline as Growth Substrates. International Journal of Molecular Sciences. 2014; 15(5):9134-9148. https://doi.org/10.3390/ijms15059134
Chicago/Turabian StyleLi, Yan, Bing Li, Cui-Ping Wang, Jun-Zhao Fan, and Hong-Wen Sun. 2014. "Aerobic Degradation of Trichloroethylene by Co-Metabolism Using Phenol and Gasoline as Growth Substrates" International Journal of Molecular Sciences 15, no. 5: 9134-9148. https://doi.org/10.3390/ijms15059134




