Chemical Characterization and Antitumor Activities of Polysaccharide Extracted from Ganoderma lucidum
Abstract
:1. Introduction
2. Results and Discussion
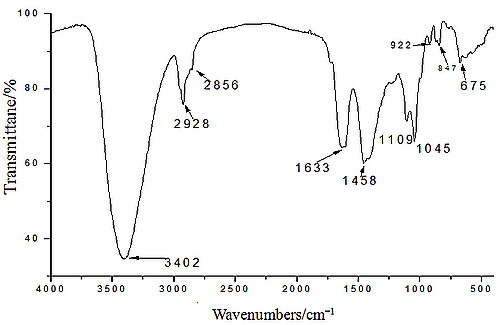
2.1. Yield, Purity and Characterization of Polysaccharide
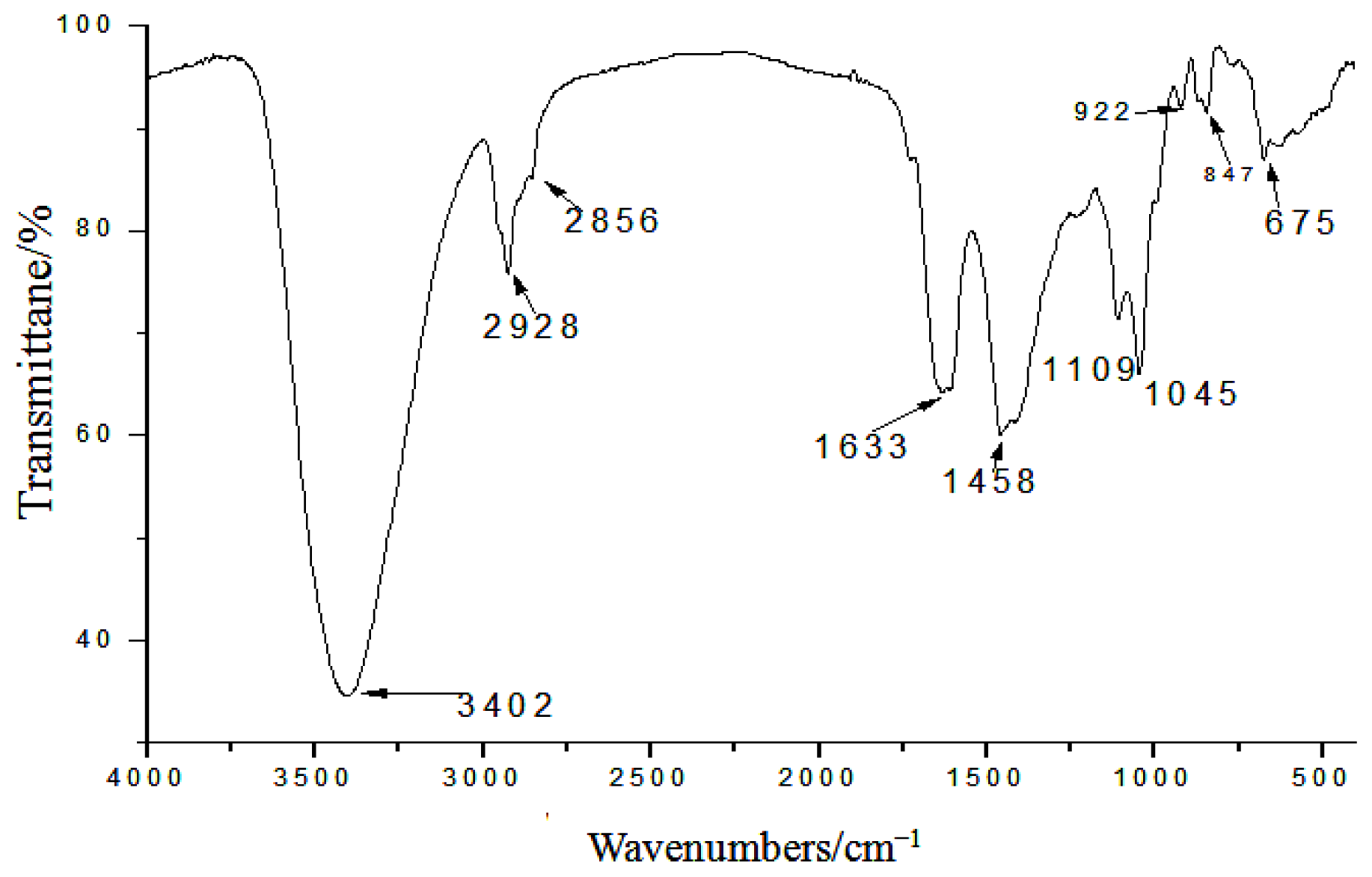
2.2. Effects of Ganoderma lucidum Polysaccharide (GLP) on HCT-116 Cell Viability
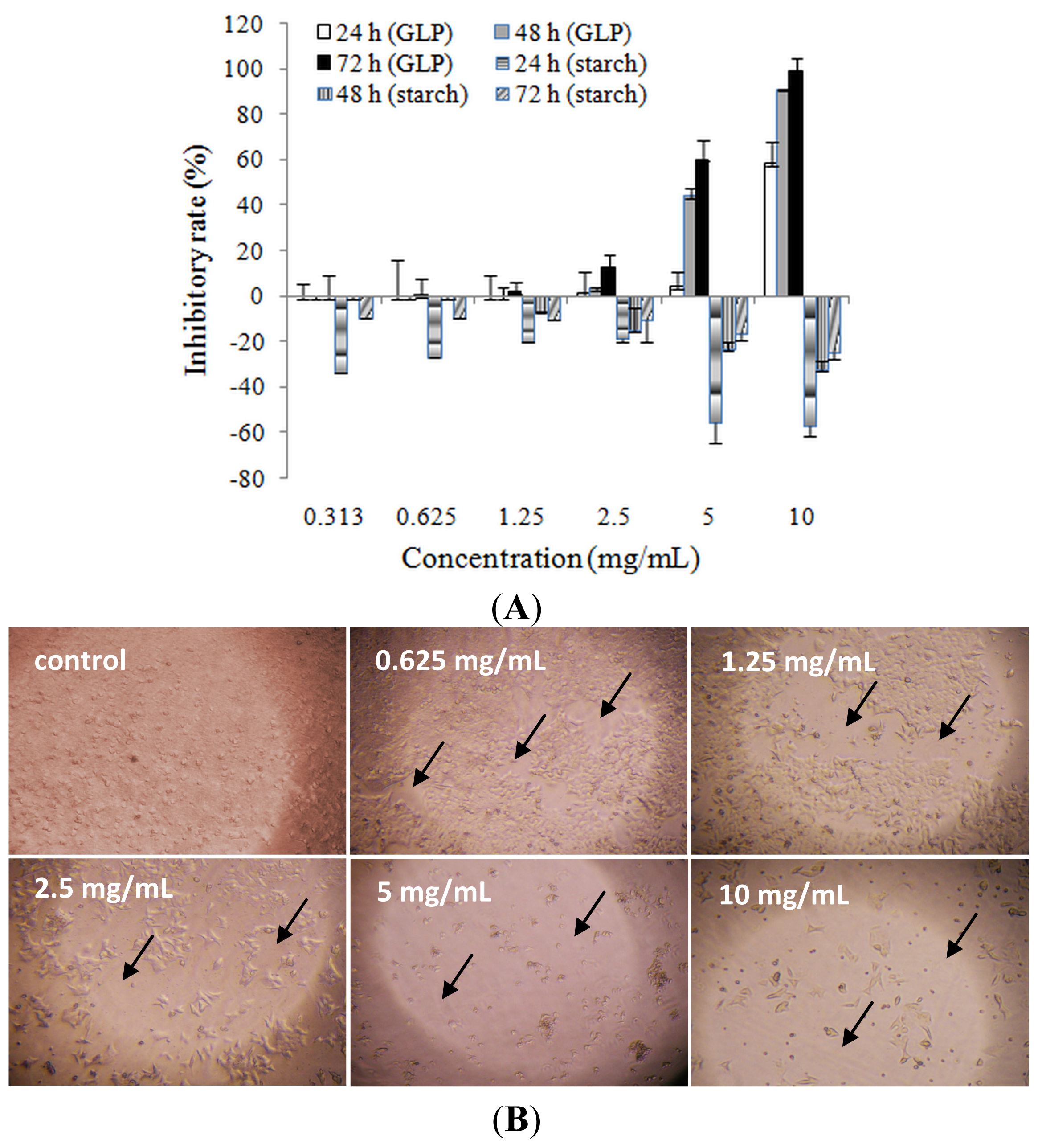
2.3. Effects of GLP on HCT-116 Cell Apoptosis
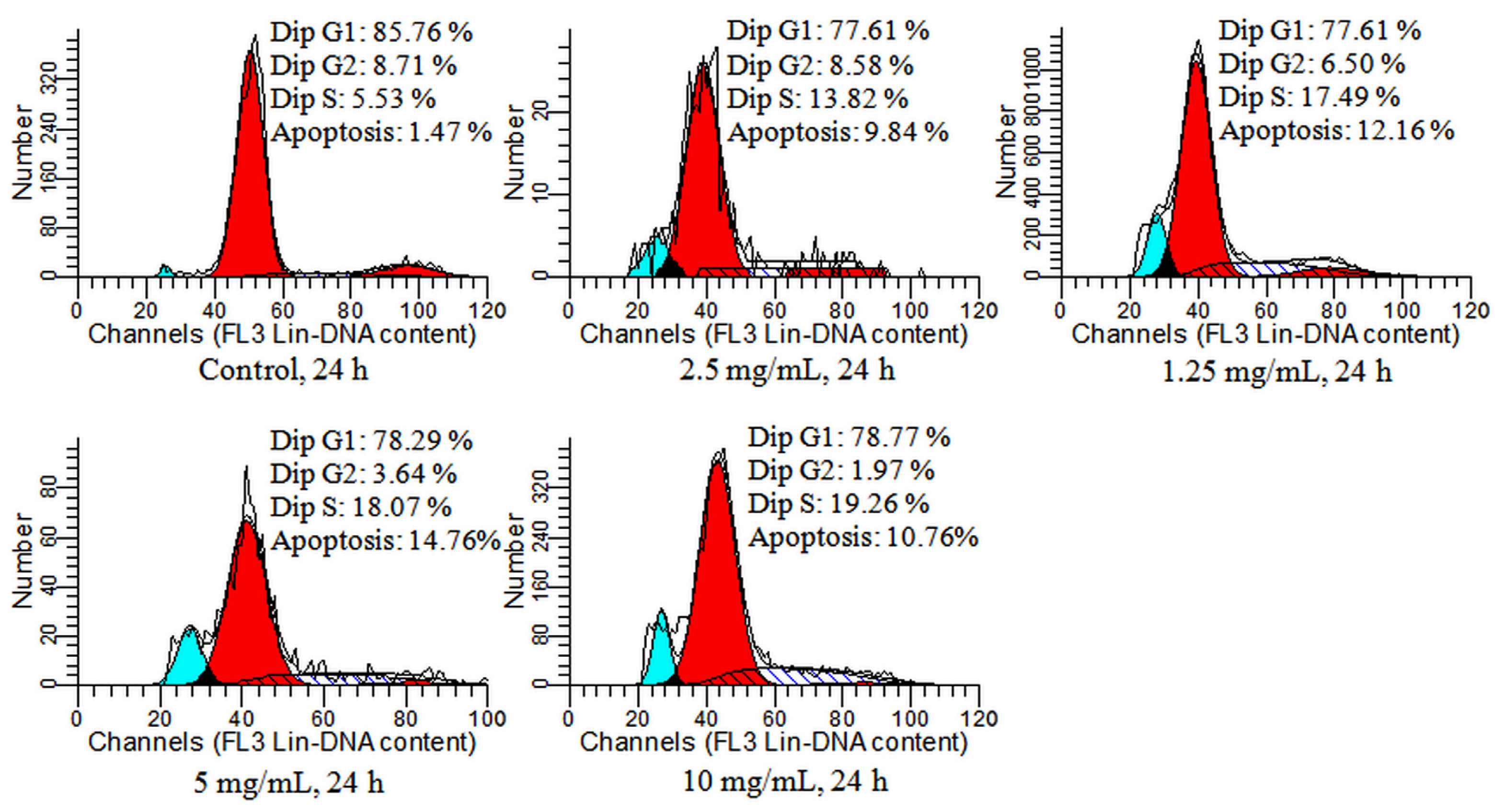
2.4. Detection of Cell Cycle and Apoptosis by Flow Cytometry
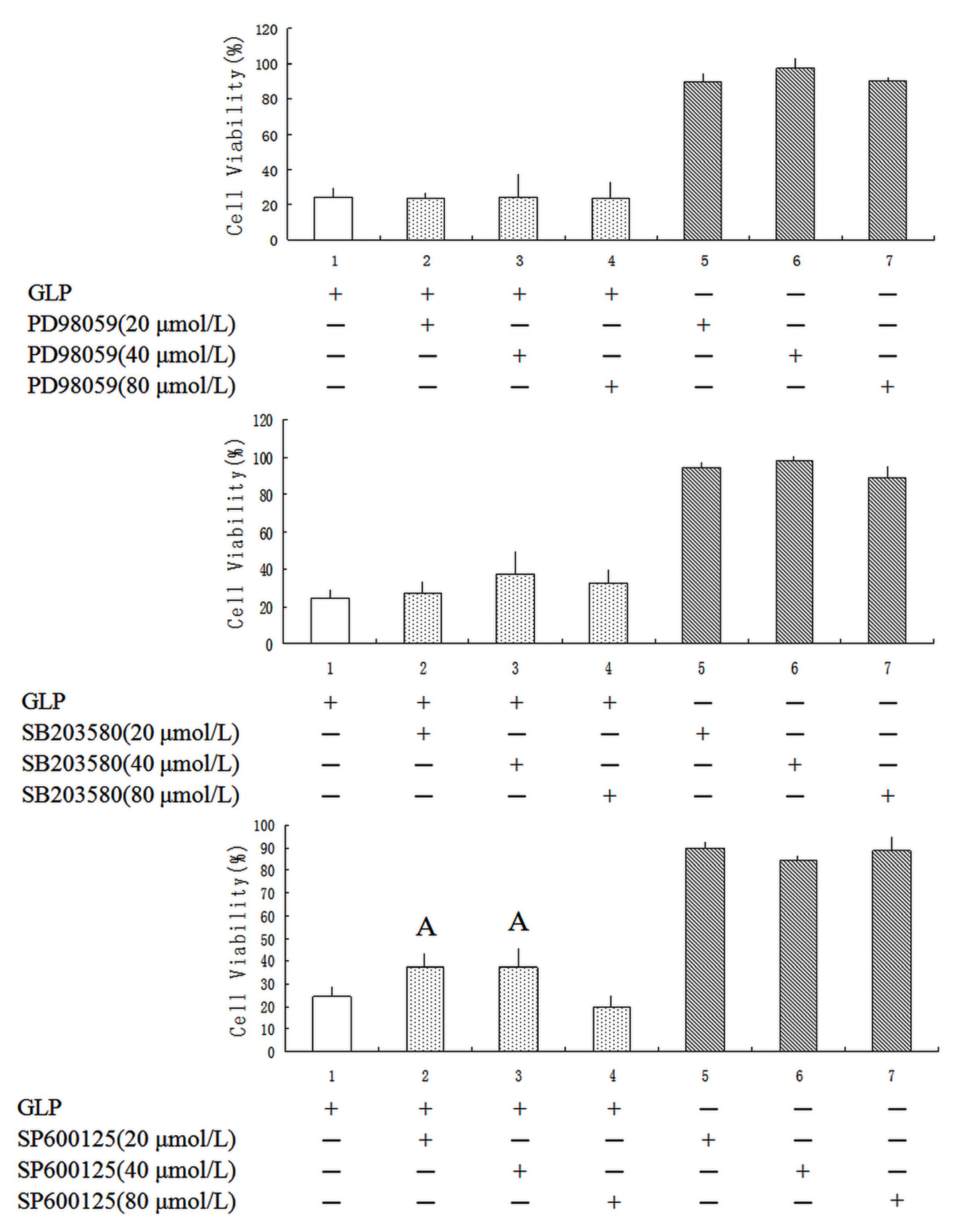
2.5. Effects of GLP on the Mitogen-Activated Protein Kinase (MAPK) Pathway
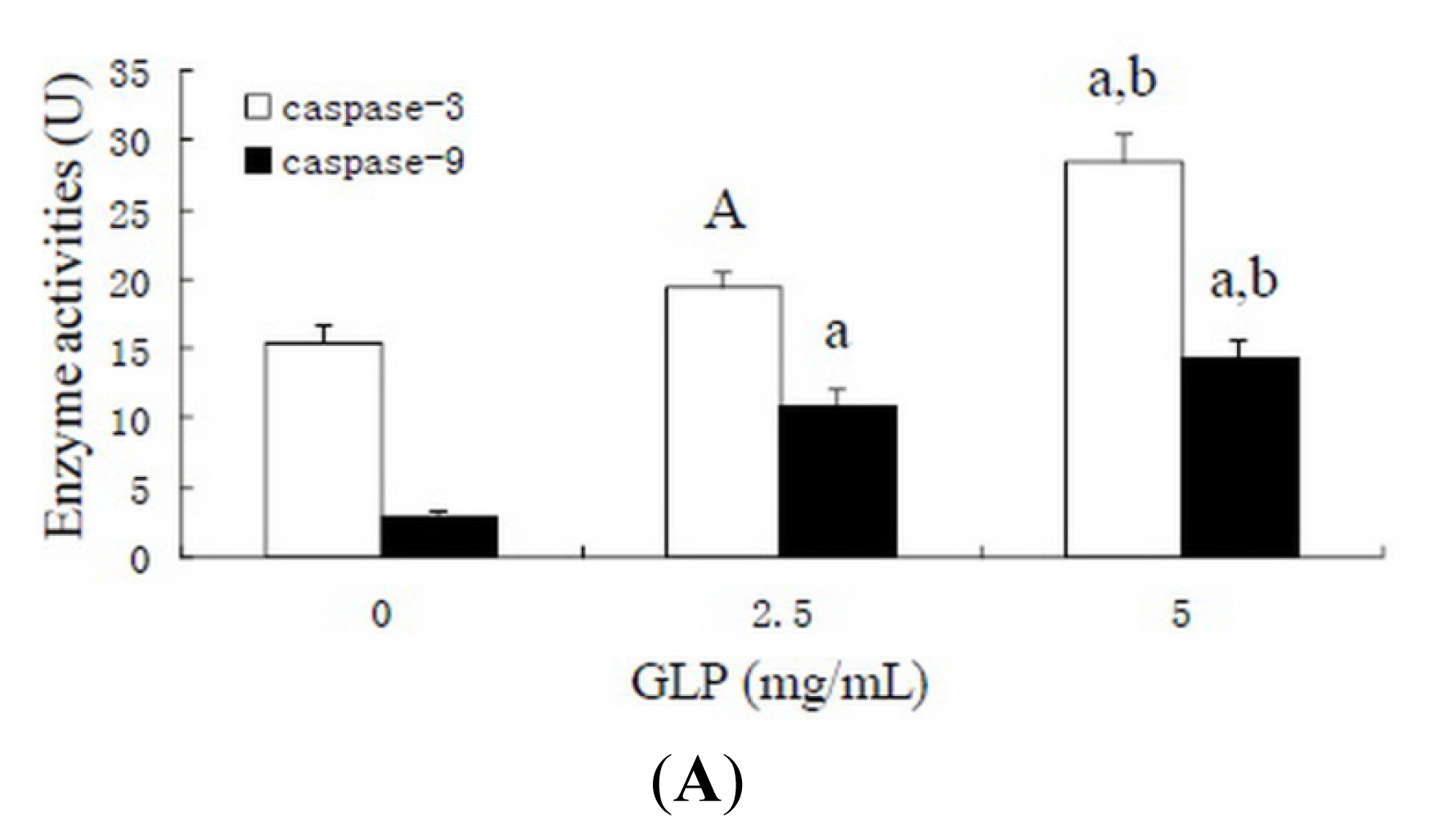
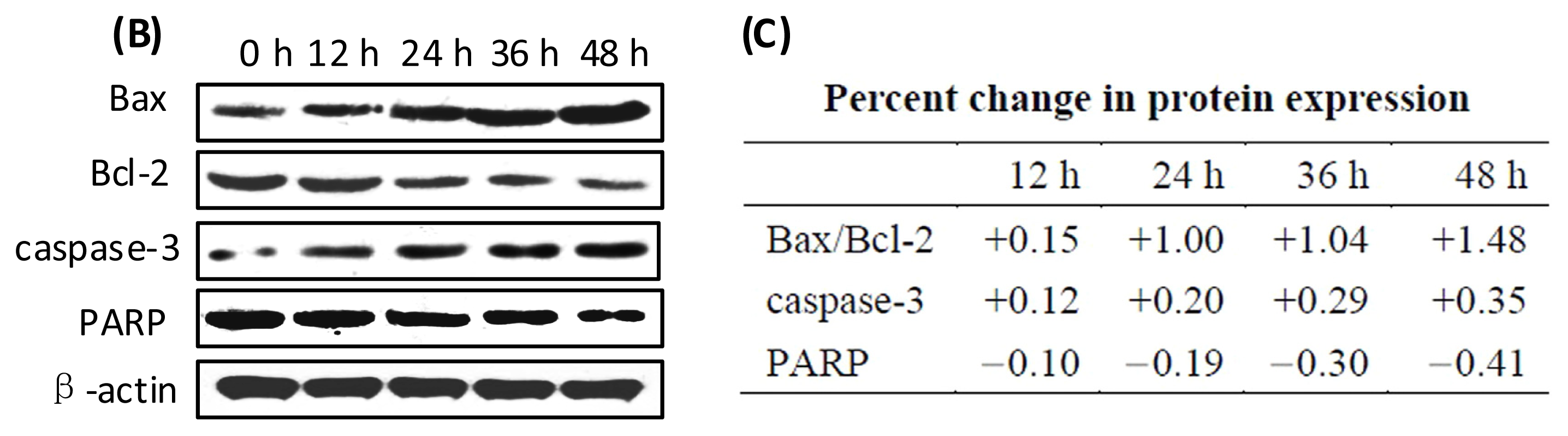
2.6. Effects of GLP on Apoptosis-Related Proteins
3. Experimental Section
3.1. Materials
3.2. Isolation and Purification of GLP
3.3. Chemical Properties
3.4. Cell Viability Assay
3.5. Hoechst 33258 Staining
3.6. Measurement of DNA Fragmentation
3.7. Mitochondrial Membrane Potential Analysis
3.8. Caspase Activity Assay
3.9. Flow Cytometry
3.10. MAPK Inhibitors Assay
3.11. Western Blot Analysis
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsXingyao Xiong, Youjin Yi, Rencai Wang and Zengenni Liang conceived and designed the study. Zengenni Liang, Yutong Guo and Qiulong Hu performed the experiments. Zengenni Liang wrote the paper. Xingyao Xiong, Rencai Wang, and Youjin Yi reviewed and edited the manuscript. All authors read and approved the manuscript.
References
- Atkin, W.S.; Edwards, R.; Kralj-Hans, I.; Wooldrage, K.; Hart, A.R.; Northover, J.M.; Parkin, D.M.; Wardle, J.; Duffy, S.W.; Cuzick, J. -only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet 2010, 375, 1624–1633. [Google Scholar]
- Cutsem, E.V.; Nordlinger, B.; Cervantes, A. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann. Oncol 2010, 21, 93–97. [Google Scholar]
- Siegel, R.; Desantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin 2012, 62, 220–241. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin 2012, 62, 10–29. [Google Scholar]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res 2011, 30, 87–100. [Google Scholar]
- Kim, K.N.; Heo, S.J.; Kang, SM.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol in Vitro 2010, 24, 1648–1654. [Google Scholar]
- Sykiotis, G.P.; Papavassiliou, A.G. Apoptosis: The suicide solution in cancer treatment and chemoprevention. Expert Opin. Investig. Drug 2006, 15, 575–577. [Google Scholar]
- Huerta, S.; Goulet, E.J.; Livingston, E.H. Colon cancer and apoptosis. Am. J. Surg 2006, 191, 517–526. [Google Scholar]
- Chen, X.P.; Wang, W.X.; Li, S.B.; Xue, J.L.; Fan, L.J.; Sheng, Z.J.; Chen, Y.G. Optimization of ultrasound-assisted extraction of Lingzhi polysaccharides using response surface methodology and its inhibitory effects on cervical cancer cells. Carbohydr. Polym 2010, 80, 944–948. [Google Scholar]
- Xu, Z.T.; Chen, X.P.; Zhong, Z.F.; Chen, L.D.; Wang, Y.T. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med 2011, 39, 15–27. [Google Scholar]
- Sun, L.X.; Lin, Z.B.; Li, X.J.; Li, I.; Lu, J.; Duan, X.S.; Ge, Z.H.; Song, Y.X.; Xing, E.H.; Li, W.D. Promoting effects of Ganoderma lucidum polysaccharides on B16F10 cells to activate lymphocytes. Basic Clin. Pharmacol 2011, 108, 149–154. [Google Scholar]
- Li, F.L.; Zhang, Y.M.; Zhong, Z.J. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int. J. Mol. Sci 2011, 12, 6135–6145. [Google Scholar]
- Li, S.G.; Zhang, Y.Q. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohydr. Polym 2009, 78, 343–348. [Google Scholar]
- Xu, L.; Li, H.G.; Xu, Z.Y.; Wang, Z.Q.; Liu, L.S.; Tian, J.H.; Sun, J.; Zhou, L.; Yao, Y.; Jiao, L.; et al. Multi-center randomized double-blind controlled clinical study of chemotherapy combined with or without traditional Chinese medicine on quality of life of postoperative non-small cell lung cancer patients. BMC Complement. Altern. Med 2012, 12, 112. [Google Scholar]
- Chan, K.K.; Yao, T.J.; Jones, B.; Jones, J.F.; Zhao, F.K.; Leung, C.Y.; Lau, S.K.; Yip, M.W.; Ngan, H.Y. The use of Chinese herbal medicine to improve quality of life in women undergoing chemotherapy for ovarian cancer: A double-blind placebo-controlled randomized trial with immunological monitoring. Ann. Oncol 2011, 22, 2241–2249. [Google Scholar]
- Lam, W.; Bussom, S.; Guan, F.; Jiang, Z.; Zhang, W.; Gullen, E.A.; Liu, S.H.; Cheng, Y.C. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci. Transl. Med 2010, 2, 45–59. [Google Scholar]
- Liang, Z.; Yi, Y.J.; Guo, Y.T.; Wang, R.C.; Xiong, X.Y. Effect of combined Ganoderma lucidum polysaccharides and fluorouracil on proliferation and apoptosis in human colon carcinoma HCT-116 cells. Food Sci 2012, 33, 310–314. [Google Scholar]
- Li, Y.Q.; Fang, L.; Zhang, K.C. Structure and bioactivities of a galactose rich extracellular polysaccharide from submergedly cultured Ganoderma lucidum. Carbohydr. Polym 2008, 77, 323–328. [Google Scholar]
- Ram, V.J.; Kumari, S. Natural products of plant origin as anticancer agents. Drug News Perspect 2001, 14, 465–482. [Google Scholar]
- Hu, Q.P.; Xu, J.G. Active components and antimicrobial activity in two Sedum species grown in Shanxi Huoshan, China. J. Food Agric. Environ 2012, 10, 172–174. [Google Scholar]
- Dai, Y.; Grant, S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin. Cancer Res 2010, 16, 376–383. [Google Scholar]
- Kuerbitz, S.J.; Plunkett, B.S.; Waklsh, W.V.; Kastan, M.B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc. Natl. Acad. Sci. USA 1992, 89, 7491–7495. [Google Scholar]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar]
- Ahcene, B.; Xavier, R.; Jean, B. Chalcones derivatives acting as cell cycle blockers: Potential anti cancer drugs? Curr. Drug Targets 2009, 10, 363–371. [Google Scholar]
- Li, J.J.; Lei, L.S.; Yu, C.L.; Zhu, Z.G.; Zhang, Q.; Wu, S.G. Effect of Ganoderma lucidum polysaccharides on tumor cell nucleotide content and cell cycle in S180 ascitic tumor-bearing mice. J. South Med. Univ 2007, 27, 1003–1005. [Google Scholar]
- Hsu, J.W.; Huang, H.C.; Chen, S.T.; Wong, C.H.; Juan, H.F. Ganoderma lucidum polysaccharides induce macrophage-like differentiation in human leukemia THP-1 cells via caspase and p53 activation. Evid-Based Complement. Altern 2011, 2011, 1–13. [Google Scholar]
- Tidyman, W.E.; Rauen, K.A. The RASopathies: Developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev 2009, 19, 230–236. [Google Scholar]
- Olson, M.; Kornbluth, S. Mitochondria in apoptosis and human disease. Curr. Mol. Med 2001, 1, 91–122. [Google Scholar]
- Sharma, S.; Singh, R.L.; Kakkar, P. Modulation of Bax/Bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem. Toxicol 2011, 49, 770–779. [Google Scholar]
- Soldani, C.; Scovassi, A.I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis 2002, 7, 321–328. [Google Scholar]
- Shang, D.J.; Li, Y.; Wang, C.; Wang, X.M.; Yu, Z.; Fu, X.A. Novel polysaccharide from Se-enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol. Rep 2011, 25, 267–272. [Google Scholar]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2010, 477, 107–110. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, Z.; Yi, Y.; Guo, Y.; Wang, R.; Hu, Q.; Xiong, X. Chemical Characterization and Antitumor Activities of Polysaccharide Extracted from Ganoderma lucidum. Int. J. Mol. Sci. 2014, 15, 9103-9116. https://doi.org/10.3390/ijms15059103
Liang Z, Yi Y, Guo Y, Wang R, Hu Q, Xiong X. Chemical Characterization and Antitumor Activities of Polysaccharide Extracted from Ganoderma lucidum. International Journal of Molecular Sciences. 2014; 15(5):9103-9116. https://doi.org/10.3390/ijms15059103
Chicago/Turabian StyleLiang, Zengenni, Youjin Yi, Yutong Guo, Rencai Wang, Qiulong Hu, and Xingyao Xiong. 2014. "Chemical Characterization and Antitumor Activities of Polysaccharide Extracted from Ganoderma lucidum" International Journal of Molecular Sciences 15, no. 5: 9103-9116. https://doi.org/10.3390/ijms15059103